Abstract
Despite the effectiveness of chemoimmunotherapy (CIT), in most cases the clinical course of chronic lymphocytic leukemia (CLL) is characterized by consecutive episodes of disease progression and need for therapy. Treatment possibilities for patients with CLL in whom CIT fails whose disease progresses after initial CIT include pathway inhibitors (PIs) and, for selected patients, cellular therapy (ie, allogeneic stem cell transplant, chimeric antigen receptor T cells). PIs (ie, Bruton tyrosine kinase inhibitors, phosphatidylinositol 3-kinase inhibitors, and BCL2 inhibitors) are revolutionizing the treatment of CLL. PIs have proved to be more effective than CIT, both as upfront therapy and for relapsed/refractory disease, largely because they may overcome the negative impact of adverse biomarkers (eg, TP53 aberrations, unmutated IGHV) on outcomes and because of their acceptable toxicity. In this article, the management of patients with relapsed/refractory CLL is discussed, with a particular emphasis on the role of PIs.
Learning Objectives
Describe treatment strategies and the role of pathway inhibitors in patients with relapsed/refractory chronic lymphocytic leukemia
Understand factors influencing therapeutic choices
Introduction
In the last few decades, the outcome of patients with chronic lymphocytic leukemia (CLL) has dramatically improved, and a fraction of patients may now expect to experience prolonged remission (>10 years). However, the cure of CLL is still elusive, and usually the course of the disease is punctuated by consecutive episodes of disease progression and need for therapy. Consequently, the overall survival (OS) of patients with CLL depends on the response to different treatments during the course of the disease.
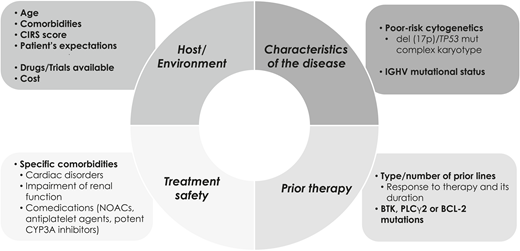
Historically, treatment options for patients with relapsed/refractory (R/R) CLL were limited and treatment results unsatisfactory. This scenario has changed since the introduction of pathway inhibitors (PIs), including Bruton tyrosine kinase inhibitors (BTKis; ibrutinib, acalabrutinib), phosphatidylinositol 3-kinase inhibitors (PI3Kis; idelalisib, duvelisib), and time-limited therapy with venetoclax-based regimens. Selecting therapy for R/R CLL requires clinicians to take into consideration several patient, disease, prior therapy, and socioeconomic aspects (Figure 1).
Patient-related, disease-related factors, and prior therapies need to be taken into consideration to select treatment modality.
Patient-related, disease-related factors, and prior therapies need to be taken into consideration to select treatment modality.
Clinical case part I
A 60-year-old woman with relapsed CLL was referred to our center for evaluation. She had received frontline chemoimmunotherapy (CIT) with FCR (fludarabine, cyclophosphamide, and rituximab) for 6 cycles, and a complete response (CR) was achieved. Her laboratory test results immediately before starting fist treatment revealed the presence of poor prognostic variables, including del(11q), serum β2-microglobulin 6 mg/dL, and unmutated IGHV genes.
Three years later, the patient presented with progressive lymphocytosis with an absolute lymphocyte count (ALC) of 50 × 109/L, hemoglobin (Hb) level of 110 g/L, and platelet count of 111 × 109/L. She was completely asymptomatic. Her physical examination revealed small lymph nodes of 2 to 3 cm that were palpable in all peripheral areas. Fluorescence in situ hybridization shows isolated del(11q) but no del(17p). No TP53 mutations were present.
Prognostic factors
Bulky disease, treatment refractoriness, extensive prior therapy, and adverse biomarkers (eg, TP53 aberrations, unmutated IGHV) are poor prognostic factors. In a large retrospective study based on 2475 patients with R/R CLL treated in 6 PI trials, a prognostic model was used that consisted of 4 factors (1 point each for serum β2-microglobulin >5 mg/dL, lactate dehydrogenase greater than the upper limit of normal, Hb <110 g/L for women or <120 g/L for men, and time from initiation of last therapy <24 months), separating patients into low (score 0-1), intermediate (score 2-3), and high (score 4) risk groups. The most important predictor is a short interval from treatment initiation to relapse.1,2 An important caveat is that this model was generated in cohorts of patients treated with CIT, and treatment consisted of different PIs. Because prognostic factors may be treatment dependent, this is a limitation. Also, prognostic models for patients initially treated with PIs are needed.
Treatment options in R/R CLL
Treatment should be initiated only when International Workshop on Chronic Lymphocytic Leukemia criteria are met in the presence of signs or symptoms of disease activity, as in newly diagnosed patients.3 Relapsed, symptomatic, and refractory (or resistant) disease should not be employed as synonymous terms. Disease relapse (ie, reappearance of the disease after a period of remission) is not necessarily an indication for therapy, because many patients in this situation are asymptomatic and can be followed up with no intervention for a long period of time. Likewise, refractory disease (ie, disease that does not respond to therapy) should not be confounded with disease progression (ie, symptomatic, relapsed disease). The main results of pivotal studies conducted in R/R CLL are summarized in Table 1.4-11
Phase III trials comparing targeted therapies including BTKi, PI3Ki, and BCL2i vs anti-CD20 monoclonal antibodies or chemoimmunotherapy regimens in patients with R/R CLL
| Treatment . | Median age (y) . | TP53 aberrations . | Prior lines of therapy, median (range) . | 3-y PFS . | Neutropenia grade ≥3 AEs . | Infection grade ≥3 AEs . | Any nonhematologic grade ≥3 AEs* . | Median follow-up . |
|---|---|---|---|---|---|---|---|---|
| IBRU (vs ofatumumab)4,5 | 67 | 32% del(17p) | 3 (1-12) | 59%† | 25% | 30% | Hypertension (9%), AF (6%), major bleeding (4%) | 59 mo (final analysis of the study) |
| IDELA-R (vs R)6,7 | 71 | 43% either del(17p) or TP53 mutation | 3 (1-12) | Median 19.4 mo | 13% | 53.6% | Diarrhea (16%), transaminitis (5%-9%), colitis (8%), pneumonitis (6%) | 18 mo (final analysis of the study) |
| VEN-R (vs BR)8,9 | 65 | 26% del(17p), 25% TP53 mutation | 2 (1-4) | 71.4% | 58.8% | 10% | TLS (2%), hyperglycemia (2%) | 23.8 mo (last update up to 4 y) |
| A (vs IDELA-R or BR)10 | 67 | 16% del(17p), 24% TP53 mutation | 1 (1-8) | Median NR | 17% | 20% | AF (1%), hemorrhage (3%), hypertension (3%) | 16 mo |
| DUV (vs ofatumumab)11 | 68 | 21% del(17p), 20% TP53 mutation | 3 (2-8) | Median 15.7 mo | 23% | 13% | Diarrhea (23%), colitis (11%), pneumonia (11%) | 22 mo |
| Treatment . | Median age (y) . | TP53 aberrations . | Prior lines of therapy, median (range) . | 3-y PFS . | Neutropenia grade ≥3 AEs . | Infection grade ≥3 AEs . | Any nonhematologic grade ≥3 AEs* . | Median follow-up . |
|---|---|---|---|---|---|---|---|---|
| IBRU (vs ofatumumab)4,5 | 67 | 32% del(17p) | 3 (1-12) | 59%† | 25% | 30% | Hypertension (9%), AF (6%), major bleeding (4%) | 59 mo (final analysis of the study) |
| IDELA-R (vs R)6,7 | 71 | 43% either del(17p) or TP53 mutation | 3 (1-12) | Median 19.4 mo | 13% | 53.6% | Diarrhea (16%), transaminitis (5%-9%), colitis (8%), pneumonitis (6%) | 18 mo (final analysis of the study) |
| VEN-R (vs BR)8,9 | 65 | 26% del(17p), 25% TP53 mutation | 2 (1-4) | 71.4% | 58.8% | 10% | TLS (2%), hyperglycemia (2%) | 23.8 mo (last update up to 4 y) |
| A (vs IDELA-R or BR)10 | 67 | 16% del(17p), 24% TP53 mutation | 1 (1-8) | Median NR | 17% | 20% | AF (1%), hemorrhage (3%), hypertension (3%) | 16 mo |
| DUV (vs ofatumumab)11 | 68 | 21% del(17p), 20% TP53 mutation | 3 (2-8) | Median 15.7 mo | 23% | 13% | Diarrhea (23%), colitis (11%), pneumonia (11%) | 22 mo |
A, acalabrutinib; AEs, adverse events; AF, atrial fibrillation; BR, bendamustine and rituximab; DUV, duvelisib; IBRU, ibrutinib; IDELA, idelalisib; NR, not reached; PFS, progression-free survival; R, rituximab; TLS, tumor lysis syndrome; VEN, venetoclax.
Focused on diverse events of clinical interest.
Median PFS 44.1 mo in the final analysis (6 years of follow-up).
Clinical case part II
The patient was asymptomatic, and no therapy was provided. Nevertheless, 4 months later, she presented with extreme fatigue. Her blood test results revealed ALC 120 × 109/L, Hb 96 g/L, and platelet count 60 × 109/L. The result of a direct antiglobulin test was negative. The fluorescence in situ hybridization test was repeated and showed del(11q) but not del(17p). No TP53 mutations were detected. Different treatment options were discussed with the patient.
BTKis
The significant clinical activity observed through the inhibition of Bruton tyrosine kinase (BTK), an important enzyme in the amplification of B-cell receptor signaling, represented a firm first step into the era of targeted therapies in CLL. BTKis such as ibrutinib and acalabrutinib disrupt B-cell receptor signaling and other circuits between CLL cells and the microenvironment. Ibrutinib was approved in 2014 as therapy for patients with CLL who had received at least one prior line of therapy, based on the initial results of the phase Ib/II PCYC-1102, which were further confirmed by the multicenter randomized RESONATE study in patients with previously treated CLL.4,12 Long-term follow-up data of both trials demonstrated that ibrutinib administered as a single agent resulted in a significant benefit in R/R CLL, the estimated progression-free survival (PFS) and OS at 7 years being 34% and 55%, respectively, in the phase I-IIb study, and PFS and OS at 5 years of 40% and 60%, respectively, in the RESONATE study. The benefit of ibrutinib was observed across all risk groups, although patients with TP53 aberrations and complex karyotype had shorter PFS and OS.5,13
On the downside, ibrutinib is associated with side effects that, in some cases, can be severe. The most serious adverse events are bleeding and arrhythmias, mainly atrial flutter and atrial fibrillation; ventricular arrhythmias can also occur.14,15 The most frequent toxicities, however, include arthralgias and pneumonia, and these are manageable. Other adverse events are diarrhea, hypertension, fatigue, cough, and skin lesions.16 Ibrutinib must be discontinued in a significant proportion of patients (20%-50%). In a long-term analysis, only 28% of patients with R/R disease continued on ibrutinib beyond 5 years of initiating therapy; 33% discontinued treatment because of disease progression and 21% due to ibrutinib-associated toxicities, particularly frequent in older and heavily pretreated patients. This study showed that most adverse events tend to decrease over time, except hypertension, and that patients discontinue treatment mainly because of atrial fibrillation, diarrhea, infections, and bleeding events.13 In a real-world scenario, treatment discontinuation was observed in 50% of patients with R/R disease.17
Second-generation BTKis are designed to improve upon first-generation agents such as ibrutinib by having less cardiotoxicity, fewer adverse events requiring treatment discontinuation, and fewer off-target effects. Acalabrutinib is a second-generation BTKi approved by the US Food and Drug Administration in 2019 for treatment of patients with CLL. In R/R CLL, acalabrutinib administered as a single agent showed a significant benefit in PFS compared with control arms (bendamustine with rituximab or idelalisib with rituximab). With a median follow-up of 22 months, the estimated 18-month PFS was 82% vs 48%; no differences in PFS were observed across risk groups.10
Although the follow-up is short, acalabrutinib seems as effective as ibrutinib, and its tolerance is likely better; atrial fibrillation, hypertension, and bleeding have been reported to be less frequent than with ibrutinib. Indeed, acalabrutinib is considered a therapeutic alternative in patients who do not tolerate ibrutinib.18 The most frequent adverse event associated with acalabrutinib is headache, which can be easily managed. Other possible adverse events are neutropenia, diarrhea, cough, and upper tract respiratory infection. The acalabrutinib discontinuation rate has not been studied extensively. In one study, treatment discontinuation due to adverse events occurred in 16% of patients after a median follow-up of 22 months.10 The results of a randomized phase III study, ACE-CL-006, comparing acalabrutinib with ibrutinib in previously treated patients with high-risk CLL, defined by the presence of del(11q) or del(17p), are eagerly awaited because they could be practice changing.
Another selective BTKi in advanced phases of clinical development is zanubrutinib. A phase I/II study reported a promising safety profile, with atrial fibrillation and major bleeding being infrequent. Ninety-five percent patients continued treatment after a median follow-up of 14 months, and only 2% discontinued therapy due to adverse events. Zanubrutinib at a dose of 160 mg twice daily achieved a steady inhibition of BTK. In the R/R setting, the overall response rate (ORR) was 93%, and the estimated PFS at 12 months was 100%.19 A phase II study in patients with R/R CLL showed similar results; after a median follow-up of 15 months, the ORR was 84.6%, and the estimated PFS and OS at 12 months were 92.9% and 96%, respectively. Nine percent of patients discontinued zanubrutinib due to adverse events.20 Updated results of the initial phase I/II study with a follow-up of 24 months showed a persistent benefit in PFS, regardless of the presence of del(17p).21 A randomized phase III trial is comparing ibrutinib with zanubrutinib in patients with R/R CLL, but no data are yet available.
As mentioned above, a proportion of patients must discontinue treatment with BTKis due to disease progression. Although mechanisms of resistance are not fully understood, the acquisition of secondary mutations of BTK leading to impaired binding of the BTKi is the most common cause of resistance to BTKis. Acquired mutations in BTK involving Cys481 or phospholipase C-γ2 have been reported in >80% of patients with progressive disease treated with ibrutinib22 and in 69% in a small cohort of patients treated with acalabrutinib.23 Recently, a novel mutation of BTK involving Leu528Trp has been found along with Cys481 mutations in patients who experienced progression under zanubrutinib therapy.24 Furthermore, in patients showing resistance to BTKis, other non-BTK mutations (ie, TP53, SF3B1, and CARD11) have been described.25 Several strategies aimed at overcoming resistance associated with mutations of BTK are in development, such as the use of reversible noncovalent BTKis, including LOXO-305 and ARQ-531.26 Also, several combinations of BTKis with CIT and targeted therapies are under investigation. An important objective of these studies is to shift continuous therapy to time-limited therapy, to achieve deeper responses, and to prevent resistance to therapy.
PI3Kis
The selective inhibitor of phosphatidylinositol 3-kinase δ, idelalisib, is another treatment for patients with CLL who experience relapse after CIT. Idelalisib in combination with rituximab significantly improved PFS and OS compared with rituximab alone in a pivotal study including patients with R/R CLL and comorbidities.6 In addition, a post hoc analysis suggested that the benefit of this combination extends to patients with complex karyotype and TP53 aberrations.27 However, immune-mediated adverse events (ie, hepatitis, colitis) and risk of infections can be a limiting factor, and because of this, idelalisib is generally reserved for later lines of therapy.28
Duvelisib is an oral dual phosphatidylinositol 3-kinase δ and γ inhibitor approved as treatment of patients with CLL who have received at least 2 prior lines of therapy, based on the results of the DUO trial. This study showed significant benefit in PFS in patients receiving duvelisib compared with the control arm; the median PFS was 13.3 months vs 9.9 months, respectively.11 In addition, duvelisib showed a high response rate (77%) and a benefit in PFS in patients who experienced progression under the control arm (ofatumumab) and were subsequently treated with duvelisib in the DUO trial. However, duvelisib is associated with PI3Ki characteristic toxicity.29
Umbralisib is a second-generation PI3Ki with promising activity and moderate toxicity, especially hepatotoxicity and colitis. Combinations of umbralisib with novel anti-CD20 monoclonal antibodies, BTKis, or BCL2 inhibitors (BCL2is) are being investigated.30-32
BCL2is
Venetoclax is an oral selective inhibitor of the antiapoptotic BCL-2 protein that was first approved as monotherapy for patients with relapsed disease or with 17p deletion.33 This agent was approved as a fixed-duration regimen in combination with rituximab for R/R CLL, based on the results of the MURANO trial.8 This regimen consists of venetoclax for 2 years combined with rituximab for 6 months and is the first “time-limited therapy” in the era of targeted therapies. The last update of the MURANO trial with 4 years of follow-up demonstrated a consistent benefit in PFS and OS compared with bendamustine and rituximab in previously treated patients with CLL; the PFS at 4 years was 57.3% vs 4.6%. The progression rate after treatment discontinuation is 32% at 2 years. Patients with high-risk features, particularly those with TP53 aberrations, are those more likely to experience progression.9,34
The combination venetoclax plus rituximab eradicates the disease in a high proportion of patients. Importantly, those patients with undetectable minimal residual disease (uMRD) at the end of therapy have a significantly better outcome than those in whom leukemic cells remain detectable, emphasizing the importance of minimal residual disease (MRD) as a treatment goal not only in patients treated with CIT but also in those receiving PIs.35 Many trials are exploring combinations of PIs with uMRD as an endpoint and landmark to discontinue therapy. In patients with bulky disease previously treated with BTKis or having received >3 lines of therapy, treatment results are poorer.34
In early trials, tumor lysis syndrome (TLS) was observed in a not negligible proportion of patients.36 Currently, a 5-week ramp-up dosage and adequate general prophylaxis have drastically limited TLS (<1% of patients), which, in most cases, is only a laboratory finding. In clinical practice, the risk for TLS (ie, subjects with bulky disease with any lymph node ≥10 cm or ≥5 cm with an ALC ≥25 × 109/L) can be considered as inconvenient, but the risks of TLS are counterbalanced by the high efficacy of venetoclax. The most frequent toxicity associated with venetoclax is neutropenia that needs to be managed with granulocyte colony-stimulating factor. Less frequent events are diarrhea, nausea, fatigue, anemia, and infections.37 In real-world data, venetoclax therapy was discontinued in 29% of patients (20.5% due to adverse events and 53.8% due to disease progression). The most frequent adverse event leading to discontinuation was hematological toxicity.38
The main limitation of treatment with venetoclax is disease progression. Although there is little information on mechanisms of resistance to venetoclax, the acquisition of the Gly101Val point mutation in BCL2 and other alternative BCL2 mutations at clonal and subclonal levels interfere with the engagement of venetoclax in the binding site of BCL2.39 However, these mutations are not found in all patients resistant to venetoclax, and other mechanisms, such as upregulation of antiapoptotic proteins such as MCL-1 and BCL-xL overexpression, might cause treatment resistance.40,41
CIT
CIT could still be considered a treatment option for patients experiencing late relapses (ie, >24 to 36 months) and in those initially treated with poorly effective regimens (eg, chlorambucil, rituximab). However, the short- and long-term toxicity, mainly hematologic and secondary malignancies associated with CIT, are of concern.42 Because of this, CIT is gradually replaced by PIs. In patients with R/R CLL, CIT could still be an option when PIs are not available.
Clinical case part III
To sum up, this patient with CLL experienced relapse and had symptomatic disease after 3 years of receiving CIT. Of note, the patient did not have prior comorbidities except for hypercholesterolemia. Among the prognostic factors with which the patient presented was unmutated IGHV gene del(11q). After discussing the different therapeutic choices mentioned above, the patient started oral ibrutinib at a standard dose of 420 mg daily. The patient’s tolerance of treatment was good; she only complained of fatigue that got worse during the first 2 months but progressively improved after 3 months of receiving ibrutinib. After 1 year of treatment, her blood test results revealed recovery of her Hb level and platelet count with an ALC of 6 × 109/L, consistent with partial remission.
Combination strategies and cellular therapies
The emergence of mutations driving treatment refractoriness to ibrutinib and venetoclax is a compelling argument to combine different PIs and/or anti-CD20 monoclonal antibodies. Overall, combinations of PIs (ibrutinib and idelalisib) with anti-CD20 monoclonal antibodies and CIT regimens in R/R CLL have been shown to increase CR rates, including in patients with uMRD. However, the improvement of the quality of response has not always been followed by a significant benefit in PFS and OS. For example, the addition of rituximab to ibrutinib did not improve PFS and OS compared with ibrutinib alone.43 In contrast, the addition of the novel anti-CD20 monoclonal antibody ublituximab to ibrutinib improved not only the quality of response but also PFS in patients with high-risk CLL [(del(17p), del(11q), and/or a TP53 mutation] compared with single-agent ibrutinib.44 Further studies with long follow-up are necessary to determine whether second-generation anti-CD20 monoclonal antibodies in combination with BTKi or PI3Ki agents provide a benefit as compared with single-agent therapy.
Another strategy consists of combining BTKis with BCL2is, based on the synergy found in preclinical models. Several trials are combining ibrutinib as a single agent during the first 2 or 3 cycles to mobilize CLL cells from microenvironment niches and reduce tumor burden, followed by the addition of venetoclax for a limited number of cycles. The main endpoint of most of these trials is the eradication of MRD to guide treatment discontinuation. A recent report has shown impressive results, with MRD negativity rates of 53% in peripheral blood and 36% in bone marrow in patients with R/R CLL after 12 months of combined therapy with ibrutinib and venetoclax. Although the follow-up is still short, only 1 of 53 patients experienced progression.45 The same approach is being investigated with PI3Kis such as umbralisib or duvelisib with venetoclax with or without anti-CD20 monoclonal antibodies. The main results of all these trials are summarized in Tables 2 and 3.45-52
PI3Ki combinations with venetoclax with or without anti-CD20 monoclonal antibodies in patients with R/R CLL
| Trial phase . | Treatment . | No. of patients . | TP53 aberrations . | Schedule . | Strategy . | uMRD rate . | Discontinuation rate . | Median follow-up . |
|---|---|---|---|---|---|---|---|---|
| Phase I/II46 | Umbralisib-venetoclax-obinutuzumab | 21 | 38%* | UMBRA cycle 1-12 + UBLI cycles 1-3 + VEN cycles 4-12 | BM uMRD at cycle 12, discontinue all therapy BM MRD-positive at cycle 12, continue UMBRA monotherapy | 4 patients with BM uMRD (19%) | 4.7% | 4.2 mo |
| Phase I47 | Duvelisib-venetoclax | 12 | 25% del(17p) and 42% TP53 mutation | DUV days 1-7, then DUV+VEN for 12 cycles | uMRD on 2 assessments, discontinue all therapy; resume VEN monotherapy at MRD recrudescence MRD-positive, continue VEN monotherapy | 22% uMRD in BM and PB | 25% | Reported with median number of cycles 6 (range, 1-9) |
| Trial phase . | Treatment . | No. of patients . | TP53 aberrations . | Schedule . | Strategy . | uMRD rate . | Discontinuation rate . | Median follow-up . |
|---|---|---|---|---|---|---|---|---|
| Phase I/II46 | Umbralisib-venetoclax-obinutuzumab | 21 | 38%* | UMBRA cycle 1-12 + UBLI cycles 1-3 + VEN cycles 4-12 | BM uMRD at cycle 12, discontinue all therapy BM MRD-positive at cycle 12, continue UMBRA monotherapy | 4 patients with BM uMRD (19%) | 4.7% | 4.2 mo |
| Phase I47 | Duvelisib-venetoclax | 12 | 25% del(17p) and 42% TP53 mutation | DUV days 1-7, then DUV+VEN for 12 cycles | uMRD on 2 assessments, discontinue all therapy; resume VEN monotherapy at MRD recrudescence MRD-positive, continue VEN monotherapy | 22% uMRD in BM and PB | 25% | Reported with median number of cycles 6 (range, 1-9) |
BM, bone marrow; c, cycles; DUV, duvelisib; PB, peripheral blood; UBLI, ublituximab; UMBRA, umbralisib; VEN, venetoclax.
Also includes patients with del(11q).
BTKis combined with venetoclax with or without anti-CD20 monoclonal antibodies in patients with R/R CLL
| Trial phase . | Treatment . | No. of patients . | TP53 aberrations . | Schedule . | Strategy . | uMRD* rate . | Discontinuation rate . | Median follow-up . |
|---|---|---|---|---|---|---|---|---|
| Phase II48 | IBRU-VEN | 80 | 30% del(17p) and TP53 mutation | IBR for 3c, IBRU+ VEN 4-27c | MRD+ in BM could continue ibrutinib | 67% uMRD in BM at 24 c | 19% | 22 mo |
| Phase II45 | IBRU-VEN | 53 | 22% del(17p) | IBRU 1-2c, IBRU+VEN after cycle 2 | PB/BM uMRD at cycle 8: cease therapy after cycle 14; PB/BM uMRD between cycles 14 and 26: cease therapy after cycle 26; MRD-positive at cycle 26: continue IBR monotherapy | uMRD 53% in PB at 12 mo; uMRD 36% in BM at 12 mo | Not reported | 21 mo |
| Phase II49 | IBRU-VEN | 51 | 18% del(17p) and TP53 mutation | IBRU 1-2 c, IBR + VEN 3-15 c | > PR and BM uMRD after cycle 15 randomized to observation or IBR monotherapy*; MRD recrudescence retreated with combination | uMRD 55% in PB; uMRD 39% in BM | 16% | 15 mo |
| Phase II50 | IBRU-VEN | 24 | 50% del(17p)/50% TP53 mutation | IBR+VEN for up to 2 y | uMRD CR on 2 assessments: discontinue all therapy or continue IBR monotherapy*; patients MRD-positive or less than CR: continue IBR monotherapy | 67% uMRD in BM at 12 mo (in 15 evaluable patients) | 12% | NA |
| Phase Ib51 | IBRU-VEN-OBINU | 12 | 8% del(17p) | OBINU 1-8 c + IBRU 2-14 c + VEN 3-14 c | After cycle 14, can continue IBR monotherapy | 100% uMRD in PB/50% uMRD in BM at 14 c | Not reported | 24 mo |
| Phase II52 | IBRU-VEN-OBINU | 25 | Not reported | OBINU 1-8 c + IBRU 2-14 c + VEN 3-14 c | After cycle 14, can continue IBR monotherapy | 100% uMRD in PB/50% uMRD in BM at 14 c | Not reported | 18 mo |
| Trial phase . | Treatment . | No. of patients . | TP53 aberrations . | Schedule . | Strategy . | uMRD* rate . | Discontinuation rate . | Median follow-up . |
|---|---|---|---|---|---|---|---|---|
| Phase II48 | IBRU-VEN | 80 | 30% del(17p) and TP53 mutation | IBR for 3c, IBRU+ VEN 4-27c | MRD+ in BM could continue ibrutinib | 67% uMRD in BM at 24 c | 19% | 22 mo |
| Phase II45 | IBRU-VEN | 53 | 22% del(17p) | IBRU 1-2c, IBRU+VEN after cycle 2 | PB/BM uMRD at cycle 8: cease therapy after cycle 14; PB/BM uMRD between cycles 14 and 26: cease therapy after cycle 26; MRD-positive at cycle 26: continue IBR monotherapy | uMRD 53% in PB at 12 mo; uMRD 36% in BM at 12 mo | Not reported | 21 mo |
| Phase II49 | IBRU-VEN | 51 | 18% del(17p) and TP53 mutation | IBRU 1-2 c, IBR + VEN 3-15 c | > PR and BM uMRD after cycle 15 randomized to observation or IBR monotherapy*; MRD recrudescence retreated with combination | uMRD 55% in PB; uMRD 39% in BM | 16% | 15 mo |
| Phase II50 | IBRU-VEN | 24 | 50% del(17p)/50% TP53 mutation | IBR+VEN for up to 2 y | uMRD CR on 2 assessments: discontinue all therapy or continue IBR monotherapy*; patients MRD-positive or less than CR: continue IBR monotherapy | 67% uMRD in BM at 12 mo (in 15 evaluable patients) | 12% | NA |
| Phase Ib51 | IBRU-VEN-OBINU | 12 | 8% del(17p) | OBINU 1-8 c + IBRU 2-14 c + VEN 3-14 c | After cycle 14, can continue IBR monotherapy | 100% uMRD in PB/50% uMRD in BM at 14 c | Not reported | 24 mo |
| Phase II52 | IBRU-VEN-OBINU | 25 | Not reported | OBINU 1-8 c + IBRU 2-14 c + VEN 3-14 c | After cycle 14, can continue IBR monotherapy | 100% uMRD in PB/50% uMRD in BM at 14 c | Not reported | 18 mo |
BM, bone marrow; c, cycles; IBR, ibrutinib; IBRU, ibrutinib; NA, not available; OBINU, obinutuzumab; PB, peripheral blood; PR, partial response; VEN, venetoclax.
uMRD defined as < 1 CLL cell per 10000 leukocytes.
However, whether any additional benefit is derived from adding CIT or anti-CD20 monoclonal antibodies in the form of “doublets” and “triplets” to PIs administered as single agents remains unclear due to the lack of randomized clinical trials in the R/R setting. Treatment costs (“financial toxicity”) and treatment alternatives once these regimens fail are of concern.
Another field that is moving forward is CD19-targeted, chimeric antigen receptor (CAR)-engineered T-cell immunotherapy. Early phase I /II clinical trials have shown encouraging results with durable responses in patients with R/R CLL who are resistant to ibrutinib and venetoclax. In line with this, the combination therapy of BTKi (ibrutinib) and CAR T cells is appealing. Preclinical studies suggest that ibrutinib may improve T-cell function and result in better CAR T-cell proliferation and antitumor effect. Results of a pilot study showed an ORR of 83%, with 61% cases with uMRD in bone marrow; the 1-year OS and PFS were 86% and 59%, respectively. Of interest, the concurrent administration of ibrutinib and CD19-targeted CAR T cells was well tolerated with lower severity of cytokine release syndrome than that observed with CD19-targeted CAR T cells without ibrutinib.53-55
Which therapeutic strategy should be used in patients experiencing relapse after CIT?
Whenever possible, patients should be included in clinical trials. In those patients not included in trials, choosing the optimal treatment strategy depends on several factors (Figure 1):
Host factors: Age, comorbidities, Cumulative Illness Rating Scale score,56 and patient’s expectations.
Characteristics of the disease: Bulky disease, extensive prior therapy (≥3 regimens), and a short PFS (<24 months) are high-risk features that predict poor outcome in patients initially treated with CIT. Other adverse factors that should be considered are TP53 aberrations and complex karyotype. Patients with complex, high-risk genetic lesions should be included in clinical trials. In selected cases, T-cell therapy (allogeneic stem cell transplant, CAR T cells) can be an option.57 Of note, disease relapse or progression may reveal Richter syndrome, whose therapy is not covered in this review.
Treatment safety: Specific comorbidities such as cardiac disorders, impairment of renal function or certain comedications (eg, oral anticoagulants, antiplatelet agents, potent CYP3A inhibitors) may interfere with PIs.
Practical aspects: Availability of drugs and their cost.
Resistance to PI: Presence of mutations associated with refractoriness to targeted therapies (ie, BTK, phospholipase C-γ2, or BCL-2 mutations).22,23,40
Goals of therapy: The goals of therapy are prolonging survival and improving quality of life. Achieving response with uMRD is associated with longer PFS and OS. The importance of obtaining uMRD, however, depends on the target population and treatment objectives. For example, achieving uMRD is not critical in very old patients (>80 years), in whom disease palliation is the most reasonable objective. In contrast, in younger patients, it can be of paramount importance. In the allotransplant setting, achieving uMRD is important because results are better in patients in CR. In such cases, CIT and/or venetoclax plus rituximab, if not previously administered, are the best options to cross the bridge.57 Importantly, ibrutinib may be effective in patients experiencing progression after transplant.58
How should patients be managed during the coronavirus disease 2019 pandemic?
The coronavirus disease 2019 (COVID-19) pandemic is challenging the management of patients with cancer. CLL mainly affects older people with comorbidities. This fact, along with the severe immunosuppression inherent to the disease, suggests that in patients with CLL, severe acute respiratory syndrome coronavirus 2 infection could be more severe than in the general population. A detailed discussion of the management of patients with CLL with associated COVID-19 infection is beyond the scope of this review and has been addressed thoroughly by different organizations.59,60 Nevertheless, in those patients with active CLL, treatment should not be withheld, because there are data showing that the outcome of patients with COVID-19 is better if their cancer is under control. In patients requiring intervention, BTKis may represent the preferred therapeutic option.61
Conclusions
The management of patients with R/R CLL is changing with the advent of PIs (BTKis, PI3Kis, and BCL2is). Continuous therapy with BTKis and fixed duration of venetoclax with anti-CD20 monoclonal antibodies have significantly improved the outcomes of patients with R/R CLL. However, most patients with CLL must discontinue PIs because of treatment-related toxicity, treatment failure, or disease progression . To overcome this, second-generation BTKis are being incorporated into CLL therapy, and newer combinations of PIs with other agents are being investigated. Achieving uMRD is increasingly employed as an endpoint and to define treatment duration. The inclusion of patients in clinical trials, large databases, and meta-analyses is critical to continue advancing CLL therapy.
Correspondence
Carol Moreno, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, and Biomedical Research Institute, IIB Sant Pau, Barcelona, Spain; e-mail: cmorenoa@santpau.cat.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.