Abstract
The treatment of chronic lymphocytic leukemia (CLL) embodies one of the great success stories in translational research, with the development of therapies aimed at disrupting crucial pathways that allow for the survival and proliferation of the malignant clone. The arrival of targeted agents into our armamentarium, along with the advent of novel monoclonal antibodies that can achieve deeper remissions, has steered the field to a new treatment paradigm. Given the panoply of therapeutic options available, the question arises whether chemotherapy still has a role in the management of CLL. The novel targeted agents, which include the Bruton’s tyrosine kinase inhibitors, ibrutinib and acalabrutinib, along with the B-cell lymphoma-2 inhibitor, venetoclax, are highly effective in achieving a response with improved remission duration and survival, particularly in high-risk patients. Despite this major progress, the new agents bring a unique set of toxicities unlike those associated with cytotoxic chemotherapy. There is a paucity of head-to-head comparisons among all of the novel agents, because their approval was based on randomization against traditional chemoimmunotherapeutic regimens. Parallel to the increase in the number of available targeted agents, there has been a significant improvement in quality of life and life expectancy of the patients with a CLL diagnosis over the last decade. Our review will examine whether “chemotherapy-free” frontline treatment approaches are worth the associated risks. Our goal is to help identify optimal treatment strategies tailored to the individual by reviewing available data on monotherapy vs combination strategies, depth of response, treatment duration, and potential toxicities.
Learning Objectives
Review the current “chemotherapy-free” regimens available for the management of patients with treatment-naive chronic lymphocytic leukemia
Review the prognostic markers that identify which patients may benefit from chemoimmunotherapy based approach vs the use of targeted agents
Recognize criteria for selection of the optimal treatment strategy
Introduction
In 2020, the Surveillance, Epidemiology and End Result program database estimated 21 040 new cases of chronic lymphocytic leukemia (CLL) in the United States, with 4060 deaths attributed to this disease.1 The natural history of CLL is variable, and outcomes are influenced by patient characteristics, clinical factors at the time of diagnosis, and the intrinsic biology of the tumor. Given the heterogeneity of the disease, there is no “one size fits all” recommendation. Until recently, systemic chemoimmunotherapy (CIT) had been considered the standard of care for frontline management. The CIT regimens of fludarabine/cyclophosphamide/rituximab (FCR) and bendamustine/rituximab (BR) had demonstrated excellent response rates, progression-free survival (PFS), and overall survival (OS) in the patients who could tolerate these regimens.2,3 Nonetheless, significant myelosuppression and infectious complications made their use difficult to tolerate in elderly patients with comorbidities. Additionally, patients with a deletion 17p (del17p) or TP53 mutation (TP53mut) were considered ultrahigh risk,4 because these patients progressed more rapidly and invariably relapsed shortly after CIT. The frontline treatment paradigm changed with the approval of the first-in-class Bruton’s tyrosine kinase inhibitor (BTKi), ibrutinib, in patients with del17p.5 This approval was a revolution in the treatment of del17p/TP53mut, achieving an OS never seen with prior therapies.6-8 Shortly after, ibrutinib was approved for all patients and, later, other targeted agents followed, including the second-generation BTKi, acalabrutinib, and the B-cell lymphoma-2 (BCL-2) inhibitor, venetoclax. All of these agents are now preferred regimens in the United States for the initial treatment of CLL, with or without del17p.9
In the era of multiple available targeted agents and CIT approaches, the following questions remain: Which patients benefit most from each regimen? Are the novel regimens worth their risk for potential complications? Is there a role for early intervention now that these novel agents are available? Are these drugs best used sequentially or in combinations?
Clinical case part 1
A 63-year-old man with a history of hypertension and hyperlipidemia presents for evaluation after an incidental finding of lymphocytosis (8000 lymphocytes per microliter) on routine complete blood count with normal hemoglobin and platelet count. On examination, he has mildly enlarged axillary and inguinal lymph nodes (∼1.5-2 cm). No hepatosplenomegaly is appreciated on physical examination. He denies any constitutional symptoms. Flow cytometry reveals a CD5+ CD10− CD19+ CD20dim CD23+ CD200+ and κ-restricted monoclonal B cell population, confirming a diagnosis of CLL. Additional prognostic testing reveals unmutated immunoglobulin heavy chain (UM-IGHV) with a normal β-2 microglobulin level. Fluorescence in situ hybridization (FISH) testing is positive for trisomy 12. Next-generation sequencing does not reveal TP53mut.
Management of treatment-naive CLL in the era of targeted therapies
The role of prognostic factors
Risk stratification gives us the tools to deliver appropriately targeted care in a disease with a clinical presentation as varied as CLL. Outcomes are influenced by comorbidity burden, clinical factors, and the genetics of the tumor. Two staging criteria, the Rai10 and Binet11 systems, are widely used in clinical practice because of their simplicity (only a physical examination and a complete blood count are needed) and accuracy in predicting outcomes. High-risk group patients (Rai III/IV, Binet C) have a more aggressive clinical course associated with shorter survival. Further risk stratification is based on molecular and genomic studies, including IGHV mutation status12 and cytogenetic analysis with FISH testing, including del17p,13 del11q, trisomy 12, and del13q.14 Complex karyotype, defined as ≥3 to 5 chromosomal abnormalities is associated with a worse prognosis.15,16 Genomic mutations, particularly in TP53,17,18 mark a more aggressive disease course. In 2016, the CLL International Prognostic Index (CLL-IPI) incorporated important genomic factors (IGHV, TP53 mutational state) with Rai/Binet stage, β-2 microglobulin level, and age to predict survival.19 The validity of the CLL-IPI has been confirmed across several series, including mostly younger patients treated with CIT.20 This novel prognostic index appears to be predictive of time to first therapy20 in early-stage disease, thus identifying high-risk cohorts that may benefit from participation in early-intervention clinical trials. Nonetheless, the utility of the CLL-IPI to predict OS in patients starting targeted therapy remains uncertain.21
Timing and selection of therapeutic strategy
Consensus guidelines of the International Workshop on Chronic Lymphocytic Leukemia recommend initiating treatment only at the onset of constitutional symptoms (Table 1).22 Data are lacking at this time to suggest a benefit of early intervention based on prior studies utilizing CIT at the time of diagnosis.23,24 Trials studying the role of novel agents for patients with high-risk CLL are ongoing,25-27 with a trial on ibrutinib against placebo presented in abstract form in 2019. The primary end point was event-free survival, defined as time from randomization until occurrence of active disease, new treatment, or death. At a median observation time of 31 months, event-free survival was 47.8 months in the placebo arm vs not reached in the ibrutinib arm (hazard ratio [HR], 0.25; 95% confidence interval [CI], 0.14-0.43; P < .0001). PFS was 14.8 months in the placebo arm vs not reached in the ibrutinib arm (HR, 0.18; 95% CI, 0.12-0.27). Time to next treatment was longer in the ibrutinib arm.27 Until data from the full survival analysis are available, continued active surveillance of early-stage patients who have an increased risk for progression remains the standard of care.
International Workshop on Chronic Lymphocytic Leukemia 2018 indications for therapy
| Progressive marrow failure, as evidenced by development or worsening anemia < 10 g/dL or thrombocytopenia (<100 000 platelets per liter) |
| Massive or progressive symptomatic splenomegaly |
| Massive lymph nodes (>10 cm) or progressive symptomatic lymphadenopathy |
| Rapidly increasing lymphocytosis defined as an increase of 50% over a 2-mo period or a lymphocyte doubling time < 6 mo* |
| Autoimmune complications (anemia, thrombocytopenia) poorly responsive to corticosteroids |
| Symptomatic extranodal involvement |
| Constitutional symptoms |
| Unintentional weight loss > 10% within 6 months |
| Progressive fatigue |
| Temperature > 100.5°F for >2 weeks without another cause |
| Night sweats for >1 month without alternative etiology |
| ECOG PS > 2 if progressive/worsening |
| Progressive marrow failure, as evidenced by development or worsening anemia < 10 g/dL or thrombocytopenia (<100 000 platelets per liter) |
| Massive or progressive symptomatic splenomegaly |
| Massive lymph nodes (>10 cm) or progressive symptomatic lymphadenopathy |
| Rapidly increasing lymphocytosis defined as an increase of 50% over a 2-mo period or a lymphocyte doubling time < 6 mo* |
| Autoimmune complications (anemia, thrombocytopenia) poorly responsive to corticosteroids |
| Symptomatic extranodal involvement |
| Constitutional symptoms |
| Unintentional weight loss > 10% within 6 months |
| Progressive fatigue |
| Temperature > 100.5°F for >2 weeks without another cause |
| Night sweats for >1 month without alternative etiology |
| ECOG PS > 2 if progressive/worsening |
ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Absolute lymphocytosis alone is not an indication for treatment but can be used to determine disease pace; leukostasis rarely occurs in patients with CLL.
Clinical case part 2
Based on the above review of clinical presentation and prognostic markers, our patient did not meet criteria for initiation of CLL-directed therapy at the time. Over the next 4 years, the patient’s absolute lymphocyte count increases to 120 000 cells per microliter, and he develops symptomatic anemia with a hemoglobin of 9.8 g/dL and thrombocytopenia (platelets, 98 000 per microliter). He now has stage 3 chronic kidney disease (glomerular filtration rate, 50 mL/min per 1.73 m2) attributed to hypertension. His Eastern Cooperative Oncology Group (ECOG) performance status is 0, and he remains active. He has palpable cervical (3×3cm) and axillary (3×4-cm) lymphadenopathy, and his spleen tip is palpable 3 cm below the costal margin. Repeat FISH testing reveals trisomy 12 without del17p, and next-generation sequencing is negative for TP53mut. He understands that he is in need of therapy and wishes to discuss available treatments.
Brief overview of chemotherapeutic approaches
Fludarabine-based chemotherapy regimens were standard of care for patients younger than 65 to 70 years of age based on the data from the CLL8 trial, which demonstrated an improvement in PFS for patients treated with FCR compared with fludarabine-cyclophosphamide.2,28 Patients with mutated immunoglobulin heavy chain (M-IGHV) had the longest PFS, with the subset of patients achieving undetectable minimal residual disease (uMRD, defined as no evidence of CLL at a level of 1 in 10,000 cells) showing no evidence of disease relapse, even a decade posttreatment.29 However, this regimen is generally too toxic for older patients and those with comorbidities. CLL10 compared FCR with BR in patients with CLL who were fit to receive CIT.30 In the intention-to-treat population, BR was found to be inferior to FCR, with the exception of the subset of patients older than 65 years where the PFS was equivalent. Furthermore, FCR was associated with a higher rate of severe neutropenia and infectious complications, and this was more pronounced in patients above the age of 65 years. Based on these findings, BR was frequently used as a standard of care for patients over 65 years of age who were fit to receive intensive CIT. To determine optimal therapy for older unfit patients, CLL11 randomized patients who were not candidates for CIT to chlorambucil (Chl) monotherapy, chlorambucil-rituximab, or chlorambucil-obinutuzumab (ChlO).31 ChlO demonstrated a superior overall response rate (ORR) of 75.5% compared with ChlR (65.9%) and chlorambucil-rituximab (30%), with a median PFS of 29.2 months for ChlO.32 Given the shorter PFS compared with historical data for FCR and BR, Chl-based combinations were traditionally reserved for patients who were unable to tolerate intensive CIT.
The new era of chemotherapy-free frontline treatment regimens: ibrutinib, acalabrutinib, venetoclax, and beyond
The success of the BTKi, ibrutinib, in the relapsed/refractory setting,33 particularly for patients with high risk features, such as del17p/TP53mut, allowed for its quick approval as frontline treatment. This was followed by the RESONATE-2 trial, the frontline trial for patients over the age of 65 years who were unable to tolerate intensive regimens. Patients were randomized to receive ibrutinib vs Chl monotherapy. At 18.4 months of follow-up, median PFS for patients treated with ibrutinib was not reached compared with 18.9 months for patients treated with Chl, with a corresponding decrease in the risk of death of 84% in the ibrutinib group (HR, 0.16; P = .001).5 Long-term follow-up (median, 60 months) demonstrated a sustained PFS and OS benefit for patients treated with ibrutinib compared with Chl (5-year PFS: 70% vs 12%; HR, 0.146; 5-year OS: 83% vs 68%; HR, 0.45).34 Improvement in PFS was seen across subgroups, including del11q and UM-IGHV. There were more hematologic adverse events with Chl monotherapy and more nonhematologic adverse events with ibrutinib. Six percent of patients developed atrial fibrillation, and 4% of patients developed major hemorrhage. After 58 months of follow-up, the most common adverse events were diarrhea (50%), cough (36%), and fatigue (36%).34 Rates of atrial fibrillation did not increase over time, but rates of hypertension remained consistent over 5 years. Ibrutinib yields long-term responses and is well tolerated for long durations of continuous therapy. Responses are improved in patients across all risk groups, including patients with historically poor prognostic factors, such as del11q and UM-IGHV.
Two pivotal trials confirmed the benefit of upfront treatment with ibrutinib in older patients (age ≥ 65-70 years) compared with chemotherapy. A047102, an Alliance-led National Clinical Trials Network study, compared ibrutinib with ibrutinib-rituximab (IR) and with BR in patients over the age of 65 years.35 At a median follow-up of 38 months, ibrutinib and IR demonstrated superior PFS compared with BR (HR, 0.38 and 0.39, respectively). There was no difference in PFS between ibrutinib and IR, and 2-year PFS was 87% with ibrutinib, 88% with IR, and 78% with BR. PFS was improved in patients with del17p (median not reached in ibrutinib arms vs 7 months with BR). Zap70 methylation did not show a significant difference in PFS among the 3 cohorts. No differences in OS between the ibrutinib-containing arms and BR were reported, although patients initially treated with BR were allowed to crossover to ibrutinib upon confirmed disease progression. Nonhematologic toxicities were more common in the ibrutinib-containing arms. Atrial fibrillation occurred in 14% and 17% of patients treated with ibrutinib and IR, respectively. Other common side effects in the ibrutinib-containing arms are hypertension (∼30% of patients), hematologic problems (anemia, neutropenia, thrombocytopenia), and infections. The rates of serious bleeding events were <5%. It is important to note that a numerically higher number of unexplained deaths occurred in the ibrutinib-containing arms. When compared against CIT, treatment with an ibrutinib-containing regimen improves PFS in older patients. Although ibrutinib is generally considered to be well tolerated, patients still require close monitoring for the potential development of toxicities, particularly cardiovascular toxicity.
The iLLUMINATE trial tested ibrutinib in combination with obinutuzumab (IO) in patients older than 65 years (or in patients younger than 65 years with comorbidities unsuitable for fludarabine-based CIT). Patients were randomized to IO or ChlO.36 At a median follow-up of 31.3 months, PFS was not reached for patients treated with IO vs 19 months for patients treated with ChlO; 30-month PFS was 79% for IO and 31% for ChlO. ORR was higher for patients on IO vs ChlO (88% vs 73%), with more patients achieving a complete response (CR; 41% vs 16%). In patients with del17p, median PFS was not reached with IO compared with 11.3 months with ChlO. Similarly, patients with UM-IGHV had improved PFS (not reached vs 14.6 months), but there was no difference in PFS in patients with M-IGHV. Hematologic adverse events were similar between the 2 treatments. Patients treated with IO had higher rates of hypertension (17%), atrial fibrillation (6%), upper respiratory tract infections (14%), and musculoskeletal toxicities (35%). These frontline ibrutinib trials demonstrate that ibrutinib (with or without an anti-CD20 antibody) improve PFS in older patients (or those unfit to receive CIT). Importantly, atrial fibrillation and hypertension remain 2 of the common toxicities that need close monitoring, particularly in an older patient population. In summary, ibrutinib improves the outcomes of elderly patients across prognostic groups compared with CIT regimens.
The ECOG 1912 trial sought to compare IR with FCR in younger patients (age < 70 years) fit to receive intensive CIT.37 Patients were assigned 2:1 to IR (n = 354) or to FCR (n = 175). Notably, patients with del17p by FISH were excluded from the study, given the anticipated poor outcomes with FCR. The primary and secondary end points were PFS and OS, respectively. At a median follow-up of 34 months, PFS and OS favored ibrutinib-based therapy. Specifically, PFS was 89.4% (95% CI, 86-93) in the IR arm compared with 72.9% (95% CI, 65.3-81.3) in the FCR arm (HR, 0.35; P < .001). Although the number of deaths in both arms was limited, a statistically significant improvement in 3-year OS was also observed for IR vs FCR (98.8% vs 91.5%; HR for death, 0.17; 95% CI, 0.05-0.54; P < .001), with the majority of deaths in the FCR cohort attributed to CLL progression. On subset analysis, the PFS advantage observed with ibrutinib was statistically significant for UM-IGHV but did not reach statistical significance for M-IGHV. Higher rates of hematologic toxicity, including febrile neutropenia, occurred in the FCR group, and higher rates of hypertension, atrial fibrillation, and arthralgias were seen with IR. Updated results presented at the American Society of Hematology Annual Meeting in 2019 showed that, after a median follow-up of 48 months, IR continued to demonstrate superiority with regard to PFS and OS.38 With extended follow-up, 73% of patients treated with IR remained on therapy, with the majority of treatment discontinuations attributed to drug toxicities rather than to progression. Patients who discontinued IR prior to progressive disease (PD) or death did not progress for a median of 23 months after the last dose of ibrutinib. Grade 3 and above treatment-related adverse events throughout the entirety of the study period were observed in 70% of IR-treated patients and 80% of FCR-treated patients (odds ratio, 0.56; 95% CI, 0.34-0.90; P = .013). Grade 3 or above events of interest, including hypertension and atrial fibrillation, occurred in 8.5% and 2.8% of IR-treated patients vs 1.9% and 0% of FCR-treated patients, respectively. There were higher rates of major bleeding with IR (1.1%) compared with FCR (0%). Lower rates of hematologic toxicities, including neutropenic fever, were seen in the IR group compared with FCR. Other common toxicities (all grades) with IR include upper respiratory tract infections (29%) and musculoskeletal toxicities (61%). IR demonstrates excellent clinical efficacy in younger patients, particularly those with UM-IGHV. Although the risk is smaller in younger patients, the commonly observed side effects of atrial fibrillation, hypertension, bleeding events, and infections remain a reason for drug discontinuation.
Notwithstanding the fact that ibrutinib is well tolerated overall, the toxic effects have been attributed, in part, to off-target inhibition of other kinases.39 As such, several second-generation BTKi’s with more selectivity have been developed in an attempt to improve the safety profile. Acalabrutinib, a second-generation covalent BTKi, was recently approved for frontline CLL treatment based on results from the ELEVATE-TN trial.40 In this study, patients were randomized to receive acalabrutinib monotherapy, acalabrutinib-obinutuzumab (AO), or ChlO. At a median follow-up of 28.3 months, median PFS was not reached for AO vs ChlO (22.6 months), with a 90% reduction in relative risk of death or progression (HR, 0.10; 95% CI, 0.06-0.17; P > .0001). Similar PFS improvements were seen in patients treated with acalabrutinib (not reached) compared with ChlO (22.6 months), with an 80% risk reduction in death or progression (HR, 0.2; 95% CI, 0.13-0.30; P < .0001). Estimated 24-month PFS was 93% in the AO arm, 87% for acalabrutinib, and 47% for ChlO. Best ORR was 94% for AO, 86% for acalabrutinib, and 79% for ChlO. CRs were 13% for AO, 5% for ChlO, and 1% for acalabrutinib. AO demonstrated improved estimated 24-month PFS compared with ChlO across prespecified subgroups, including UM-IGHV (88% vs 76%), M-IGHV (96% vs 76%), and del17p (88% vs 22%). It is important to note that this trial was not powered to compare acalabrutinib vs AO, so the importance of adding an anti-CD20 antibody to upfront therapy remains unknown. Adverse events were equal across treatment arms, with headache (60-70%), diarrhea (62-69%), and upper respiratory tract infections (18-21%) being more common in the acalabrutinib-containing arms compared with ChlO. Neutropenia was more frequent with ChlO (45%) compared with AO (31.5%) and acalabrutinib (10.6%). With >2 years of follow-up, 79.3% of the patients in both acalabrutinib-containing arms remain on single-agent acalabrutinib, demonstrating the drug’s activity and tolerability in treatment-naive patients, including patients with poor prognostic factors (eg, UM-IGHV and del17p).
Venetoclax, a novel orally bioavailable small molecule inhibitor for selective targeting of BCL-2, has proven efficacy and safety in CLL. The drug was initially approved by the US Food and Drug Administration (FDA) as continuous monotherapy for the treatment of patients with relapsed CLL with del17p.41 Approval was then extended to all relapsed patients in combination with rituximab as a fixed-duration regimen42 ; more recently, it was approved for frontline therapy in combination with obinutuzumab based on the CLL14 trial.43 CLL14 randomized patients with significant medical comorbidities to receive a fixed duration of 1 year of treatment with venetoclax-obinutuzumab (VenO) or 6 months of ChlO.44 At a median follow-up of 28.1 months, 24-month PFS was 88.2% in the VenO group and 64.1% in the ChlO group (HR, 0.39; 95% CI, 0.22-0.44; P < .0001). At 39.6 months, recently updated data has demonstrated continued PFS benefit (not reached vs 35.6 months) for VenO and ChlO.45 In patients with del17p/TP53mut, median PFS has not been reached for VenO, whereas it is 19.8 months for ChlO. For patients with UM-IGHV, median PFS was not reached for the VenO group, and it was 26.3 mo for ChlO. A PFS benefit was also seen for patients with M-IGHV with VenO (not reached) compared with ChlO (42.9 months).45 Median OS was not reached in either arm, and no difference in OS was noted during this limited observation period. Three patients treated with VenO had laboratory evidence of tumor lysis syndrome (TLS) during treatment with obinutuzumab, but there were no TLS events reported during venetoclax ramp-up. Overall safety for hematologic and nonhematologic adverse events was similar in both groups, with neutropenia being the most common hematologic adverse event. Further follow-up is needed to determine the duration of remission and time to next treatment. Additionally, it remains unknown whether patients can be successfully rechallenged with venetoclax after disease relapse after therapy discontinuation, although data from an early-phase clinical trial suggests that this is possible.46 Similar to CIT regimens, this fixed-duration regimen offers the opportunity for planned treatment discontinuations with demonstrated improvement across prognostic groups, with a 3-year PFS of 82% (2 years posttreatment cessation).
Many combination strategies involving BCL-2 inhibitors and BTKi’s, with or without anti-CD20 antibodies, are under study with the goal to induce deeper remissions with time-limited therapy. Ibrutinib-venetoclax has been studied in 2 trials. In a single-center phase 2 trial, the combination of ibrutinib-venetoclax was given for 12 cycles; it demonstrated an 88% CR or CR with incomplete count recovery (CRi) rate, with 61% of patients achieving uMRD.47 Similar findings were noted in a multicenter phase 2 trial (CAPTIVATE) in which patients received ibrutinib-venetoclax for a total of 12 cycles. There were similar rates of MRD-negative responses in the peripheral blood and bone marrow (75% and 72%, respectively).48 A single-center phase 2 study of the combination of ibrutinib, venetoclax, and obinutuzumab for frontline CLL treatment also demonstrated an ORR of 96%, with 63% of patients achieving uMRD.49 Twenty-eight percent of patients achieved a CR with uMRD. Further studies looking at second-generation BTKi’s are underway.50 The triplet acalabrutinib-venetoclax-obinutuzumab showed deep responses, with 75% of patients achieving uMRD.51 More mature data are needed to determine whether time-limited treatment with doublet or triplet therapy can improve long-term outcomes without increased toxicity burden.
Considerations determining upfront therapy
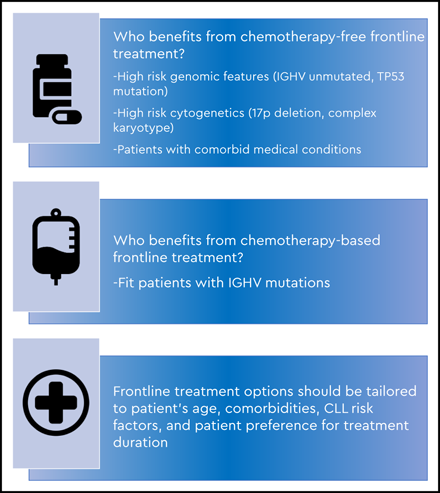
As discussed above, several factors are important when determining frontline treatment of CLL, including age, CLL prognostic factors, comorbidities, concomitant medications, and patient preferences on treatment duration. Data from phase 3 trials have demonstrated that CLL patients with high-risk features and UM-IGHV benefit from chemotherapy-free frontline regimens, and these options should be offered as the new standard of care.
Patients with M-IGHV CLL, in the absence of del17p or TP53mut, may still benefit from treatment with FCR or BR, and these regimens should be discussed as an option for this specific subgroup, taking into consideration their toxicity profiles and the patient’s desire for time-limited therapy with the potential for long-term remission (Figure 1). Other frontline treatment options include a BTKi with or without anti-CD20 antibody or VenO (for patients who would prefer a fixed-duration regimen), because outcomes are excellent, and both regimens are well tolerated.
As a class, BTKi’s are known for nonhematologic toxicities, particularly atrial fibrillation,52 bleeding risk,53 and hypertension54 (Table 2). Ventricular arrhythmias and sudden cardiac deaths have also been reported.35,37,55,56 It is important to consider the patient’s age, cardiac risk factors (history of atrial fibrillation, hypertension on multiple medications), and risk for bleeding (concomitant anticoagulation, history of severe bleeding). The risks of potential side effects must be carefully weighed against the benefits of therapy on a case-by-case basis. Patients on BTKi’s (in particular, ibrutinib) remain at risk for hypertension and quality of life toxicities, such as musculoskeletal toxicities, for the duration of treatment.57 These toxicities may not be reversible upon discontinuation. For patients who are at high risk for cardiac complications, we involve a cardiologist who understands the potential risks of these agents. Infectious complications can occur, most commonly upper respiratory tract infections. Opportunistic infections have been reported, although they are more frequent in the relapsed/refractory setting.58 Currently, there are no guidelines regarding infectious prophylaxis; thus, it should tailored to the patient’s unique clinical presentation.
Summary of significant nonhematologic adverse events on clinical trials with BTKi’s
| BTKi clinical trial . | Arthralgias, % . | Atrial fibrillation, % . | Bleeding/hemorrhage, % . | Hypertension, % . | Infection, % . |
|---|---|---|---|---|---|
| RESONATE-2: ibrutinib34 (N = 136) | 26 | 16 | 11 | 26 | 12* |
| A041202 | |||||
| Ibrutinib35 (n = 180) | 1 | 17 | 2* | 29* | 20* |
| Ibruitnib-rituximab35 (n = 181) | 2 | 14 | 4* | 34* | 20* |
| iLLUMINATE: Ibrutinib-Obintuzumab36 (N = 113) | 22 | 12 | NR | 17 | 14* |
| ECOG E1912: Ibrutinib-rituximab7 (N = 352) | 4.8* | 7.4 | NR | 18.8* | 9.4† |
| ELEVATE-TN | |||||
| Acalabrutinib40 (n = 179) | 11.2 | 3.9 | 1.7‡ | 4.5 | 14† |
| Acalabrutinib-obinutuzumab40 (n = 179) | 9.5 | 3.4 | 2.2‡ | 7.3 | 20.8† |
| BTKi clinical trial . | Arthralgias, % . | Atrial fibrillation, % . | Bleeding/hemorrhage, % . | Hypertension, % . | Infection, % . |
|---|---|---|---|---|---|
| RESONATE-2: ibrutinib34 (N = 136) | 26 | 16 | 11 | 26 | 12* |
| A041202 | |||||
| Ibrutinib35 (n = 180) | 1 | 17 | 2* | 29* | 20* |
| Ibruitnib-rituximab35 (n = 181) | 2 | 14 | 4* | 34* | 20* |
| iLLUMINATE: Ibrutinib-Obintuzumab36 (N = 113) | 22 | 12 | NR | 17 | 14* |
| ECOG E1912: Ibrutinib-rituximab7 (N = 352) | 4.8* | 7.4 | NR | 18.8* | 9.4† |
| ELEVATE-TN | |||||
| Acalabrutinib40 (n = 179) | 11.2 | 3.9 | 1.7‡ | 4.5 | 14† |
| Acalabrutinib-obinutuzumab40 (n = 179) | 9.5 | 3.4 | 2.2‡ | 7.3 | 20.8† |
All data are percentages.
NR, not reported.
Reported grade 3 or higher adverse event.
†Upper respiratory tract.
‡Grade 3 or any grade in central nervous system.
Despite its robust clinical efficacy, BTKi monotherapy seldom leads to CRs.40,59 Hence, continuous therapy is recommended to prevent disease progression in the absence of unacceptable toxicity. In the E1912 trial, more than half of the patients discontinued ibrutinib use because of an adverse event.38 Real-world evidence confirms this finding.60,61 Nevertheless, patients have longer PFS than do patients who discontinue for progression of disease.61 Second-generation BTKi’s, which are designed to have fewer off-target effects, may be able to successfully overcome a toxicity after rechallenge. Most patients treated with acalabrutinib after ibrutinib discontinuation due to intolerance were able to be successfully treated without recurrence or an increase in the severity of the adverse events.62,63 It is possible that the most common BTKi side effects are a class effect. There are several ongoing noninferiority phase 3 trials comparing second-generation BTKi’s against ibrutinib that will help to answer this question.
In cases in which there is the potential for severe cardiovascular adverse events, our preference would be a venetoclax-based regimen. Although higher rates of neutropenia are observed, these respond to growth factor support. TLS remains the adverse event of highest concern when venetoclax is used, and frequent laboratory monitoring in real-time is required during the ramp-up period to recognize and treat acute laboratory changes at the earliest signs. For many patients, admission to the hospital is required for safe monitoring. Initial debulking therapy with obinutuzumab appears to decrease the rates of TLS and is a potential strategy to minimize the number of inpatient stays required.44 The VenO regimen also offers the advantage of a time-limited treatment option. It is unknown whether there are patients with high-risk disease who may not benefit from this regimen. Combination strategies of BTKi with venetoclax appear to have higher rates of cytopenias than co either drug alone, and this should be factored into clinical decision making. This combination is not yet approved by the FDA, but the initial results seem very promising. Long-term follow-up for all of these trials is eagerly awaited.
The optimal treatment strategy for patients with del17p/TP53mut remains unknown. Venetoclax and BTKi regimens have both demonstrated improvement in outcomes compared with CIT. Mature data using continuous BTKi8 or venetoclax64 have demonstrated durable responses. In CLL14, the majority of progression events occurred in patients with del17p/TP53mut44 ; hence, it is unclear whether time-limited regimens would offer the same benefit to this cohort of patients. Ongoing clinical trials with time-limited therapies will answer this question in the future.
Currently, there are no available head-to-head data to determine which chemotherapy-free frontline regimen provides superior ORR, PFS, and/or OS. The CLL17 trial in Germany will compare ibrutinib, VenO, and ibrutinib-venetoclax to answer the question: What is the optimal chemotherapy-free frontline regimen? Results will not be available for years, but they will provide the answers to the important questions: Is treatment with BTKi or BCL-2i better in the frontline setting? Is the addition of an anti-CD20 antibody necessary? Many trials are underway to determine the best frontline therapeutic approaches. The phase 3 FLAIR trial in the United Kingdom is currently comparing FCR against ibrutinib monotherapy vs IR vs ibrutinib-venetoclax in the frontline setting. In the United States, phase 3 intergroup trials are underway for younger and older patients evaluating IO with or without venetoclax. These data will help to determine who may benefit most from monotherapy/doublet/triplet strategies. Triplet therapy appears to have higher rates of adverse events, although most patients are able to complete the intended duration of therapy. Combination strategies address continuous dosing by utilizing MRD-driven durations of treatment, but they come at the cost of higher incidences of hematologic adverse events upfront. As evidenced by all of these ongoing trials, frontline chemotherapy-free regimens have become the new standard of care. Further study of these combinations, particularly in older patients, will be needed to determine whether the side effect profile is acceptable for widespread use.
Clinical case part 3
Given his UM-IGHV status, our patient is not a candidate for CIT therapy. Potential treatment choices were discussed, including participation in a clinical trial, ibrutinib, acalabrutinib, or venetoclax with obinutuzumab because of his age, minimal comorbidities, and overall fitness. The patient chose the combination VenO, given its fixed-duration strategy. Staging computed tomography scans revealed a retroperitoneal lymph node conglomerate that measured 5.3 × 6.1 cm. Based on absolute lymphocyte count and tumor size, he is at high risk for TLS. He received the first cycle of obinutuzumab, which was only complicated by a grade 2 infusion reaction with test dose. He was admitted to the hospital for close TLS monitoring when venetoclax was started. After 3 cycles of therapy, his peripheral lymphadenopathy resolved, and his complete blood count showed improvement, specifically white blood cells (4400 per microliter), absolute lymphocyte count (1000 per microliter), absolute neutrophil count (1300 per microliter), hemoglobin (12.4 g/dL), and platelets (154 000 per microliter). Postcompletion of therapy, he is in a CR and remains under active surveillance.
Summary
Frontline treatment of CLL has dramatically evolved in the last few years with the rapid approvals of several novel targeted agents. Patients with high-risk genomic features have greatly benefited from this paradigm shift. CIT, once the backbone of treatment, has largely been replaced by targeted agents as a result of the PFS benefits seen across several phase 3 trials (Tables 3 and 4). To date, patients with M-IGHV without del17p or TP53mut remain the only group who may benefit (and potentially may achieve a functional “cure”) with FCR or BR, although this may come at the expense of secondary malignancies, myelosuppression, and increased infection risk. Ongoing and upcoming studies are exploring regimens that can achieve better efficacy with deeper remissions to allow the patient a “treatment holiday” (Table 5). It is possible that achieving a deep response with a more intensive approach may be more beneficial to certain groups of patients (ie, fit with high-risk disease). Trial data comparing the efficacy of different chemotherapy-free frontline regimens are important to develop a more tailored approach to treatment selection.
Selected phase 3 trials for FDA-approved frontline regimens for unfit patients
| Trial . | Duration . | ORR, % . | PFS, % . |
|---|---|---|---|
| RESONATE-2*: chlorambucil33,34 | Continuous | 37 | 12 (est. 60 mo) |
| RESONATE-2*: ibrutinib33,34 | Continuous | 92 | 70 (est. 60 mo) |
| A041202: IR35 | Continuous ibrutinib; 6 cycles of rituximab | 94 | 88 (est. 24 mo) |
| A041202: Ibrutinib35 | Continuous | 93 | 87 (est. 24 mo) |
| A041202: BR35 | Up to 6 cycles | 81 | 73 (est. 24 mo) |
| iLLUMINATE: IO36 | Continuous | 88 | 79 (est. 30 mo) |
| iLLUMINATE: ChlO36 | Up to 6 cycles | 73 | 31 (est. 30 mo) |
| CLL14: VenO44 | Obinutuzumab 6 cycles, venetoclax 12 cycles | 84.7 | 82 (est. 36 mo) |
| CLL14: ChlO44 | Obinutuzumab 6 cycles, chlorambucil 12 cycles | 71.2 | 50 (est. 36 mo) |
| ELEVATE-TN: acalabrutinib40 | Continuous | 86 | 87 (est. 24 mo) |
| ELEVATE-TN: AO40 | Continuous | 94 | 93 (est. 24 mo) |
| ELEVATE-TN: ChlO40 | Up to 6 cycles | 79 | 47 (est. 24 mo) |
| Trial . | Duration . | ORR, % . | PFS, % . |
|---|---|---|---|
| RESONATE-2*: chlorambucil33,34 | Continuous | 37 | 12 (est. 60 mo) |
| RESONATE-2*: ibrutinib33,34 | Continuous | 92 | 70 (est. 60 mo) |
| A041202: IR35 | Continuous ibrutinib; 6 cycles of rituximab | 94 | 88 (est. 24 mo) |
| A041202: Ibrutinib35 | Continuous | 93 | 87 (est. 24 mo) |
| A041202: BR35 | Up to 6 cycles | 81 | 73 (est. 24 mo) |
| iLLUMINATE: IO36 | Continuous | 88 | 79 (est. 30 mo) |
| iLLUMINATE: ChlO36 | Up to 6 cycles | 73 | 31 (est. 30 mo) |
| CLL14: VenO44 | Obinutuzumab 6 cycles, venetoclax 12 cycles | 84.7 | 82 (est. 36 mo) |
| CLL14: ChlO44 | Obinutuzumab 6 cycles, chlorambucil 12 cycles | 71.2 | 50 (est. 36 mo) |
| ELEVATE-TN: acalabrutinib40 | Continuous | 86 | 87 (est. 24 mo) |
| ELEVATE-TN: AO40 | Continuous | 94 | 93 (est. 24 mo) |
| ELEVATE-TN: ChlO40 | Up to 6 cycles | 79 | 47 (est. 24 mo) |
Did not enroll patients with del17p.
Selected phase 3 trials for FDA-approved frontline regimens for fit patients
| Trial . | Duration . | ORR, % . | PFS . |
|---|---|---|---|
| CLL8: FCR29 | Up to 6 cycles | 90 | 56.8 mo (est. 36-mo PFS, 65%) |
| CLL10: FCR30 | Up to 6 cycles | 95 | 55.2 mo (est. 36-mo PFS, 70%) |
| CLL10: BR30 | Up to 6 cycles | 96 | 41.7 mo (est. 36 mo PFS, 58%) |
| E1912*: IR37 | Continuous ibrutinib, rituximab 6 cycles | 96 | Est. 36-mo PFS, 89% |
| E1912*: FCR37 | Up to 6 cycles | 81 | Est. 36-mo PFS, 73% |
| Trial . | Duration . | ORR, % . | PFS . |
|---|---|---|---|
| CLL8: FCR29 | Up to 6 cycles | 90 | 56.8 mo (est. 36-mo PFS, 65%) |
| CLL10: FCR30 | Up to 6 cycles | 95 | 55.2 mo (est. 36-mo PFS, 70%) |
| CLL10: BR30 | Up to 6 cycles | 96 | 41.7 mo (est. 36 mo PFS, 58%) |
| E1912*: IR37 | Continuous ibrutinib, rituximab 6 cycles | 96 | Est. 36-mo PFS, 89% |
| E1912*: FCR37 | Up to 6 cycles | 81 | Est. 36-mo PFS, 73% |
est./Est., estimated.
Did not enroll patients with del17p.
Selected ongoing phase 2 clinical trials of frontline chemotherapy-free regimens
| Regimen . | Trial . | Cycles . |
|---|---|---|
| Venetoclax + ibrutinib49 | CAPTIVATE trial; 150 patients. Primary end point: ibrutinib use in uMRD with assessment of disease-free survival at 1 y (n = 11). 55% CR; 100% uMRD in PB. | 12, cont. ibrutinib based on MRD |
| Venetoclax + ibrutinib48 | 80 patients with high-risk features. Primary end point: CR/CRi. 92% CR/CRi at 12 mo. | 24 |
| Ibrutinib + venetoclax + obinutuzumab50 | 25 patients. Primary end point: MRD-negative CR at EOT. 52% CR/CRi; 58% uMRD. | 14 |
| Phase 3: Alliance 041702 for elderly patients, ECOG 9161 for young fit patients | ||
| Acalabrutinib + venetoclax + obinutuzumab52 | 72 patients. Primary end point: uMRD CR at EOT. 75% uMRD at EOT. | 15-24, based on MRD |
| Zanubrutinib + venetoclax + obinutuzumab52 | 77 patients. Primary end point: establish rate of uMRD CR (time frame: 1 y). 68% uMRD at 8 mo. | Up to 12, based on MRD |
| Regimen . | Trial . | Cycles . |
|---|---|---|
| Venetoclax + ibrutinib49 | CAPTIVATE trial; 150 patients. Primary end point: ibrutinib use in uMRD with assessment of disease-free survival at 1 y (n = 11). 55% CR; 100% uMRD in PB. | 12, cont. ibrutinib based on MRD |
| Venetoclax + ibrutinib48 | 80 patients with high-risk features. Primary end point: CR/CRi. 92% CR/CRi at 12 mo. | 24 |
| Ibrutinib + venetoclax + obinutuzumab50 | 25 patients. Primary end point: MRD-negative CR at EOT. 52% CR/CRi; 58% uMRD. | 14 |
| Phase 3: Alliance 041702 for elderly patients, ECOG 9161 for young fit patients | ||
| Acalabrutinib + venetoclax + obinutuzumab52 | 72 patients. Primary end point: uMRD CR at EOT. 75% uMRD at EOT. | 15-24, based on MRD |
| Zanubrutinib + venetoclax + obinutuzumab52 | 77 patients. Primary end point: establish rate of uMRD CR (time frame: 1 y). 68% uMRD at 8 mo. | Up to 12, based on MRD |
cont, continuous; EOT, end of therapy; PB, peripheral blood.
Acknowledgments
American Society of Hematology Grant (064542), Conquer Cancer Foundation Young Investigator Award (15032), and Paul Foundation Innovation Award.
Correspondence
Jacqueline C. Barrientos, CLL Research and Treatment Center, 410 Lakeville Rd, Suite 212, Lake Success, NY 11042; e-mail: jbarrientos@northwell.edu.
References
Competing Interests
Conflict-of-interest disclosure: J.M.R. has served as a consultant for Verastem, AstraZeneca, Pharmacyclics, and Abbvie/Genentech. J.C.B. has served as a consultant for Verastem, AstraZeneca, Pharmacyclics, and Abbvie/Genentech and received research support from Oncternal and Velosbio. She has received honoraria from Janssen.
Author notes
Off-label drug use: The authors discuss off label drug use for ibrutinib, venetoclax, obinutuzumab, acalabrutinib and zanubrutinib.