Learning Objectives
Review the literature examining outcomes for CLL patients after progression on venetoclax-based treatment
Discuss a patient-specific approach to therapy selection following CLL progression on venetoclax
Clinical case
Two years after his initial diagnosis, a 65-year-old man with chronic lymphocytic leukemia (CLL) developed progressive fatigue and anemia requiring initiation of therapy per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines. Mutational profiling before treatment was initiated revealed mutated IGHV, del(13q), and normal karyotype. There was no del(17p) or TP53 mutation. He was treated with 6 cycles of chemoimmunotherapy (fludarabine-cyclophosphamide-rituximab) and achieved complete remission (CR). However, 24 months after completion of therapy, he developed progressive lymphadenopathy that required initiation of second-line therapy. Repeat mutational profiling again revealed no evidence of TP53 disruption. Venetoclax-rituximab was chosen for treatment, and the patient completed a total of 6 months of rituximab and 24 months of venetoclax per the MURANO study regimen. He initially had a CR, but 12 months after stopping venetoclax, he now has progressive lymphadenopathy and worsening anemia. What would be the next treatment that you would select for this patient?
Introduction
The BCL2 inhibitor venetoclax has shown deep and durable responses in treating CLL, including in traditionally high-risk patients such as those with del(17p) or complex karyotype.1-7 On the basis of the results of the CLL14 and the MURANO trials, venetoclax in combination with an anti-CD20 antibody is now a standard-of-care treatment option in both the first-line and relapsed/refractory (R/R) settings.2,3 Given its increasing use in clinical practice, it is expected that more patients will eventually require a subsequent therapy after discontinuing a venetoclax-based regimen given in earlier lines of therapy. Herein, we discuss reasons for progression on venetoclax, review the existing literature on clinical outcomes of treatment after progression on venetoclax, and highlight patient-specific considerations for selecting the next therapy.
Reasons for progression during or after venetoclax-based therapies and mechanisms of resistance
Data from clinical trials, registries, and real-world series demonstrate that most patients discontinue venetoclax because of progressive disease (PD) rather than because of adverse events.7-10 Patients whose disease progresses on venetoclax have been shown to have traditionally high-risk features. A retrospective analysis of 445 patients treated with venetoclax on 4 early-phase clinical trials showed that patients who had received previous therapy with a Bruton tyrosine kinase inhibitor (BTKi), 3 or more previous lines of therapy, or had bulky lymphadenopathy were less likely to have a durable response to venetoclax-based therapy. In addition, del(17p), TP53 mutations, NOTCH1 mutations, and unmutated IGHV status predicted less durable responses to venetoclax.11 A separate analysis of 67 patients on 4 early-phase venetoclax clinical trials showed that complex karyotype and fludarabine refractoriness were associated with progression on venetoclax.8
Minimal residual disease (MRD) status also seems to be an important predictor of progression for patients who have completed venetoclax-based therapy. At a median follow-up of 36 months for patients who completed 24 months of therapy with venetoclax-rituximab on the MURANO trial, patients with detectable MRD at the end of treatment were more likely to have PD than those with undetectable MRD (uMRD). In addition, patients with a partial response (PR) to venetoclax-rituximab with MRD status at the end of treatment had an inferior progression-free survival (PFS) from 18 months onward from the end of combination treatment compared with patients with uMRD, suggesting that MRD status, regardless of iwCLL response, may best predict progression after cessation of venetoclax-based therapy.4
Early preclinical in vitro studies suggested that the development of a mutation to prevent venetoclax from binding to its target site on BCL2 may serve as a mechanism of acquired resistance,12,13 and a recurrent mutation (Gly101Val) in BCL2 was recently identified in patients progressing on venetoclax.14 In a study of 15 CLL patients with paired pre-venetoclax and progression samples, 7 patients had a Gly101Val mutation in BCL2 at progression. Of note, BCL2 Gly101Val was first detected after 19 to 42 months of receiving venetoclax therapy; variant allele frequencies were low at this time, and detection of the mutation preceded clinical progression by up to 25 months.14 The authors demonstrated that Gly101Val decreases Bcl2 affinity for venetoclax by ∼180-fold.14 Given the late occurrence of BCL2 mutations while receiving venetoclax therapy, it is unclear how relevant BCL2 mutations will be for patients with progression after fixed-duration venetoclax regimens of 12 to 24 months. Another study examined CLL cells from patients who had persistent MRD after 1 year of treatment with venetoclax-rituximab; of 6 patients with persistent MRD and PD, a BCL2 Gly101Val mutation was identified in 3 patients (50%). The variant allele frequencies of the identified BCL2 mutations were less than 20%. In this study, CLL cells from patients with PD also had higher levels of ROR1 and BCL2 expression and greater cancer-stemness gene expression on transcriptome analysis.15
Other studies suggest additional factors that may contribute to venetoclax resistance. A recent study performed whole-exome sequencing on 8 specimens from a CLL patient that were collected before treatment with venetoclax and at the time of progression while receiving venetoclax and did not detect BCL2 Gly101Val mutations. However, an increasing number of acquired copy number mutations or aneuploidy were identified at progression, which demonstrated that heterogeneous patterns of clonal evolution and increased genomic instability occur with exposure to venetoclax.16 Another recent study also did not find BCL2 Gly101Val mutations at progression in 6 CLL patients, but it demonstrated MCL-1 overexpression in venetoclax-resistant cells as well as increased oxidative phosphorylation. This suggests that metabolic reprogramming may also contribute to venetoclax resistance.17
Re-treatment with venetoclax
For patients with an initial response to venetoclax-based therapy who progress after completing therapy and have no identifiable acquired resistance mutation (ie, BCL2 Gly101Val), an unanswered clinical question is whether re-treatment with venetoclax should be considered. This question is particularly relevant because the MURANO and CLL14 studies used fixed-duration treatment regimens (continuous venetoclax therapy for 24 months in the MURANO study and 12 months of therapy in the CLL14 study).2,3 At a median follow-up of 4.9 years from the original phase 1b study of venetoclax-rituximab, 18 patients stopped venetoclax treatment in deep response, and 4 patients (2 with MRD-positive CR, 2 with uMRD CR) had progressive disease after stopping venetoclax and were subsequently re-treated with venetoclax or venetoclax-rituximab. Of 3 patients who had a repeat response assessment, all achieved at least a PR and 2 had ongoing responses.18 In 4-year follow-up data from the MURANO trial, 14 of 64 patients with PD in the venetoclax-rituximab arm received subsequent venetoclax or were re-treated with venetoclax-rituximab.19 Six of 11 patients with evaluable responses had a response for an overall response rate (ORR) of 55%.20 Notably, in follow-up data from the CLL14 trial, data on re-treatment with venetoclax are not available.21 Early data suggests that re-treatment with venetoclax yields responses in select patients, but further study is needed to validate this approach. It is not yet known whether the activity of venetoclax re-treatment depends on the duration and/or depth of previous response to venetoclax, and future studies evaluating venetoclax re-treatment should take these factors into consideration.
Covalent BTKi’s after venetoclax
Covalent BTKi’s, including ibrutinib,22-26 acalabrutinib,27,28 and zanubrutinib,29-31 have transformed the treatment of CLL in both the R/R and first-line settings. However, despite many studies demonstrating excellent efficacy of BTKi’s in R/R settings, initial studies largely preceded clinical trials and the subsequent approvals of venetoclax. Therefore, data are limited on the efficacy of BTKi therapy after venetoclax-based treatment.
A recent retrospective study examined outcomes for ibrutinib-naïve patients who had been treated with ibrutinib after they progressed following treatment with venetoclax.32 This study included 27 patients with a median of 2 therapies before venetoclax, including 1 patient treated with another BTKi. Notably, this was a high-risk population, with 12 (60%) of 20 patients with del(17p), 12 (50%) of 24 patients with complex karyotype, and 13 (86.7%) of 15 patients with unmutated IGHV. Of the 27 patients, 18 had discontinued venetoclax because of PD. The ORR to ibrutinib was 56.0% (PR, 13 of 25; CR, 1 of 25). Time to progression on ibrutinib ranged from 3 to 53 months, and the median duration of therapy was 18.3 months, suggesting that ibrutinib has clinical activity when used after venetoclax.
Another single-institution study examined the outcomes of 23 heavily pretreated patients who had progressed on venetoclax therapy and subsequently received a BTKi (ibrutinib or zanubrutinib).33 Notably, these patients had received a median of 4 previous lines of therapy, with 91% having received previous fludarabine-cyclophosphamide-rituximab. The population was also high risk from a genetic perspective, with 76% of patients with TP53 disruption and 68% with a complex karyotype. Of 23 patients treated with a BTKi, 20 patients had ORRs of 90%, with 15 PRs or PRs with lymphocytosis and 4 with a CR. Twelve patients had discontinued treatment with a BTKi (8 because of PD and 4 because of toxicity), and 11 patients continued to receive BTKi therapy at a median follow-up of 33 months. In subset analyses, previous CR or uMRD during venetoclax therapy (hazard ratio, 0.029) and ≥24 months during venetoclax therapy (hazard ratio, 0.044) were associated with longer PFS after initiation of a BTKi. Notably, 8 of 19 tested patients had a BCL2 Gly101Val mutation; at a median follow-up of 33 months, the median PFS while receiving a BTKi had not been reached for these 8 patients. This small study suggests that a BTKi has clinical efficacy for patients with acquired resistance to venetoclax.
There are other small retrospective reports of using a BTKi after venetoclax. A pooled analysis of venetoclax-treated patients from early clinical trials reported that 6 of 8 patients with progressive CLL received ibrutinib after venetoclax and 5 had a PR.8 Another report of 11 patients showed that 10 of 11 patients achieved PRs when treated with ibrutinib after venetoclax.34 In addition, an analysis of patients treated with venetoclax in clinical practice reported 23 patients receiving therapy after venetoclax, with 5 patients receiving ibrutinib and 1 with a PR, 2 with stable disease, and 2 with PD.9
Long-term follow-up from the MURANO study reported outcomes for 8 patients who were given ibrutinib after PD following treatment with venetoclax-rituximab.35 All 8 patients had a response to ibrutinib (7 PR and 1 very good PR) and, at last follow-up, 4 patients continued to receive ibrutinib and 4 had discontinued treatment. The median duration of treatment with ibrutinib was 15 months.
To date, the largest cohort series is a retrospective international, multicenter study that reported treatment outcomes for patients requiring treatment after venetoclax discontinuation.10 Notably, this cohort included both patients who were BTKi naïve and those with previous exposure to BTKis. The study included 326 patients who discontinued venetoclax, 188 (58%) of whom went on to subsequent treatment. BTKis were the most common therapy after venetoclax (74 of 188 patients: 44 BTKi-naïve, 30 with previous exposure to BTKis). Among BTKi-naïve patients, the estimated PFS for post-venetoclax BTKi treatment was 32 months compared with not reached in BTKi-intolerant patients and 4 months in BTKi-resistant patients (median follow-up, 7.7 months). These data suggest that BTKis after venetoclax are active and produce durable remissions, particularly in BTKi-naïve or -intolerant patients. Responses to BTKis were not durable after venetoclax for patients with known BTKi resistance. However, for patients with previous BTKi exposure who discontinued because of intolerance, a trial with an alternative BTKi is a feasible option.36
In summary, BTKis should be considered after venetoclax therapy for BTKi-naïve patients; however, prospective sequencing data are still needed. Alternative therapies should be considered for patients with previous exposure to BTKis and known failure or resistance.
Noncovalent BTKis after venetoclax
Resistance to ibrutinib, an irreversible, covalent BTKi, is mediated by an acquired cysteine-to-serine mutation in BTK.37,38 Reversible, noncovalent BTKis, including GDC-0853,39 LOXO-305,40 ARQ 531,41 and vecabrutinib,42 may overcome BTKi resistance. Although trials of noncovalent BTKis are ongoing and in early phases, preliminary data suggest that these agents have clinical activity in heavily pretreated populations; however, the exact number of patients who have received previous treatment with venetoclax is not reported.40-42 In the preliminary results from the phase 1 dose-escalation trial of LOXO-305, there was a reported response to the noncovalent BTK in an R/R CLL patient who had an acquired BCL2 Gly101Val mutation after venetoclax therapy.40
Phosphatidylinsositol-3 kinase inhibitors after venetoclax
Existing data from small retrospective series suggest that phosphatidylinsositol-3 kinase inhibitors (PI3Kis) have limited activity after treatment with venetoclax, particularly in patients who have progressed on both BTKis and venetoclax (double progressors). In a recent retrospective series, 17 CLL patients received idelalisib or duvelisib after venetoclax. At a median follow-up of 5 months, there was an ORR of 46.9% with a median PFS of 5 months and a discontinuation rate of 78%, suggesting that PI3Ki therapy did not produce durable responses and was difficult to tolerate; all of these patients had previous exposure to BTKis.10 Notably, the clinical trials leading to the approval of idelalisib and duvelisib did not include patients with previous exposure to venetoclax.43,44
Cellular therapy: allo-HSCT and CAR T-cell therapy
Allogeneic stem cell transplantation (allo-HSCT) remains the only potential curative therapy for CLL, although little is known about outcomes in the era of novel agents. A recent multicenter retrospective cohort study showed a PFS rate of 60% and an overall survival rate of 82% at 24 months for CLL patients undergoing allo-HSCT after being treated with 1 or more novel agents.45 In the largest multicenter international case series of patients receiving treatment after venetoclax, 18 patients subsequently received anti-CD19–directed chimeric antigen receptor (CAR) T-cell therapy with an ORR of 66.6%, including 33.3% of patients with CRs; notably, all of these patients had previous exposure to BTKi’s.10 The phase 1/2 study of R/R CLL patients treated with the anti-CD19–directed CAR T-cell product lisocabtagene maraleucel (TRANSCEND-CLL-004) included 9 patients who had progression with BTKi therapy and for whom venetoclax had failed. Four of these patients had ongoing responses (3, PR; 1, CR/CR with incomplete hematologic recovery [CRi]) at the time of a presentation at the 2019 American Society of Hematology meeting.46 In addition, a pilot study of 19 CLL patients treated with CD19-targeted CAR T cells with concurrent ibrutinib after ibrutinib therapy had failed included 11 patients with previous venetoclax treatment, 6 of whom had progression during treatment with venetoclax. Although outcomes of patients treated with venetoclax are not reported separately, the high 1-year PFS of 59% suggests that ibrutinib in combination with anti-CD19–directed CAR T-cell therapy could be a promising strategy in the future.47 These data suggest that cellular therapy with either allo-HSCT or CAR T-cell therapy may have a role in select, fit patients with high-risk disease. Whether CAR T-cell therapy should precede allo-HSCT is not known.
Conclusion: considerations for selection of the next therapy
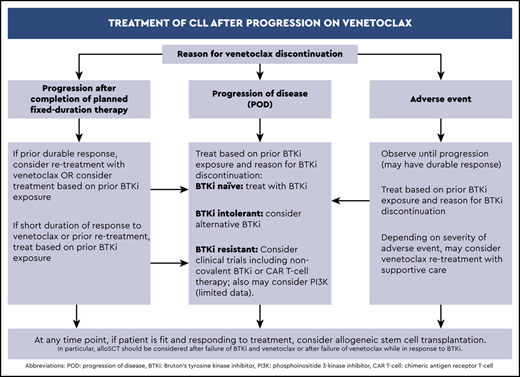
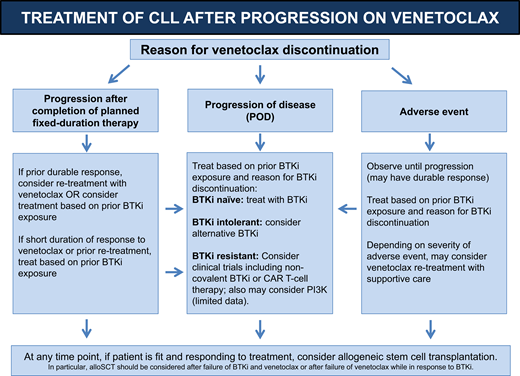
Our approach to therapy selection after venetoclax has been discontinued is summarized in Figure 1. Considering the reason for venetoclax discontinuation and taking an inventory of previous lines of CLL therapies are essential steps in selecting a therapy after venetoclax. For patients without previous exposure to BTKis, retrospective data support using a BTKi after venetoclax. Specifically, ibrutinib has the most data to support its use in this setting.
Algorithm for treatment of CLL after progression on venetoclax-based therapy.
In the clinical case presented here, the patient is BTKi naive, and therefore we recommend initiation of ibrutinib. However, this patient also had an initial response to venetoclax and had a treatment-free interval of 12 months after completion of therapy. Re-treatment with venetoclax may be an option in such patients with an initial response and without known resistance to venetoclax. Before re-treatment, the duration of previous response to venetoclax should be considered, as well as the treatment-free interval after completion of therapy. Longer-term follow-up from clinical trials and prospective studies is required to investigate the efficacy of this approach. If fit patients achieve disease control with venetoclax retreatment or BTKis, allo-HSCT may be considered for select patients.
For patients with previous exposure to a BTKi, the reason for discontinuation of the BTKi is important. For patients who have discontinued BTKi treatment because of intolerance rather than PD, it is reasonable to next try an alternate BTKi such as acalabrutinib. If the patient does not tolerate acalabrutinib or has no response to it, treatment on a clinical trial with a noncovalent BTKi (eg, LOXO-305 [NCT03740529], ARQ 531 [NCT03162536], or zanubrutinib [NCT04116437]) should be considered.
For patients who have developed PD while receiving a BTKi with or without a known acquired resistance mutation, allo-HCST may be considered in select fit patients. Investigational therapies that include CD19-directed CAR T-cell therapy or noncovalent BTKis are other options if they are available on clinical studies. There are few data to support the efficacy of PI3Kis after exposure to both BTKis and venetoclax in earlier lines of therapy, but PI3Ks should be considered if other options are not available.
In the currently available data on heavily pretreated patients, there is no evidence supporting chemotherapy or immunotherapy for PD after venetoclax progression. However, there are no data exploring the role of chemoimmunotherapy in the R/R setting in patients treated via chemotherapy-free pathways (ie, BTKi → venetoclax → PI3Ki). For IGHV-mutated patients, chemoimmunotherapy could be considered. The following are graded recommendations: (1) For patients with R/R CLL and PD during or after treatment with venetoclax, a BTKi should be chosen as the next therapy if the patient is BTKi naïve (evidence grade: moderate). (2) For patients with R/R CLL who responded to and have completed venetoclax-based therapy and then experience PD while not receiving therapy, re-treatment with venetoclax may be considered (evidence grade: low).
Correspondence
Anthony R. Mato, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: matoa@mskcc.org.
References
Competing Interests
Conflict-of-interest disclosure: A.R.M. has served as a consultant for Celgene, Acerta, and Janssen; has served as a consultant for and received research funding from AbbVie, Loxo, Genentech, Pharmacyclics, AstraZeneca, Sunesis, and Johnson & Johnson; has received research funding from DTRM Biopharma and Gilead; and has served as a consultant for, received research funding from, is a DSMB member, and other for TG Therapeutics. M.C.T. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.