Abstract
The transfusion support of hematological malignancies considers 2 dimensions: the quantity of what we order (in terms of triggers, doses, targets, and intervals), and the special qualities thereof (with respect to depths of matching and appropriate product modifications). Meanwhile, transfusion-related enhancements in the quantity and quality of life may not be dose dependent but rather tempered by unintended patient harms and system strains from overexposure. Evidence and guidelines concur in endorsing clinically noninferior conservative red blood cell (RBC) transfusion care strategies (eg, triggering at hemoglobin <7-8 g/dL and in single-unit doses for stable, nonbleeding inpatients). However, the unique subpopulation of patients with hematological malignancies who are increasingly managed on an outpatient basis, and striving at least as much for quality of life as quantity of life, is left on the edges of these recommendations, with more questions than answers. If a sufficiently specific future wave of evidence can satisfy the concerns (and contest the assumptions) of the remaining proponents of liberalism, and if conservatism is broadly adopted, savings may be potentially immense. These savings can then be reinvested to address other gaps and inconsistencies in RBC transfusion care, such as the best achievable degrees of prophylactic antigen matching that can minimize alloimmunization-related service delays and reactions.
Learning Objectives
Identify appropriate triggers for RBC transfusion in patients with hematological malignancies and the advantages specific to conservative treatment
Understand blood product matching and modification options relevant to patients with hematological malignancies
Introduction
Excluding the microbiome, adults have 37 trillion cells, with erythrocytes comprising 25 × 1012 (>67%) when we are healthy.1 Given that 90% of human cells derive from the hematopoietic lineage, the acquired and therapy-related bone marrow derangements in patients with hematological malignancies draw heavily on banked supplies of red blood cells (RBCs) and platelets (PLTs). These patients are often the dominant users of cellular blood components in high-income countries,2 but they have been the subjects of fewer RBC dosing/triggering studies than other populations.
In relying on stored donor RBCs to address anemia (that is not otherwise responsive to transfusion-sparing tactics), the goal is not to rapidly rebuild a normal red cell mass (Figure 1), but to minimize morbidity and mortality with the lowest possible exposure. Two critical questions on RBC transfusion support arise: quantity (how much: when/where/why/how often) and quality (how to serve it to minimize the disadvantages arising from its complex nature). These are the most fundamental elements of our patients’ 12 transfusion rights (the right reason, recipient, testing, product, place, timing, volume, rate, response, documentation, regulation, and cost).3
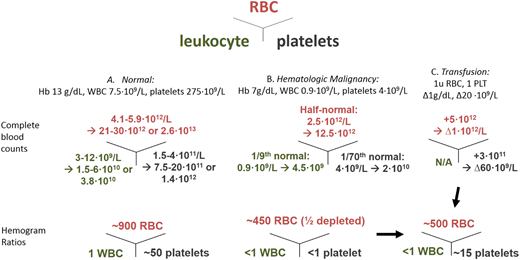
The hemogram: cell numbers in health, disease, and after transfusion. (A) Normal. (B) Counts typical for hematological malignancy. (C) Transfusion impact/goal. Assumptions: 5-L blood volume and middistribution values, 5 trillion cells per RBC unit (u), and 300 billion PLTs per adult dose concentrate. Hb, hemoglobin; WBC, white blood cell.
The hemogram: cell numbers in health, disease, and after transfusion. (A) Normal. (B) Counts typical for hematological malignancy. (C) Transfusion impact/goal. Assumptions: 5-L blood volume and middistribution values, 5 trillion cells per RBC unit (u), and 300 billion PLTs per adult dose concentrate. Hb, hemoglobin; WBC, white blood cell.
Consideration 1: getting quantity right
Conservative vs liberal triggers
When to trigger RBC transfusion and how much to give are interwined questions (Figure 2) and may be associated with the frequency with which a patient seeks RBCs for symptomatic anemia (in conservative approaches), as well as with the odds of per-sitting multicomponent-related adverse outcomes and inventory consumption (in liberal approaches). Conservatism tends not to raise objections when an inpatient is resting, hemodynamically stable, free of bleeding, and close to both the surveillance technology (complete blood counts) and component provisions (RBCs and PLTs from the blood bank). However, the outpatient who is removed from the security net of hospital resources and whose anemia-related fatigue may limit quality of life and/or function at home4,5 has long argued for liberalism. This has included higher Hb targets for the sake of a posttransfusion zenith that does not depreciate so quickly as to require disruptive returns for top-ups.
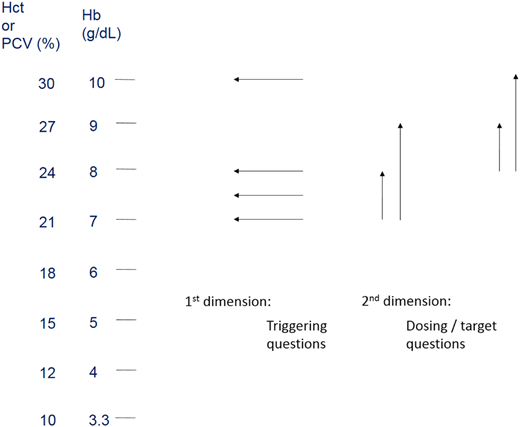
The 2 dimensions of quantity. Triggering questions relate to how low a patient’s red cell mass must fall before justifying a transfusion. Dosing questions relate to how high the posttransfusion effect should be. Hct, hematocrit; PCV, packed cell volume.
The 2 dimensions of quantity. Triggering questions relate to how low a patient’s red cell mass must fall before justifying a transfusion. Dosing questions relate to how high the posttransfusion effect should be. Hct, hematocrit; PCV, packed cell volume.
Notwithstanding these considerations, the literature confirms the safety of lower triggers in terms of uniform mortality rates, with no observable advantages at higher Hb. In 23 randomized controlled trials (RCTs) involving >10 500 patients (medical or surgical, adult or pediatric), death at 30 days was not different between restrictive (Hb ≤7-8 g/dL) and liberal (Hb ≤9-10 g/dL) approaches (9.0% vs 9.3%; relative risk, 0.97; 95% confidence interval [CI], 0.81-1.16), while outcomes for the 2 conservative thresholds (14 trials at ≤8 g/dL and 9 trials at ≤7 g/dL) were also indistinguisable.6 The collective magnitude of evidence argues that triggering is 1 of the best studied (if not already settled) matters in medicine.
The fact that many defer transfusion to Hb <7 g/dL also implies a tolerance for the loss of half of an individual’s ideal RBC mass, assuming a baseline Hb of 14 g/dL. This acknowledges the fact that we typically use only a quarter of the oxygen carried by Hb, although the myocardium is an exception, where extraction ratios may be several-fold higher. Anemia brings the supply-demand stresses to a perilous synergy, because its compensation relies on increased cardiac output despite reduced oxygen carrying capacity, and coronary perfusion may be further limited in those with heart disease. Despite these concerns, MINT (an RCT in acute myocardial infarction) is under way, and TRICSIII (a multinational RCT in cardiac surgery [7.5 vs 8.5-9.5 g/dL])7 now challenges the hypothesized advantages of higher Hb in patients with cardiac pathologies.
RCTs in the hematological malignancy patient
In the Cochrane analysis by Gu et al8 of liberal vs conservative RBC triggers in myelodysplastic syndrome (MDS), aplastic anemia, and congenital bone marrow failure syndromes, little could be concluded. In its 1 featured trial with results, a Dutch abstract (13 MDS patients enrolled [from a target of 200], with Hb triggers at 7.2 vs 9.6 g/dL), CIs were simply too wide on any outcome to ascertain a difference. ISRCTN26088319 (a UK study of 38 patients with MDS ± myeloproliferative neoplasms targeting Hb to either >10 or 8.5-10 g/dL) has not yet been published. The EnhanceRBC Study (registered at www.clinicaltrials.gov as #NCT02099669 [Canada]) of 30 MDS patients is targeting Hb to either 11 to 12 g/dL or 8.5 to 10 g/dL, with 22 enrolled (as of 15 June 2018), for an anticipated trial end in December 2018.
The TRIST RCT (#NCT01237639 [Canada]; with results in abstract form)9 randomized 300 patients undergoing stem-cell transplantation (SCT; 150 autologous, 150 allogeneic) to Hb triggers of either <7 or <9 g/dL for 100 days. Conservative triggering was noninferior in the primary outcome of quality of life (health-related quality of life/Functional Assessment of Cancer Therapy–Bone Marrow Transplant score, −1.6%; 95% CI, −0.07 to 0.04; P < .001), with liberal use also consuming more RBCs. Additionally, there were no differences found in any of the other explored outcomes.
The published feasibility pilot RCT by DeZern et al10 on acute leukemia (#NCT02086773 [Johns Hopkins, Baltimore, MD]) recently enrolled 90 adults over 2 years in a 2:1 randomization to <7 vs <8 g/dL, respectively. Of these, 73 were evaluable, with 51 in the conservative arm and 23 in the liberal arm. Group-specific Hct differences were preserved, despite noncompliance ranging from 12% in the former arm (mean pre-RBC Hb, 6.8 g/dL) to 7% in the latter arm (mean pre-RBC Hb, 7.7 g/dL). Fatigue, bleeding of any grade, and neutropenic fevers were similar, but RBC units consumed were significantly different in the course of care of each arm (8 vs 11.7; P < .0003), despite how close these 2 triggering ranges were to each other. If translated out to the 55 000 cases of acute leukemia in the United States per year, the savings (from RBC units spared with conservatism) project to >US$100 million.
Taken together, this (mixed inpatient/outpatient) evidence has been too limited for the AABB to make recommendations on RBC thresholds in outpatients with hematological malignancies or in those with chronic transfusion-dependent anemia.6
Problem of high Hcts
The idea that higher Hcts (ie, >25% [Hb >8.3 g/dL]) not only deliver more oxygen but also mechanically enhance PLT-endothelial hemostasis was recently reinvigorated in an exploratory analysis of data from the PLADO (PLT dose) RCT.11 However, because sufficiently powered RCT evidence of Hct-associated bleeding outcomes does not yet exist,12 hemostasis is not an agreed-upon goal of RBC transfusion. The impact that this report might have on reversing the gains made in conservative transfusion care is yet to be determined.
Patients at higher Hct levels may also feel fewer of the adverse effects of anemia, but in the example of erythropoiesis-stimulating agents, this advantage came at the serious price of vascular thrombotic events.13 It is challenging to predict the real-life failures and disappointments of contrived Hcts in the effort of seizing the benefits of naturally robust Hb levels. One sobering example is provided by Robitaille et al14 in a trial that was aborted at 6 participants (from an enrollment goal of 62). In the context of pediatric SCT, with randomization either to liberally triggered (<12 g/dL) or conservatively triggered (<7 g/dL) RBC transfusions, 3 of 3 in the former group developed veno-occlusive disease, whereas 0 of 3 in the latter were free of this outcome (P = .05). The question that remains (in this study and in general) is how high is too high if 12 g/dL produced this end, and other meta-analyses have not shown any clinical disadvantages of liberalism at 9 to 10 g/dL.
Finally, the commonsense arguments against transfusing liberally to high Hcts include the nonnegligible risks of minor to severe acute transfusion reactions and the delayed or cumulative harms of sensitizing (RBC and HLA) antigen exposures, as well as the more predictable complication of iron overload. The odds of transfusional hemochromatosis and the need for chelation therapy are proportional to the quantity of RBCs transfused. Left unmitigated, the most significant degrees of iron loading may also equate to avoidable mortal hazards in SCT.15
Fear of undertransfusion
Evidence of undertransfusion is rare in sufficiently resourced environments,16 with fears and motivators in blood ordering now being the subjects of formal study in behavioral theory frameworks.17
Whether chronic anemias may achieve a form of remote ischemic preconditioning, with the effect of limiting the breadth of infarctions when perfusion is compromised from independent vascular obstructions, is not known. The extent to which such an adaptation may explain some subgroup advantages (or the absence of disadvantages) in austere transfusion subgroups is enticing, but not a justification to withhold RBCs to levels significantly below the currently accepted triggers. Among those who cannot be transfused, practitioner willingness to offer standard myelotoxic regimens is variable, because the nadir Hb may fall beneath the endurable limits. These vary with age and general health, and they influence the range of achievable physiologic adaptations to anemia. The survival of the most extreme case to date was described in a 22-year-old male Jehovah’s witness with acute lymphoblastic leukemia, whose Hb was <5 g/dL for 41 days, with 2 days at <1.5 g/dL within this interval.18 (The authors speculated that successful lymphoblast clearance was attributable in part to the impact of anoxia.) Without any guarantees, modified regimens (to minimize myelotoxicity) and erythropoiesis-stimulating agent use (to hasten red cell engraftment)19 may avert the need for RBCs altogether and thereby respect the barriers of those who are untransfusable for religious or immunologic reasons.
Choosing wisely and “why do 2 if 1 will do?”
North American standard bearers in hematology and hemotherapy have unanimously asserted these tenets of the Choosing Wisely initiative in RBC transfusion: do not transfuse RBCs if alternatives and/or observation are as effective, and do not give >1 unit to the stable nonbleeding inpatient (or give only the minimal amount needed) to relieve symptoms of anemia and to return Hb to a safe range (7-8 g/dL in stable, noncardiac inpatients).6
In an example of prescient incorporation of these principles at the University Hospital in Zurich, Berger et al20 described the use of RBCs in acute myeloid (± promyelocytic) leukemia and SCT patients between July 2007 and December 2009. The period was split into 2 eras: the conventional double-unit practice period (2 units of RBCs triggered at Hb <7 g/dL) and the conservative single-unit practice period from 2008 onward (1 unit of RBCs triggered at Hb <6 g/dL). Per treatment cycle, 25% fewer (or 2.7 fewer) RBCs were used in the conservative period. Intriguingly, outpatient RBC encounters did not increase in frequency despite the lower triggers and doses, and although typical pretransfusion Hb fell from 6.4 to 6.1 g/dL, the bleeding rate (or PLT transfusion use) was unchanged over time, and survivals were similar.
The cost of the RBC unit is at least US$200,21 not counting the activity-based costs incurred around the basic system- and staff-related inputs around testing, dispensation, bedside administration, and documentation. These estimates range from three- to fourfold higher than the production cost of the unit in the United States22 to at least 40% higher than the base value in the United Kingdom.23 The most frugal estimate of US$300 per RBC unit enables the calculation of savings for RBCs averted by conservative transfusion practices. Because triggering conservatively uses 42% fewer RBCs (relative risk, 0.53, or −0.93 units), the number needed to treat to save the cost of a single unit is roughly 2 patients. Furthermore, if, in addition to lowering the trigger, the single unit (rather than the double) is prescribed, the savings expand further. This is arguably much-needed money for reallocation in the constrained and costly care realities of patients with hematological malignancies. If 2 antineoplastic approaches consistently achieved the same outcomes, the most economical (lower risk, less time intensive) approach would dominate if there were no other interferences (eg, dubious incentive structures, culture).
At Princess Margaret Cancer Centre in Toronto, Canada, the promulgation of evidence and the Choosing Wisely campaign have been associated with a decrease in inpatient median Hb triggering to ∼7 g/dL, with most of these orders now being single unit in nature (∼90%). However, these shifts in practice have not penetrated the outpatient care setting, where triggers remain higher (7.5 g/dL), and almost a third of sittings maintain double-unit orders (C.C.-G., unpublished Hospital Transfusion Committee audits, 5 September 2018; Figure 3).
Audit of practice at a Toronto cancer hospital (Princess Margaret Cancer Centre [PMH]). (A) Median triggering Hb in inpatients has trended from 7.5 to 7.0 g/dL (with >95% of orders triggering at <8.0 g/dL), but in outpatients, it remains near 7.5 g/dL (with <80% of orders at <8 g/dL). (B) Inpatient RBC transfusions are single-unit orders >90% of the time, whereas outpatient sittings use single-unit orders <70% of the time.
Audit of practice at a Toronto cancer hospital (Princess Margaret Cancer Centre [PMH]). (A) Median triggering Hb in inpatients has trended from 7.5 to 7.0 g/dL (with >95% of orders triggering at <8.0 g/dL), but in outpatients, it remains near 7.5 g/dL (with <80% of orders at <8 g/dL). (B) Inpatient RBC transfusions are single-unit orders >90% of the time, whereas outpatient sittings use single-unit orders <70% of the time.
The rationale relates not only to the blindspots in the evidence, but also to the concern that if the RBC dose is too small, frequent returns may result (ie, 2 single-unit sittings in a week rather than a single 2-unit sitting), with the patient paying in time for a neutral consumption. Whether there is a balance between the demerits of austerity (not feeling well and returning often) to those of liberalism (using more inventory and chair time at the encounter, with additive or multiplicative risks from the number of products used) is yet to be defined and optimized in patients with hematological malignancies. In the PLADO (PLT dosing) study, the standard PLT dose ultimately won against half doses and double doses (when the similar bleeding rates were weighed against the logistic prices of the extremes)24 ; it is possible that the same may apply to RBC transfusion.
Consideration 2: getting quality right
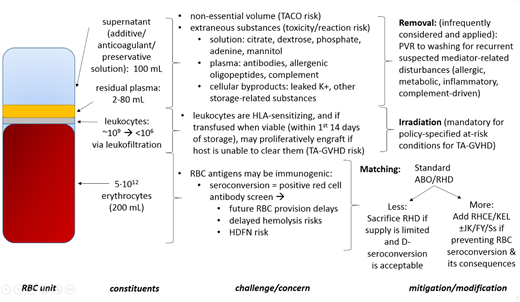
The constituent anatomy of the RBC unit (Figure 4) offers an approach to identifying various transfusion challenges and their corresponding potential modifications. In order of importance, this scan begins with the erythrocytes, the residual leukocytes and plasma from the original whole-blood donation, and the added supernatant, with each material also changing over time.
Anatomy of the RBC in a risk/mitigation framework. PVR, plasma volume reduction; TACO, transfusion-associated circulatory overload.
Anatomy of the RBC in a risk/mitigation framework. PVR, plasma volume reduction; TACO, transfusion-associated circulatory overload.
Erythrocytes: how far to take antigen matching?
Transfused RBCs are different from our recipients in 2 chief ways: they are stored ex vivo, and they come from someone else. Although banked RBCs variably exhibit a number of storage-related derangements, these have not been associated with signals of harm in human RCTs to date.6 Greater attention therefore remains fixed on the importance of antigenic differences and the extent to which we heed them. The ticket to the issue of any RBC unit is the compatibility (group and screen [G&S]) specimen, which is grouped/typed (at a minimum for the patient’s ABO/RHD type) and screened (in an interrogation for the presence of plasma antibodies). Red cell forward-type attributes are the basis for selecting ABO/RHD-compatible units, whereas the discoverable antibodies of the plasma have their specificities deduced to tally the targets that must be avoided. At a minimum, patients who are screen positive for a clinically significant antibody must indefinitely be restricted to the provision of crossmatch-compatible RBC units, which are antigen negative for the target.
If a patient has recently been exposed to foreign RBCs (in the last 1-3 months), the screen is biologically dynamic enough to justify resampling (outdating) every 3 days. Turnaround times on results and the availability of compatibility-assured RBC units therefore depend on the intrinsic complexities of the sample and the capability of the laboratory to handle them in the voluminous test environment with its finite resources. Patients with clean screens (negative for antibodies) can be serviced faster than those who have current or historic documentation of antibodies. The proportion of the latter may drive policies to solicit the G&S close enough to (but not on the day of) the intended transfusion, so the time required in prerelease work does not result in unpredictable and/or protracted delays to RBC access. However, the patient may pay in commutes for 2 encounter dates (1 with the laboratory, 1 with the infusion unit) to receive any RBCs.
Although the commonest clinically significant antibodies target no more than a dozen different antigens (D, C/c, E/c, K, Jka/Jkb, Fya/Fyb, S/s), the diversity of known antibodies now ranges in the ever-increasing hundreds.25 Many of these are uncommon because they target low-frequency antigens (unlikely to be confronted [but also challenging to identify because informative reagents are scarce]) or high-frequency antigens (not often provoked because donors and recipients usually share these structures [with the exception of the rare individual who does not and who consequently sensitizes and then needs similarly rare blood]).
Regardless of their nature, an accessible compendium of a patient’s antibodies is important to maintain. Although many antibodies may wane to nondetectability (evanesce), we do not deliberately reexpose patients to the offending antigen, because the reactivation (anamnestic) response may be stronger (ie, faster and in higher titer) than the primary seroconversion event. If (because of privacy lockdown) such laboratory information does not move as readily from hospital to hospital as a patient does for his or her care, underrecognition of an antibody repertoire creates a hazard. A downstream facility inadvertently issuing antigen-unselected units (from not having known about or seen a historical antibody) therefore adds to the patient’s risk of delayed serologic and/or hemolytic transfusion reactions. Models accounting for evanescence26 and nationalized G&S big data27 suggest that 1 in 10 blood recipients have made RBC antibodies. These antibodies are important in yet another way if the patient is a woman of childbearing potential, because they may complicate pregnancies, with hemolytic disease of the fetus/newborn (HDFN) if the offspring is cognate antigen positive.
However, not everyone carries this seroconversion risk equally; those who have positive screens (immune responders) tend to reflect a subset (13%) of recipients who on exposure to unfamiliar antigens may seroconvert (in up to 30% of such opportunities).28 Most people do not seroconvert, but those who do may do it especially well. Factors in immune responsiveness have been recently well reviewed in this forum.29
Arguments for matching more than we do
Screen-positive patients (and laboratorians) have more compatibility-assurance steps to wait for (and wade through), and these add considerably to the activity-based cost of transfusion, as shown in the HI-STAR study (US$400-US$600).30 MDS patients who are regularly transfused (and not receiving as many immune-blunting treatments as their leukemic counterparts) have a particularly high rate of RBC seroconversion (15% in 272 patients),31 the impact of which raises the question of whether something better can and should be done (beyond simply transfusing less). In MDS, Lin et al32 compared conventional (ABO/RHD only) matching with a prophylactic antigen matching (PAM) strategy (emulating host RHCE [C/c, E/e] and KEL [K]). Among 176 patients, seroconversions in 23% (without PAM) vs 11% (with PAM) were not statistically different, although as expected, the antigens that were matched for were not the targets of the antibodies that developed.
In patients for whom screening is prohibitively difficult (eg, those with obfuscating antibodies [eg, warm autoantibodies or therapeutic anti-CD38]), an increasingly popular approach is to obviate the need for antibody interrogation, by preventing alloimmunization altogether, through extended RBC antigen matching. As automated genotyping (to predict the extended blood group antigen profile) proves to be noninferior (or superior) to traditional phenotyping by serologic means and is increasingly available and cost competitive, our recipients and donors can be framed and aligned together in shared information/inventory systems when concurrent efficiencies of scale materialize.
Patients with hematological malignancies must now compete for the resource of higher fidelity matches, which are principally dedicated to those who experience seroconversion most (both in greater frequency and severity; eg, hemoglobinopathy patients) and who may already account for a large proportion of transfusion service activity at some facilities. Some of this greater frequency stems from ancestry-dependent differences in antigen profiles between donors and recipients. However, hematological malignancies do not restrict themselves to those ancestries containing both hemoglobinopathies and (paleontologically explained) antigen diversity. That is, whereas the care of those with hemoglobinopathies will naturally identify complex matching needs, the care of those with hematologic malignancies will not, unless we either gather ancestry information as a stimulus to match or regard these conditions as sufficient grounds in themselves. As certain hematological malignancy patients begin to receive investigational immune-activating checkpoint inhibitors, the effects of which include the induction of immune cytopenias,33 another byproduct may be RBC alloimmunization, particularly when compared with the attenuated risks from historically more immune suppressive regimens.34
Arguments for matching less than we do
Given the nontrivial risk of seroconversion with any single encounter in the immune responder (whose status as such is not initially apparent), what is the sense of higher fidelity matching if patients migrate from 1 site that performs it to another that does not? In the Dutch MATCH RCT, the benefits of prophylactic matching of RBC units were also offset once RBC-dependent patients became PLT dependent.35 This observation concurs with the reduced but not eliminated potency of trace residual red cells in PLTs36 to provoke an RBC-specific seroconversion. This has in turn influenced common policies to either allocate PLTs from RHD− donors to RHD− recipients (transcending attention to ABO compatibility as a result) or coadminister RHD immune globulin prophylaxis (at the price of passively inducing transiently positive screens). At the same time, the risk of RHD isoimmunization from PLTs is recognized to be low in patients with hematological malignancies (at 0%-1.4% vs 7%-23% with RBCs]),37 although the significance of this figure is in the eye of the beholder. It may not be acceptable to a female patient at risk of future HDFN, but it is increasingly acceptable in others, especially when contrasted with the 10% seroconversion rate toward the minor RBC antigens for which most transfusion services have never routinely matched RBCs.
As crossing the RHD barrier without immunoprophylaxis in PLTs becomes less objectionable, and as inventory constraints are faced, MD Anderson Cancer Center has reported on its practice of crossing the RHD barrier in RBC transfusion in those for whom HDFN would not be a concern.38 As with minor antigen matching, the provision of RHD− components is rationally and safely restricted to instances when it is obliged in seroconverts (rather than in many potential nonresponders on a primary preventative basis). How do we reconcile the innovation of matching less at some institutions (ie, disregard RHD from RBCs in PLTs or RBC units themselves) with the ambition at other centers to match more? Should facilities with higher-fidelity RBC matching practices limit them to users who rely exclusively on RBCs, or otherwise advocate for PLT apheresis donors to have extended RBC antigen profile information listed on PLT doses so as to sustain RBC matching value? Is it fair to invest in higher-fidelity matching if most patients (even in conditions noteworthy for higher seroconversion rates) are not immune responders, and we are not yet equipped to precisely identify who they are? The evolving resource costs (and associated numbers needed to treat) to keep a patient screen negative would be helpful metrics against the better-known (patient experience and system) costs of screen positivity that we care to neutralize.
Residual leukocytes
Although patients with hematologic malignancies receive prestorage leukoreduced (PSLR) components in organizations that can provide them, absolute leukocyte elimination is not yet possible. For those whose immune systems (by immaturity, disease, or treatment) are unable to clear viable passenger lymphocytes, irradiation of cellular components is indicated to mitigate the risk of transfusion-associated graft-versus-host disease (TA-GVHD), despite the powers of PSLR components.39 Pathogen-inactivation technologies (which inherently also disarm donor leukocytes) may substitute for irradiation and are being applied to PLTs, RBCs, and whole blood, but to varying jurisdiction-dependent (and often still investigational) degrees. Once a risk for TA-GVHD is recognized, the means by which this is communicated for (and/or carried by) the patient echoes the related challenge of antibody repertoire portability/RBC matching expectations. One response to the TA-GVHD threat is to irradiate empirically for patients with hematological malignancies if relevant details are prohibitively difficult to gather, given that the price of missing a qualifying case is potentially lethal. At the author’s hospital, irradiation adds ∼US$6.50 in cost per component and is applied to 35% to 40% of cellular components, a proportion of which may be unnecessary.
Noncellular volume: plasma and supernatant solution
Much of the RBC unit remains intravascularly confined after transfusion, although some of the suspension fluid (Figure 4) can be decanted (plasma volume reduction) or washed/replaced (to a limit). Despite RBC units containing 1 to 2 log less plasma than PLTs, the residual plasma may nevertheless be a mediator of allergic and other reactions. Combined with the production-related fluid suspension, the RBC medium also accumulates cell-derived materials. The febrile nonhemolytic transfusion reaction still occurs with PSLR RBCs, and although typically nonsevere, its cumulative toll on patients and systems has been underestimated.40 Whether or not the product is modified, a temptation exists (in 1.6%41 to 50%42 of audited encounters) to modify the patient with premedications for febrile nonhemolytic transfusion and allergic reactions, even though these medications may be ineffective43 or harmful (eg, antihistamine-related sedation of outpatients who drive themselves to and from clinic). If the evidence is considered flawed, this is a call for skeptical centers to take another look at whether premedication works in the PSLR component era, particularly with the less-sedating antihistamines available today.
Conclusions
Patients with hematological malignancies count on transfusion support, but the extent to which RBC prescribing practices are optimized is up for debate and warrants further research (Table 1). Although Choosing Wisely and others have endorsed conservative triggering (<7-8 g/dL) and dosing (1 vs 2 units), uptake is slowest in the gray zone of the outpatient in whom the benefits of maximizing both quantity and quality of life (including time well spent and freedom from harms) must be balanced with the most judicious use of limited health care resources. In liberalism, it is not only the system (and the donor) that pays more, but also the recipient, who pays in time and in additive risks for products that may be unnecessary and iron loading. If important outcomes in conservatively transfused patients can convincingly be shown to be noninferior to those from liberal care strategies, enormous sums of money can be reallocated to other pursuits. These may include increasing the quality and immune tolerability of the units that we dispense, should this later involve higher-fidelity (or appropriately personalized) matching; harmonizing our clinical transfusion practices; and enhancing the capabilities of our health care information systems to improve transfusion safety.
Some unanswered questions for future research
| Quantity . | Quality . |
|---|---|
| For hematologic malignancy outpatients, is there a: | Can immune responders and treatment regimens be identified or stratified? |
| General or | Can special RBC transfusion needs (matching, irradiation, volume reduction) be agreed upon and/or reliably communicated for the sake of consistency and safety? |
| Individually identifiable | At what donor and recipient testing volume (and price point) can higher-fidelity antigen matching achieve RBC seroconversion avoidance and improve patient-reported (and traditional) outcomes? |
| Best RBC trigger and dosing strategy that: | In the PSLR era and in the subset of patients who have already experienced a transfusion reaction, can an alternative (less harmful) premedication regimen achieve reductions in the quantity and/or severity of transfusion reactions? |
| Optimizes quality of life (eg, sense of wellness, best use of time, freedom from fatigue) | |
| Influences bleeding (if at all) | |
| Preserves or extends quantity of life (survival) | |
| Minimizes exposure risks | |
| Saves money/stewards inventory for system-wide sufficiency and improvements |
| Quantity . | Quality . |
|---|---|
| For hematologic malignancy outpatients, is there a: | Can immune responders and treatment regimens be identified or stratified? |
| General or | Can special RBC transfusion needs (matching, irradiation, volume reduction) be agreed upon and/or reliably communicated for the sake of consistency and safety? |
| Individually identifiable | At what donor and recipient testing volume (and price point) can higher-fidelity antigen matching achieve RBC seroconversion avoidance and improve patient-reported (and traditional) outcomes? |
| Best RBC trigger and dosing strategy that: | In the PSLR era and in the subset of patients who have already experienced a transfusion reaction, can an alternative (less harmful) premedication regimen achieve reductions in the quantity and/or severity of transfusion reactions? |
| Optimizes quality of life (eg, sense of wellness, best use of time, freedom from fatigue) | |
| Influences bleeding (if at all) | |
| Preserves or extends quantity of life (survival) | |
| Minimizes exposure risks | |
| Saves money/stewards inventory for system-wide sufficiency and improvements |
Correspondence
Christine Cserti-Gazdewich, UHN TGH BTL 3EC-306, 200 Elizabeth St, Toronto, ON M5G-2C4, Canada; e-mail christine.cserti@uhn.ca.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: Erythropoeisis-stimulating agents (in general and not by brand) are mentioned but not explicitly recommended.

![Figure 3. Audit of practice at a Toronto cancer hospital (Princess Margaret Cancer Centre [PMH]). (A) Median triggering Hb in inpatients has trended from 7.5 to 7.0 g/dL (with >95% of orders triggering at <8.0 g/dL), but in outpatients, it remains near 7.5 g/dL (with <80% of orders at <8 g/dL). (B) Inpatient RBC transfusions are single-unit orders >90% of the time, whereas outpatient sittings use single-unit orders <70% of the time.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2018/1/10.1182_asheducation-2018.1.553/2/m_hem01874f3.png?Expires=1769216803&Signature=f3qiUXPR9SB~RX0QqBtTKqWMyI8p93ouAqRZgd3Zi6YqyYxNpGaYHj-UZmr5OD4IiL4aOZl-zOc4ojEvuvBbH1Wa7xfwhpJ04XvcXjrhDGCsRkIKk0KvPQQz0NWP9SkJoxzAh-wOyf4z9NQwq6iYNv3~YbGmW4Jpzz-gynl-MxUwpdvA0pP3wEQAjUuZkctSYki3lwNLTxVzz6GPWItjfo7z1D1tbRXqkpQJD~RPHvQ~lWThBbwv~~zIU2KJXF3F9djhibzHc08QQiRjgKpIds43cbatP80m8AFRWpX7kwj5A2Ay83uZ1~krCD9mEiRy3jjbHgdJy0OF4awq6R~-AQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)