Abstract
Prophylactic platelet transfusions are used to reduce the risk of spontaneous bleeding in patients with treatment- or disease-related severe thrombocytopenia. A prophylactic platelet-transfusion threshold of <10 × 103/µL has been shown to be safe in stable hematology/oncology patients. A higher threshold and/or larger or more frequent platelet doses may be appropriate for patients with clinical features associated with an increased risk of bleeding such as high fevers, sepsis, disseminated intravascular coagulation, anticoagulation therapy, or splenomegaly. Unique factors in the outpatient setting may support the use of a higher platelet-transfusion threshold and/or dose of platelets. A prophylactic platelet-transfusion strategy has been shown to be associated with a lower risk of bleeding compared with no prophylaxis in adult patients receiving chemotherapy but not for autologous transplant recipients. Despite the use of prophylactic platelet transfusions, a high incidence (50% to 70%) of spontaneous bleeding remains. Using a higher threshold or larger doses of platelets does not change this risk. New approaches to reduce the risk of spontaneous bleeding, including antifibrinolytic therapy, are currently under study.
Learning Objectives
Define the appropriate threshold for prophylactic platelet transfusion in a stable hematology/oncology patient
List the clinical conditions for which a higher threshold may be indicated
Describe the limitations of prophylactic platelet transfusions and the potential role of additional strategies to reduce the risk of bleeding
Clinical case
A 60-year-old woman with newly diagnosed acute myelogenous leukemia (AML) is admitted for induction therapy. Her platelet count on admission is 14 × 103/µL. She reports a minor nosebleed the day before that lasted for <5 minutes. She denies headaches or visible blood in her sputum, urine, or stools. Her physical examination is only remarkable for a few scattered petechiae on both arms. Urinalysis and stool guaiac for blood is negative.
Treatment-related questions
What is the role of prophylactic platelet transfusion in this patient? What is her risk of significant bleeding at a platelet count of 14 × 103/µL? Would platelet transfusion change her risk of bleeding?
The next day, her platelet count is 5 × 103/µL. No new bleeding is identified.
More treatment-related questions
Is there a role for prophylactic platelet transfusion at this point in her course? Has her risk of bleeding changed, and would platelet transfusions reduce her risk of bleeding?
Rationale for prophylactic platelet transfusions
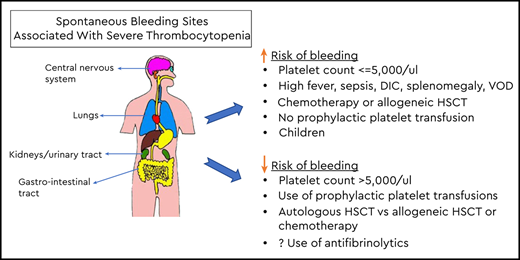
It has been known for decades that patients with treatment- or hematologic disease–related severe thrombocytopenia have an increased risk of bleeding. Although most bleeding episodes are mild, there is a risk of severe or even fatal bleeding involving critical sites such as the lungs, brain, or eyes.1 The biologic basis for prophylactic platelet transfusion was provided in a radiolabeled platelet-transfusion study, which suggested that 7 × 103/µL per day are required to maintain endothelial integrity.2 Since then, prophylactic platelet transfusions have been routinely used to reduce the risk of bleeding, and they account for nearly 50% of all platelets transfused to patients with thrombocytopenia due to hematologic/oncologic disease or treatment-related thrombocytopenia.3
Prophylactic platelet-transfusion threshold
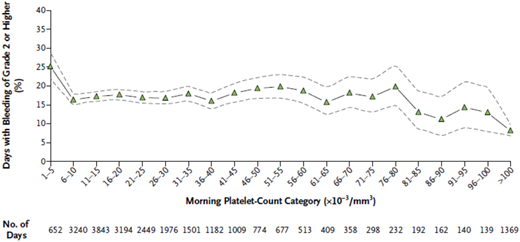
The prophylactic platelet-transfusion threshold has been evolving over the years since platelet transfusions first became available in the 1960s. For decades, a threshold of <20 × 103/µL was used for most patients. As treatment regimens changed and improvements were made in the clinical management of thrombocytopenic patients, particularly in the management of infections, it became apparent that lower platelet counts could be safely tolerated than had been previously believed. This spawned a series of observational and randomized clinical trials comparing the risk of major bleeding using a prophylactic platelet count of ≤10 × 103/µL vs <20 × 103/µL.4 Four randomized trials5-8 demonstrated the safety of a lower threshold in that there was no difference in the rate of major bleeding events using a prophylactic threshold of <10 × 103/µL vs <20 × 103/µL or higher (Table 1). The lower threshold was also safe even in patients undergoing autologous or allogeneic hematopoietic stem cell transplantation (HSCT).7,8 Admittedly, these are relatively small studies, however, additional supportive data came from a secondary analysis of a large randomized trial of the prophylactic platelet-transfusion dose.1 The Platelet Dosing (PLADO) study used a platelet-transfusion threshold of ≤10 × 103/µL in 1272 patients with over 24 000 days of bedside observation, demonstrating that bleeding occurred on 25% of the study days when the platelet count was 5 × 103/µL or lower, as compared with 17% of study days when platelet count was >5 × 103/µL (P < .001) (Figure 1). These data suggest that the risk of bleeding exhibits a threshold effect and does not appear to change once the platelet count is above 5 × 103/µL, consistent with the estimate of ∼7.1 × 103/µL platelets per day required to maintain vascular integrity in a nonfebrile, stable patient.2 The results of the randomized trials and the results of the PLADO study support the use of ≤10 × 103/µL as a prophylactic platelet-transfusion threshold in stable hematology/oncology patients.
RCTs comparing prophylactic platelet-transfusion thresholds
| Study/Year . | 10 × 103/µL threshold . | 20 × 103/µL threshold . | P . | ||
|---|---|---|---|---|---|
| No. of patients . | Major bleeding . | No. of patients . | Major bleeding . | ||
| Rebulla et al5 /1997 | 135 | 22% | 120 | 20% | .41 |
| Heckman et al6 /1997 | 37 | 4 episodes/patient | 41 | 2 episodes/patient | .12 |
| Zumberg et al7 /2002 | 78 | 14% | 81 | 17% | .66 |
| Diedrich et al8 /2005* | 79 | 18% | 87 | 14% | NS |
| Study/Year . | 10 × 103/µL threshold . | 20 × 103/µL threshold . | P . | ||
|---|---|---|---|---|---|
| No. of patients . | Major bleeding . | No. of patients . | Major bleeding . | ||
| Rebulla et al5 /1997 | 135 | 22% | 120 | 20% | .41 |
| Heckman et al6 /1997 | 37 | 4 episodes/patient | 41 | 2 episodes/patient | .12 |
| Zumberg et al7 /2002 | 78 | 14% | 81 | 17% | .66 |
| Diedrich et al8 /2005* | 79 | 18% | 87 | 14% | NS |
NS, not significant.
Compared 10 × 103/µL vs 30 × 103/µL.
Days with bleeding of grade 2 or higher in all 3 treatment groups, according to morning platelet-count categories. Days with bleeding of grade 2 or higher in all 3 treatment groups, according to morning platelet-count categories. The percentage of days on which patients had bleeding of grade 2 or higher is shown, along with the associated 95% confidence intervals (dashed lines), according to the morning platelet-count category. Data are based on the 24 309 days during the study period on which patients had both a morning platelet count and information on bleeding of grade 2 or higher. Reprinted from Slichter et al1 with permission.
Days with bleeding of grade 2 or higher in all 3 treatment groups, according to morning platelet-count categories. Days with bleeding of grade 2 or higher in all 3 treatment groups, according to morning platelet-count categories. The percentage of days on which patients had bleeding of grade 2 or higher is shown, along with the associated 95% confidence intervals (dashed lines), according to the morning platelet-count category. Data are based on the 24 309 days during the study period on which patients had both a morning platelet count and information on bleeding of grade 2 or higher. Reprinted from Slichter et al1 with permission.
The data supporting a prophylactic platelet-transfusion trigger in pediatric hematology/oncology patients are less robust. Three of the randomized trials5,7,8 included pediatric patients, but 1 study, by Rebulla et al,5 only included children who were age 16 years or older; another trial, by Zumberg et al,7 did not state how many of the 159 subjects were pediatric patients nor their mean age. Only Diedrich et al8 indicated that 51 of the 166 patients in the trial were pediatric patients. The PLADO study included 198 pediatric patients transfused using a trigger of ≤10 × 103/µL.9 Currently, there is no prospective randomized controlled trial (RCT) comparing platelet-transfusion thresholds exclusively in pediatric hematology/oncology patients. Thus, there are a limited number of pediatric patients and data upon which the recent guidelines promulgated by the American Society of Clinical Oncology (ASCO)10 and the International Collaboration for Transfusion Medicine Guidelines11 have been based. These guidelines recommend 10 × 103/µL as the prophylactic platelet-transfusion trigger for stable pediatric hematology/oncology patients.
Clinical factors that may impact the prophylactic platelet-transfusion threshold
Currently, most physician and hospital transfusion guidelines recommend a threshold platelet count of ≤10 × 103/µL as an indication for platelet transfusion in stable hematology/oncology patients. The platelet count is not the only consideration in determining bleeding risk in individual patients. Patients may have clinical factors associated with an increased risk of bleeding requiring an individualized approach. Increased platelet consumption and/or an increased risk of bleeding has been reported in patients with high fever, sepsis/infections, antifungal therapy, splenomegaly, coagulopathy, anticoagulant therapy, graft-versus-host disease, and veno-occlusive disease/sinusoidal obstruction syndrome.12-14 A higher prophylactic platelet-transfusion threshold (eg, <20 × 103/µL) and/or larger or more frequent doses is a reasonable approach to ensure that the nadir platelet count prior to the next transfusion is maintained above the critical 5 × 103/µL. For prophylactic platelet transfusions in the outpatient setting, logistics, a lack of daily patient observation, and a potential delay in receiving therapy should bleeding occur need to be taken into consideration when formulating transfusion decisions. Outpatients may benefit from using a higher threshold or larger doses to lengthen the intervals between transfusion1 ; however, this strategy has been extrapolated from inpatient studies and has not been studied in outpatients.
The prophylactic platelet-transfusion threshold data from the RCTs5-8 and other supportive observational trials4 demonstrate that the threshold can be safely lowered to ≤10 × 103/µL for stable patients including HSCT recipients who do not have clinical factors for increased risk of bleedings such as high fever, sepsis, anatomical lesions, or other abnormality of hemostasis.10,11,15 There are limitations to the available data when applied to subsets of hematology/oncology patients such as pediatric patients and outpatients.
Optimal dose of prophylactic platelets
The dose of platelets used for prophylaxis has gradually declined over the years from 10 whole-blood platelets in a pool to as few as 4 U in a pool, roughly equivalent to a single unit of apheresis platelets.16 The impact of prophylactic platelet dose on bleeding outcomes has been assessed in 6 RCTs.17 The largest and most recent of these studies is PLADO.16 This trial randomized 1272 evaluable patients to 1 of 3 platelet-dosing strategies: low dose (1.1 × 1011 platelets per m2) vs medium dose (2.2 × 1011 platelets per m2) vs high dose (4.4 × 1011 platelets per m2), which are equivalent to roughly one-half unit of apheresis platelet vs 1 U of apheresis platelet vs 2 U of apheresis using a prophylactic platelet-transfusion threshold of ≤10 × 103/µL. The study’s primary end point was World Health Organization (WHO) grade ≥2 bleeding assessed daily through physical examination and medical record review. The study found that the bleeding rate was the same in each of the 3 arms, occurring in ∼70% of subjects regardless of platelet-dose strategy. The higher-dose arm not only had the longest intertransfusion interval but also used the most platelets. A subset analysis of the PLADO study limited to 198 pediatric patients also found no difference in bleeding rates between the groups but did find a higher overall rate of bleeding of 84% compared with 67% in adults.9 A meta-analysis of the 6 RCTs concluded that a low-dose strategy for prophylactic platelet transfusion was not associated with an increased risk of bleeding compared with medium- or higher-dose strategies.17
These data support the safety of a low-dose platelet strategy for prophylactic platelet transfusion for stable adult and pediatric hematology/oncology inpatients including HSCT recipients. In clinical practice, low-dose platelets are typically reserved for periods of platelet shortages. The low-dose strategy may not be appropriate for outpatients in whom a longer transfusion interval would be desirable as was seen with the higher-dose strategy in PLADO.1 These data derived in the prophylactic setting should not be extrapolated to patients who need platelet transfusions for active bleeding as lower-dose platelets in this setting have not been adequately studied.
Prophylactic vs therapeutic platelet transfusion
The strategy of using platelets prophylactically balances the benefits of reducing the risk of bleeding vs the risks and expense of multiple platelet transfusions. As treatment regimens evolve, supportive care improves, and the duration of severe thrombocytopenia declines, particularly for autologous stem cell transplants, investigators have questioned whether a prophylactic platelet-transfusion strategy is still beneficial. The alternative would be to only transfuse platelets when patients exhibit evidence of bleeding; this is known as a therapeutic platelet-transfusion strategy. This concept is not new as 3 small RCTs totaling 99 patients were performed >30 years ago and did not find a difference in bleeding outcomes between the prophylactic and therapeutic transfusion strategies.17 More recently, 2 larger RCTs compared bleeding outcomes between a prophylactic vs therapeutic platelet-transfusion strategy (Table 2).18,19 Wandt et al18 studied 391 adults with AML undergoing chemotherapy or autologous stem cell transplantation primarily for multiple myeloma or lymphoma. The primary end point of the study was the number of platelet transfusions. The therapeutic strategy resulted in a 33.5% reduction in number of transfusions, however, the incidence of WHO grade 2 or higher bleeding was significantly higher in the therapeutic group (42%) vs the prophylactic group (19%). Importantly, the rates of more serious grade 3 and grade 4 bleeding were also higher in the therapeutic group. Grade 4 bleeding, including central nervous system bleeding events, occurred in 5% in the therapeutic group vs 1% in the prophylactic group (P = .16). Subgroup analyses in the AML group were similar to the overall results. However, in the 201 patients who underwent autologous stem cell transplantation, grade 2 hemorrhages were more common in the therapeutic group, but there were no differences in the more serious grade 3 or 4 hemorrhages between groups. The TOPPS trial by Stanworth et al19 studied 600 patients age 16 years or older who received chemotherapy or HSCT for hematologic malignancy (Table 2). This trial was designed as a noninferiority trial. The primary end point of WHO grade 2 or higher bleeding was higher in the therapeutic group (50% vs 43%), thus noninferiority was not achieved (the therapeutic strategy was inferior to the prophylactic strategy). The patients who received prophylactic platelet transfusions also had fewer days of bleeding, a shorter time to the first bleed, and a trend toward fewer serious grade 3 or 4 bleeding events (6 of 301 vs 1 of 299; P = .13). A prespecified subgroup analysis of the 410 patients (70%) in the trial who underwent autologous transplantation found no difference in WHO grade 2 or higher bleeding (47% vs 45%) between the arms.
Large RCTs of prophylactic vs therapeutic platelet transfusion
| Study/Year . | Prophylactic strategy 10 × 103/µL . | Therapeutic strategy . | P† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients . | Bleeding grade,* % . | No. of patients . | Bleeding grade,* % . | ||||||
| ≥2 . | 3 . | 4 . | ≥2 . | 3 . | 4 . | ||||
| Wandt et al18 /2012 | 194 | 19 | 3 | 4 | 197 | 42 | 7 | 14 | <.0001 |
| Stanworth et al19 /2013 | 299 | 43 | 0.3 | 0 | 301 | 50 | 1.3 | 0.7 | .06** |
| Study/Year . | Prophylactic strategy 10 × 103/µL . | Therapeutic strategy . | P† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients . | Bleeding grade,* % . | No. of patients . | Bleeding grade,* % . | ||||||
| ≥2 . | 3 . | 4 . | ≥2 . | 3 . | 4 . | ||||
| Wandt et al18 /2012 | 194 | 19 | 3 | 4 | 197 | 42 | 7 | 14 | <.0001 |
| Stanworth et al19 /2013 | 299 | 43 | 0.3 | 0 | 301 | 50 | 1.3 | 0.7 | .06** |
WHO bleeding grading system.1
P value for primary end point WHO grade 2 or higher bleeding.
P value for inferiority.
Thus, both of these trials provide evidence supporting the benefit of prophylactic platelet transfusions in reducing overall and serious bleeding events compared with a therapeutic platelet-transfusion strategy in adults. The evidence for benefit is predominantly in adult hematology/oncology patients treated with chemotherapy; interestingly, both studies found little benefit in autologous stem cell transplant recipients, prompting the ASCO to recommend a therapeutic transfusion strategy in autologous stem cell transplants recipients.10 There were too few patients who underwent allogeneic stem cell transplant to draw a conclusion about the safety of a therapeutic platelet-transfusion strategy, and pediatric patients <16 years old were not studied in either trial.
New approaches to reducing the risk of spontaneous bleeding
Acute leukemia patients and HSCT transplant recipients are at higher risk of bleeding compared with patients with other causes of severe thrombocytopenia, such as immune thrombocytopenic purpura, due to chemotherapy-induced damage to the endothelium, graft-versus-host disease, infection, and their primary disease. The PLADO1 and TOPPS19 studies used a ≤10 × 103/µL prophylactic platelet-transfusion threshold, and found the incidence of WHO grade 2 or higher bleeding was quite high, ranging from 43% to 79% in adults and up to 84% in children.9 Using a higher threshold5-8 or using a higher-dose platelet-transfusion strategy1 does not change bleeding rate. This led investigators to explore alternative strategies to enhance hemostasis. For many years, antifibrinolytic drugs such as ε amino caproic acid or tranexamic acid (TXA) have been used to reduced bleeding in thrombocytopenic patients.20,21 Anecdotal experience and observational studies suggest efficacy, but rigorous studies are lacking. There are 2 phase 3 double-blinded placebo-controlled RCTs under way studying the efficacy of TXA on bleeding in hematology/oncology patients with severe thrombocytopenia. The National Heart, Lung, and Blood Institute (NHLBI)-funded American Trial Using Tranexamic Acid in Thrombocytopenia (A-TREAT; NCT02578901) recently completed its planned enrollment of 330 evaluable adult patients with hematologic malignancy or aplasia. Patients were randomized to receive TXA or placebo when their platelet count fell below 30 × 103/µL until they experienced platelet count recovery or 30 days on study, whichever came first. The primary end point was WHO grade 2 or higher bleeding assessed daily at the bedside. The results of this trial are expected soon. The Trial to Evaluate Tranexamic Acid Therapy in Thrombocytopenia (B-TREATT; NCT03136445) is the UK version of this study. This double-blinded and placebo-controlled trial plans to enroll ∼600 patients with hematologic malignancy and severe thrombocytopenia. The study intervention is similar to A-TREAT and the primary end point is the proportion of patients with WHO grade 2 or higher bleeding or death within the first 30 days. Enrollment is ongoing but is expected to be completed in 2020. These studies should provide high-quality data defining the role of TXA in addition to prophylactic platelet transfusion in mitigating the risk of spontaneous bleeding in adult hematology oncology patients with severe thrombocytopenia. The use of TXA in children has been limited to surgical patients and has not been studied in pediatric hematology/oncology patients. Studies of TXA or other strategies to reduce bleeding in this population are needed.
Summary
In stable hematology/oncology patients, a prophylactic platelet-transfusion threshold of ≤10 × 103/µL would appear to provide a reasonable safety margin to ensure a nadir circulating platelet count above 5 × 103/µL, a level below which the risk of bleeding appears to increase. A higher threshold and/or larger or more frequent doses may be appropriate for patients with clinical features associated with an increased risk of bleeding or in the outpatient setting. There is a high incidence of spontaneous bleeding despite the use of a prophylactic platelet-transfusion strategy. New approaches to reducing the risk of bleeding including antifibrinolytic therapy are currently under study.
Clinical case revisited
Returning to the 60-year-old woman with newly diagnosed AML admitted for induction therapy, her platelet count on admission was 14 × 103/µL. She reported a minor nosebleed the day before lasting <5 minutes. She denied headaches or visible blood in her sputum, urine, or stools. Her physical examination was only remarkable for a few scattered petechiae on both arms. Urinalysis and stool guaiac for blood were negative.
What is the role of prophylactic platelet transfusion in this patient?
Since she is stable with only minor WHO grade 1 bleeding and a platelet count >10 × 103/µL, prophylactic platelet transfusion is not indicated.
What is her risk of significant bleeding at a platelet count of 14 × 103/µL?
She would be expected to have a grade 2 or higher hemorrhage on 17% of days or roughly 1 in 6 days when her platelet concentration is >10 × 103/µL (Figure 1).
Would platelet transfusion change her risk of bleeding?
No, the risk of bleeding remains unchanged even if she were to be transfused to a higher platelet count (Figure 1). The next day, her platelet count is 5 × 103/µL. No new bleeding is identified.
Is there a role for prophylactic platelet transfusion at this point in her course?
Yes, based on her platelet count, prophylactic platelet transfusion is indicated.
Has her risk of bleeding changed, and would platelet transfusions reduce her risk of bleeding?
Yes. She is at increased risk of bleeding with a platelet count of 5 × 103/µL and her risk will be decreased with a platelet transfusion (Figure 1).
Correspondence
Darrell J. Triulzi, Vitalant Clinical Services, University of Pittsburgh, 3636 Boulevard of the Allies, Pittsburgh, PA 15213; e-mail: dtriulzi@vitalant.org.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.