Learning Objectives
Understand the epidemiology of transfusion-related adverse events and their impact on care
Review the evidence for premedication prior to platelet transfusion
Clinical case
An 11-year-old boy with acute lymphoblastic leukemia undergoing induction therapy received a platelet transfusion without premedication. Midway through the transfusion, he experienced a fever of 38.5°C, increasing from a baseline of 37.1°C, accompanied by chills and rigors. His other vital signs remained stable. The transfusion was stopped, and his symptoms resolved within 30 minutes with a decline in temperature to baseline. Workup confirmed unit compatibility with the patient and did not reveal any evidence of bacteremia or hemolytic transfusion reaction. Should this patient be premedicated prior to subsequent platelet transfusion?
Discussion
Over half of pediatric oncology patients require transfusion support with platelet products during therapy for their malignancy. The median number of platelet transfusions required per patient ranges from 2 to 25 and varies on the basis of diagnosis and therapy.1 Transfusion-related adverse events (TRAEs) complicate ≤14% of all platelet transfusions.2,3 The most commonly occurring reactions are febrile nonhemolytic transfusion reactions (FNHTRs) and allergic reactions.4 FNHTRs are identified by fever (>38°C and a change in temperature of >1°C) during or shortly following transfusion and can be associated with additional symptoms such as chills, headache, nausea, or vomiting.5 Allergic reactions occur in 1% to 2% of platelet transfusions and are typically mild or moderate, with cutaneous symptoms of erythema, urticaria, and pruritus. However, severe allergic reactions, including anaphylaxis, can occur as well.5 The risk of TRAEs may be greater in pediatric patients than in adult patients. In a retrospective analysis of data from a 6-year period, children experienced an increased rate of allergic reactions (833 vs 358 per 100 000 platelet transfusions) and FNHTR (155 vs 126 per 100 000 platelet transfusions) compared with adults.6
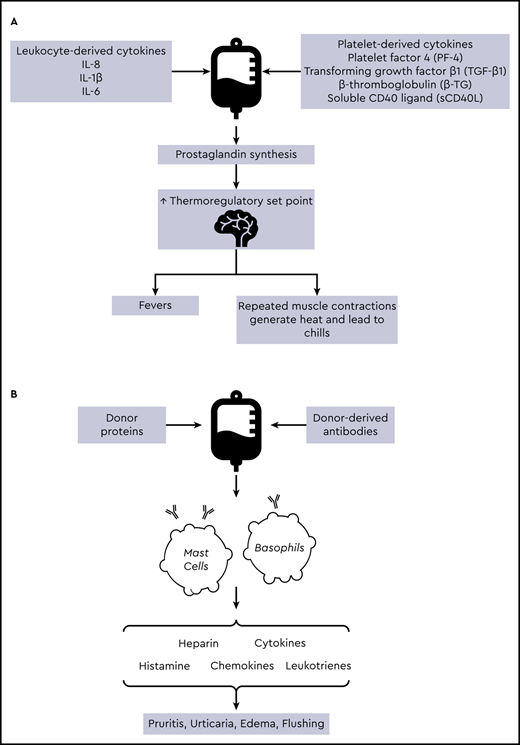
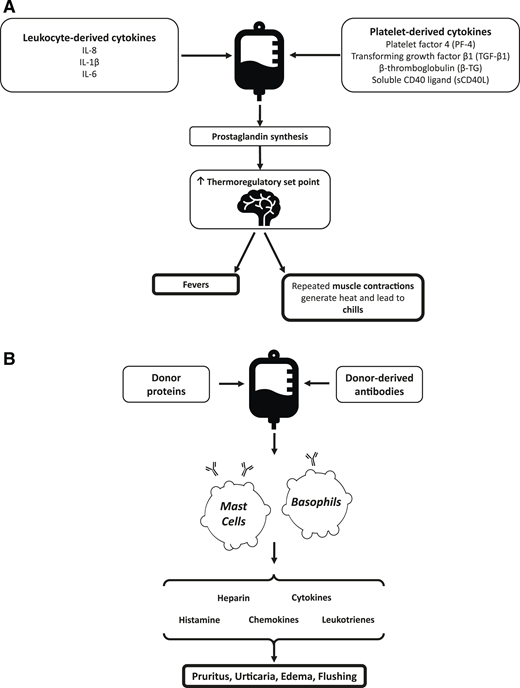
The are several factors that contribute to the pathophysiology underlying platelet transfusion reactions (Figure 1). In FNHTRs, cytokines (including interleukin-1, interleukin-6, and tumor necrosis factor-α) accumulate within plasma supernatant prior to transfusion. When administered to a recipient, these inflammatory mediators interact with the hypothalamic thermoregulatory center, leading to an increase in body temperature.5,7 Allergic reactions occur secondary to interactions between donor proteins and immunoglobulin E, leading to mast cell and basophil activation.8 Premedication with antipyretics and antihistamines has been used in an attempt to decrease the incidence of FNHTRs and allergic reactions. The earliest published research on antihistamines to prevent TRAEs was produced in the 1950s after investigators inoculated whole blood with diphenhydramine, chlorpheniramine, or other antihistamines and noted a subsequent decrease in the rate of febrile and allergic reactions.9,10 A Cochrane review on the topic of premedication for the prevention of TRAEs concluded there was insufficient evidence to support the use of premedication on the basis of existing data.11 Despite this, the use of premedication remains widespread. In a survey of pediatric physicians from 15 tertiary hospitals, the majority of respondents reported administering premedication prior to platelet transfusion in ≤25% of transfusions. Twelve percent of respondents reported administering premedication in 26% to 50% of transfusions.12 Observational studies have reported 60% to 80% of adult patients receive premedication prior to transfusion.13,14 However, data supporting the efficacy of this practice are limited.
Pathophysiology of FNHTRs (A) and allergic reactions (B) in platelet transfusions. IL, interleukin.
Pathophysiology of FNHTRs (A) and allergic reactions (B) in platelet transfusions. IL, interleukin.
Two studies have evaluated the use of premedication prior to platelet transfusion in pediatric patients.15,16 The first retrospectively reviewed all blood and platelet transfusions administered to pediatric hematology-oncology patients over the course of 1 year.15 In 385 patients receiving 8277 blood product transfusions, a total of 4280 platelet concentrates were administered. Premedication was provided in 68% of transfusions, including in 63% of patients who had no prior history of TRAEs. Allergic reactions and FNHTRs complicated 0.86% and 0.21% of platelet transfusions, respectively, and 0.61% and 0.36% of red blood cell transfusions. However, premedication with diphenhydramine did not significantly affect the development of allergic reaction (odds ratio, 1.74; 95% confidence interval [CI], 0.99-3.06). Likewise, acetaminophen premedication was not associated with a decrease in FNHTRs in multivariate analysis (odds ratio, 1.74; 95% CI, 0.71-4.23).15
Patterson et al16 collected data from both adult and pediatric patients across 5 hospitals. They reported the rate of TRAEs following platelet transfusion at 3 points in time: prior to implementation of a protocol standardizing premedication use, following protocol institution, and after applying universal prestorage leukoreduction of all platelet products. The protocol dictated that premedication was indicated only if a febrile or allergic reaction had occurred in both of the 2 prior transfusions. Following protocol implementation, the use of premedication prior to platelet transfusion decreased from 73.1% to 50%. Despite this decrease in premedication use, among pediatric patients, the number of platelet transfusions complicated by TRAEs did not significantly change (27.9% vs 25.8% of transfusions). However, a decrease in TRAEs was noted after implementing universal leukoreduction, with only 13.9% of subsequent transfusions in pediatric patients complicated by TRAEs.16
If data from the adult literature are considered, evidence for the efficacy of premedication remains limited. In a large retrospective study of 34 867 platelet transfusions over a 5-year period, Ezidiegwu et al14 found the incidence of FNHTRs within their population to be 0.09%. In their institution, acetaminophen is routinely prescribed to all patients prior to transfusion. The authors compared the incidence of FNHTR within their hospital with published rates in the literature of 0.11% to 38%, and they considered this indirect evidence of premedication efficacy. However, the results of a randomized trial conducted by Wang et al17 did not conclude premedication was effective. In this study, 55 hematology-oncology patients received 122 platelet transfusions. Randomization to premedication (consisting of 650 mg of acetaminophen and 25 mg of diphenhydramine) or placebo occurred prior to each platelet transfusion. There was no significant difference in the number of transfusions affected by nonhemolytic transfusion reactions between the 2 groups (15.4% vs 15.2%). There were only 3 cases of urticarial reaction, all of which were experienced in the placebo group.17
A second randomized trial, conducted by Kennedy et al, also did not provide convincing evidence for the routine use of premedication.18 In their study, 315 patients admitted to either the leukemia or bone marrow transplantation services were assigned to receive either placebo or a combination of 500 mg of acetaminophen and 25 mg of diphenhydramine 30 minutes prior to transfusion. A total of 2333 platelet transfusions were administered, which represented 55% of all blood products transfused to study patients. The authors found no significant difference in the incidence of allergic reactions (1.05 vs 0.68 reactions per 100 transfusions) between the intervention and placebo groups. There was a slight reduction in the incidence of FNHTRs in the intervention group (0.35 vs 0.64 reactions per 100 transfusions), which was not significant. In multivariate regression analysis, premedication was associated with decreased hazard of FNHTRs (hazard ratio, 0.48; 90% CI, 0-0.89) after adjusting for age, race, sex, and diagnosis. On the basis of these results, the authors estimated 344 transfusions would require premedication to prevent a single FNHTR.18
It is important to consider that there is significant heterogeneity between the studies discussed, which makes direct comparison of results difficult. Studies varied in terms of patient population, outcome measures, and inclusion and exclusion criteria (such as exclusion of patients with a history of prior FNHTRs). Importantly, both acetaminophen and diphenhydramine have an onset of action within 30 to 60 minutes. Of the studies described, only the one by Kennedy et al18 included a recommendation regarding the timing of premedication administration in their protocol (30 minutes prior to transfusion), though no data were provided regarding compliance with this recommendation. Other cited works did not include information on the temporal relationship between premedication and transfusions. Therefore, it can be difficult to conclude if a lack of demonstrated premedication efficacy was secondary to suboptimal use of medications. An additional historical element to take into account is a history of TRAEs with a prior transfusion, which seems to be associated with an increased risk of recurrent transfusion reactions.17 A history of reaction often influences practitioner decision making when determining if premedication use is appropriate.16,19 This group of patients was excluded from the study by Kennedy et al18 ; however, Sanders et al15 found that regardless of the number of prior reactions, premedication was not associated with a decreased incidence of TRAEs.
Although the majority of TRAEs are considered to be mild in severity, they can have serious implications for patient quality of life and cost of care. In one study describing 437 FNHTRs, 93% of patients required interruption of transfusions for evaluation, and transfusions were resumed in only 15% of cases. Blood cultures and imaging studies were frequently obtained to determine the source of symptoms, and in 15% of cases, patients required admission from the outpatient setting due to FNHTRs.20 These interventions can lead to significant cost burdens.14,20 Pediatric oncology patients are at a heightened risk of developing fevers, with febrile neutropenia as a result of myelosuppressive chemotherapy being one of the most common adverse effects of therapy. In some cases, the benefit of therapy with antipyretics prior to transfusion may lie in their efficacy in reducing temperature elevation associated with coincidental neutropenia or infection and therefore avoiding costly evaluation of fevers falsely attributed to blood products.21
Other options exist to decrease the risk of TRAEs through modifications to the platelet product itself. Prestorage leukoreduction is associated with a >90% reduction in the risk of TRAEs due to a decrease in cytokine levels stored in platelet products.22,23 Through a similar mechanism, the use of concentrated or washed products reduces transfused plasma volume and decreases TRAEs.24 The use of platelet additive solution may also be associated with fewer TRAEs.25 In conclusion, although the utility of premedication prior to transfusion is yet to be proved, careful consideration of premedications based on individual circumstances and the use of blood product modifications may be beneficial in conserving limited resources.
Correspondence
Darrell Triulzi, University of Pittsburgh Institute for Transfusion Medicine, 3636 Blvd of the Allies, Pittsburgh, PA 15213; e-mail: dtriulzi@itxm.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.