Abstract
Immune checkpoint blockade therapy (CBT) was born of the combination of several elements: the understanding of some of the important immune regulation pathways in humans; the recognition that tumors can engage those pathways to evade immune responses; and the clinical development of monoclonal antibodies targeting checkpoint receptors to restore effective anti-tumor immunity. This form of therapy, focused to date mostly on the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed-death 1 (PD-1) pathways, has already revolutionized the treatment of several solid tumors. Hematologic malignancies (HMs) offer a promising testing ground for this strategy, and several trials have already demonstrated evidence of therapeutic activity with checkpoint blockade, especially in lymphoma. This review will discuss the current clinical results of CBT in lymphoma in the context of their scientific underpinning, and build from this summary a projection of how the field may evolve in the near future.
Learning Objectives
To understand the preclinical support for checkpoint blockade in lymphoma
To know the existing clinical results in checkpoint blockade therapy in lymphoma
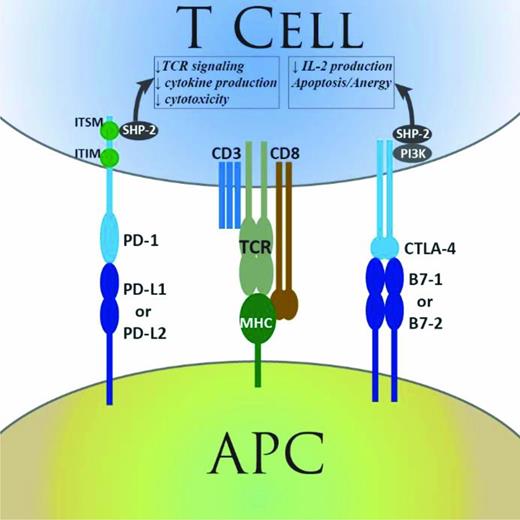
The interaction between the T-cell receptor and antigenic peptides presented in the context of the major histocompatibility complex (MHC) forms the core of the immunological synapse and the basis of antigen-restricted T-cell function. However, numerous other co-receptors and their ligands modulate the level of activation or inhibition of T-cell function, in order to preserve effective immune responses while preventing runaway or anti-self T-cell function. Two such coreceptors, the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed-death 1 (PD-1), have been extensively studied and are recognized to provide critical inhibitory signals that down-regulate T-cell function in the context of antigen recognition1-3 (Figure 1). Although the function of those immune checkpoints plays an important part in the normal regulation of immune responses, it also provides a mechanism of immune evasion for tumors. Based on the recognition of this phenomenon, therapeutic monoclonal antibodies targeting various elements of those pathways have been developed and extensively tested in humans in order to restore anti-tumor immunity at the priming stage for CTLA-4 or the effector stage for PD-1. The results of this checkpoint blockade therapy (CBT) have transformed the field of oncology, with durable responses obtained in many tumor types including melanoma, lung cancer, renal cell carcinoma, and others.4-11 Because hematologic malignancies (HMs) are well known to have inherent immune sensitivity, as exemplified by the curative potential of adoptive immunotherapy through allogeneic hematopoietic stem cell transplantation (HSCT), these tumors provide a natural target for CBT. Preliminary results are now available for trials of CBT in lymphoma. Here we will review some of the salient clinical results obtained to date, framed in the context of their scientific foundation. On that basis, we will discuss some possible future directions for this field. The groundbreaking results obtained in solid tumors, together with the promise of CBT in lymphoma, are generating much enthusiasm for checkpoint blockade trials; there are many ongoing and planned trials, and a daunting number of possible avenues for future research, especially using combination therapy. How we think about expanding our knowledge and leveraging results from both the extensive solid tumor and growing HM experience will likely strongly influence the decisions of which avenues to pursue and how successful we ultimately are at maximizing the benefit of this novel form of therapy for patients.
Summary of PD-1 and CTLA-4 function. Simplified representation of the function of the PD-1 and CTLA-4 immune checkpoint pathways. APC indicates antigen-presenting cell; TCR, T-cell receptor; MHC, major histocompatibility complex; CD, cluster of differentiation; IL-2, interleukin-2; PD-1, programmed death-1; CTLA-4, cytotoxic T-lymphocyte associated protein 4; ITIM, immunoreceptor tyrosine-based inhibitory motif; and ITSM, immunoreceptor tyrosine-based switch motif. Reprinted from Armand.38
Summary of PD-1 and CTLA-4 function. Simplified representation of the function of the PD-1 and CTLA-4 immune checkpoint pathways. APC indicates antigen-presenting cell; TCR, T-cell receptor; MHC, major histocompatibility complex; CD, cluster of differentiation; IL-2, interleukin-2; PD-1, programmed death-1; CTLA-4, cytotoxic T-lymphocyte associated protein 4; ITIM, immunoreceptor tyrosine-based inhibitory motif; and ITSM, immunoreceptor tyrosine-based switch motif. Reprinted from Armand.38
Early trials of CBT in lymphoma
The first attempts at CBT in HM were phase 1 studies using the anti-PD1 antibody pidilizumab and anti-CTLA4 antibody ipilimumab. In the pidilizumab study, 17 patients with advanced HMs were treated.12 The authors reported a “clinical benefit” rate of 33%, which mostly comprised patients with stable disease, with the notable exception of a patient with follicular lymphoma (FL) who achieved a complete remission (CR) with therapy. In the ipilimumab study, 18 patients with non-Hodgkin lymphoma (NHL) were treated, with an objective response rate (ORR) of 11%. Although the response rate itself was not impressive, there were some durable responses, one in a patient with diffuse large B-cell lymphoma (DLBCL) who achieved a CR maintained over 2.5 years, and one in a patient with FL who obtained a PR maintained for 1.5 years. Together those studies demonstrated that CBT might have activity in NHL.
The second wave
The activity observed with pidilizumab in NHL led to two further studies of this agent. The first was a phase 2 trial in patients with DLBCL who received 3 doses of pidilizumab as consolidative therapy after autologous stem cell transplantation (ASCT).13 The primary endpoint of this study was the progression-free survival (PFS) after ASCT, which is briefly discussed below. However, as some patients still had measurable disease after ASCT, it was also possible on this trial to estimate the response rate to pidilizumab among those patients. Based on computed tomography (CT) results, this response rate was 51%, with a 34% CR rate. Although this result was encouraging, it was not a predefined endpoint of the study, and it is not clear how to appropriately interpret the time course of CT-detected lesions after ASCT. Therefore, this provided only circumstantial evidence of a direct anti-DLBCL activity of the antibody.
The other trial was a phase 2 study of pidilizumab in combination with rituximab in patients with relapsed FL.14 The ORR was 66%, with a 52% CR rate. These findings support the effectiveness of pidilizumab in this disease, as suggested by the phase 1 results. Here again, the design of the study slightly complicated its interpretation. It enrolled only patients with rituximab-sensitive disease, in whom single-agent rituximab is likely to have significant activity, though probably not such a high CR rate. This provided further evidence for an anti-lymphoma activity of PD-1 blockade.
The third wave
In 2014, preliminary results from 2 large phase 1 studies of PD-1 blockade in HM were reported. The first study used the fully human IgG4 monoclonal antibody nivolumab in patients with multiple myeloma (MM), NHL and Hodgkin lymphoma (HL).15,16 The second study used the humanized IgG4 antibody pembrolizumab in patients with myelodysplastic syndromes (MDS), MM, NHL, and HL.17 At the time of this writing, the results of this study in MDS, MM, and NHL are not yet public; further, the results of nivolumab in MM will not be discussed here. By the time these 2 studies were launched, more was known about the biology of PD-1 in lymphoma, and this understanding was important both to the design of the studies and to the interpretation of their results. Interestingly, the 3 major histologies represented in the published results, namely DLBCL, FL, and HL, display fundamentally different biology with respect to PD-1.
Hodgkin lymphoma
The inclusion of independent HL expansion cohorts in both phase 1 studies is a notable departure from most early phase studies in lymphoma, and reflects the unique biology of this disease. It has long been surmised that the pathology of HL, with its isolated tumor cells surrounded by extensive but ineffective immune cells, indicates an unusual relationship between HL and the immune system of its host. Comprehensive genetic analyses identified PD-L1 and PD-L2 as the primary targets of 9p24.1 amplification, which is a recurrent genetic abnormality in HL.18 The JAK2 gene is also located at 9p24, and its amplification further drives PD-L1 transcription through JAK/STAT signaling,18 as do other rarer genetic mechanisms.19 Finally, Epstein-Barr virus (EBV) infection also leads to PD-L1 overexpression,20 which is consistent with the recognized ability of viruses to engage checkpoint pathways to avoid immune eradication. Through those overlapping mechanisms, the PD-1 ligands are expressed on the tumor cell surface in a very high fraction of classical Hodgkin lymphoma tumors.21 This makes HL unique among all malignancies heretofore subjected to PD-1 blockade, in that it appears to have a genetically determined dependence on the PD-1 pathway for survival. Based on this understanding, HL was felt to constitute a prime target for PD-1 blockade, leading to its inclusion in both phase 1 studies mentioned above. The clinical results provided a dramatic validation of the scientific hypothesis. Despite the fact that the 52 patients enrolled on the 2 studies were heavily pretreated, with most having relapsed after ASCT and after brentuximab vedotin, PD-1 blockade was associated with high response rates, 87% with nivolumab (with a 17% CR rate), and 65% with pembrolizumab (with a 21% CR rate). The responses appear to be long lasting, with a median duration not reached in either study, and the majority of patients with ongoing responses at the time of the latest data cutoffs. Furthermore, correlative studies performed in the nivolumab study confirmed the earlier genetic studies, with 100% of the tumor samples studied showing copy number gain at 9p24.1, with in all cases expression of PD-L1 and PD-L2 on the Hodgkin Reed–Sternberg cell surface.15
Diffuse large B-cell lymphoma
Unlike in HL, the frequency of PD-L1 expression on the surface of DLBCL cells is an uncommon event. In the first study to address this question systematically, only about one-quarter of primary DLBCLs displayed PD-L1 expression.22 The notable exception was primary mediastinal B-cell lymphoma (PMBL), whose biology is much more similar to that of HL. Indeed, PMBL frequently harbors genetic amplification or rearrangements involving 9p24 and leading to surface expression of PD-L1 and PD-L2.18,23,24 Although this entity was specifically included in the nivolumab and pembrolizumab studies, few patients were enrolled and the clinical results are not yet mature. Among the 11 patients with DLBCL (not including PMBL) treated with nivolumab, the ORR was 36% (including 1 CR), with a median duration of response (MDR) of 22 weeks, and 1 ongoing responder at 73 weeks. This raises an obvious question, because the response rate is close to the documented rate of PD-L1 positivity: are the responses confined to patients with PD-L1 tumor expression, and could this provide a biomarker for patient selection? At this time, the answer is not yet known, but a large ongoing phase 2 study of nivolumab in DLBCL will allow us to address this in the near future.
Further work on PD-L1 expression in DLBCL has yielded interesting clues to PD-1 biology in this disease. In a detailed analysis of many well-classified DLBCL tumors, Chen and colleagues reported that PD-L1 expression is indeed present only in a subset of tumors, but in a well-defined subset, characterized either by viral infection (especially EBV) or by histology (T-cell/histiocyte-rich large cell lymphoma; TCHRLCL).21 In those subtypes, the prevalence of PD-L1 expression was very high, whereas it was low in other DLBCLs. This suggests that PD-1 blockade in DLBCL could be most effective when directed at a defined subset including PMBL, EBV-positive disease, and TCHRLCL. Although the aforementioned ongoing studies may shed light on this question, a conclusive answer will likely require dedicated clinical trials.
Follicular lymphoma
FL provides yet another perspective on PD-1 blockade. In the study of Andorsky et al,22 FL did not in fact demonstrate PD-L1 expression on tumor cells. Yet the prior studies of pidilizumab in this disease demonstrated activity; moreover, analyses performed in the context of the pidilizumab + rituximab study showed that treatment was associated with an apparent increase in endogenous anti-tumor activity.14 In the nivolumab phase 1 study, 10 patients with FL were treated, and 4 (40%) demonstrated a response, including 1 (10%) who achieved CR. Here again the responses appear durable, with an MDR not reached at a median observation time of 62 weeks. In support of prior work, immunohistochemical analyses of tumor material from the nivolumab trial showed that PD-L1 expression in tumors appeared confined to the infiltrating macrophages and generally absent on tumor cells.16 There has been much study of the FL microenvironment, with differing conclusion about the prognostic impact associated with PD-1+ infiltrating T cells.25,26 Subsequent research has suggested that there are in fact different populations of PD-1+ T cells in the FL microenvironment, and that PD-1lo and PD-1hi subgroups play different roles in FL biology.27,28 Here again, ongoing larger studies may allow us to better understand this phenomenon and how it may relate to the therapeutic activity of PD-1 blockade in FL.
Safety and toxicity
Although the adverse effects seen with CBT are very different from those seen with conventional cytotoxic or targeted therapy, being dominated by immune-related events including pneumonitis, colitis, hypophysitis, etc, overall this therapy is associated with a low rate of treatment-related severe or life-threatening complications. An important concern is whether this will hold true in the treatment of lymphoma. Many existing lymphoma treatments have potential pneumotoxicity, including radiotherapy, bleomycin, brentuximab vedotin, and carmustine (often used in high dose for ASCT conditioning). Moreover, the lung toxicity associated with some of those agents, such as bleomycin or radiotherapy, can be delayed. Pneumonitis is a particular concern with checkpoint blockade, and has accounted for most of the drug-related fatalities seen to date. It is therefore important to show that the use of those agents in patients with extensive prior treatments will not result in excessive pulmonary (or other) toxicity. There is not enough data at present to be certain of the safety of CTLA-4 and PD-1 blocking antibodies in lymphoma; however, the results obtained so far suggest a favorable safety profile. Although the rates of pneumonitis seen in the 2 recent phase 1 studies of PD-1 blockade may be slightly higher than that seen in solid tumors, the overall rates of severe or life-threatening toxicity attributed to PD-1 blockade appear tolerably low.15-17 This will need to be confirmed in the larger ongoing studies, and carefully monitored as we incorporate CBT into combination regimens.
In search of a biomarker
It is clear from the clinical results obtained to date that PD-1 blockade by itself has important but limited activity in most lymphoma subtypes. This raises the important question of how to select patients most likely to respond to this type of therapy. Already we can appreciate not only the complexity of the answer but the fact that it may be different in different histologies. As summarized above, at this time the 3 main lymphoma histologies in which PD-1 blockade has been tested demonstrate very different relationships between scientific and clinical results. In the case of HL, preclinical results strongly suggested that there should be a high response rate to PD-1 blockade, which was resoundingly borne out by trial results. In DLBCL, laboratory studies suggest that responses could be confined to certain definable subsets; clinical results indeed suggest a limited response rate, but we do not yet know whether responding tumors are the ones predicted. In addition, in FL, we know that there is important clinical activity of PD-1 blockade but are facing the possibility that the principal determinant of response may reside in the complexity of the tumor microenvironment's composition rather than in the expression of ligands on the tumor cell surface. This difficulty reflects a similar question in solid tumors, especially melanoma and lung cancer, where some studies suggest that PD-L1 expression on the tumor cell surface is an important biomarker,29,30 whereas others suggest that microenvironmental composition may be more important.31 In this respect, it should be noted that the high response rate seen in HL could in fact not be based directly on high ligand expression but on the effect that this high ligand expression may have on the microenvironment. To answer those questions, careful correlative studies conducted in the context of large studies with diligent collection of tumor biopsy samples and analyses not only of the tumor but also of the microenvironment's architecture and composition will be essential.
In the meantime, however, we can already leverage the results obtained in HL by targeting tumors that display similar constitutive PD-L1/PD-L2 expression. As mentioned above, PMBL is an obvious candidate. Other candidates are the subsets of DLBCL demonstrating evidence of viral infection, or TCHRLCL. Finally, there may be other lymphomas of various histologies that happen to have amplification or rearrangements involving 9p24.1 and the PD-1 ligands; such tumors could be identified based on a combination of immunohistochemical and FISH assays and included in focused trials of single-agent PD-1 blockade.
This raises the question of how to optimally target the PD-1 axis. At present, there are both anti-PD-1 and anti-PD-L1 monoclonal antibodies available for clinical testing. There is little published clinical experience to date with the anti-PD-L1 antibodies, although such results are forthcoming. Although it is difficult to know which strategy will ultimately be most fruitful therapeutically, the presence of PD-L2 on the surface of tumor cells for HL and PMBL, as well as the description of other lymphoma cases harboring translocations involving PD-L2,16 suggests that blockade at the receptor level, which will disrupt both PD-1/PD-L1 and PD-1/PD-L2 interactions, may be theoretically preferable to blockade at the ligand level (which will not affect the PD-1/PD-L2 interaction), at least in the treatment of lymphoma.
Combination treatment
There may be select lymphoma subtypes, including HL and potentially others mentioned above, with particularly high sensitivity to PD-1 blockade, in which single-agent therapy could provide adequate disease control. In most patients, however, it is unlikely that this will be sufficient. There is therefore considerable interest in using CBT as part of combination therapy. In the setting of lymphoma treatment, there are several distinct and potentially useful ways to combine CBT. First, different checkpoints may be blocked or stimulated simultaneously. This strategy has already yielded impressive results in melanoma with the combination of CTLA-4 and PD-1 blockade.32 However, the single-agent activity of CTLA-4 in melanoma may be stronger than in lymphoma,4,33 and it is therefore not certain that this combination will be similarly useful in lymphoma. Already the combination of nivolumab and ipilimumab has been tested in a continuation of the nivolumab phase 1 HM study, although results are not yet available. Several other combinations are currently in testing, such as nivolumab with the CD137-targeting antibody urelumab, or with the KIR-targeting antibody lirilumab. As other checkpoint blocking agents move through early phase trials, the number of possible combinations will grow quickly.
Another potentially important CBT partner is cytotoxic therapy. There is growing interest in the immunologic impact of conventional cytotoxic agents, and already a significant scientific basis in support of the hypothesis that immune-enhancing treatments, such as CBT, could provide synergistic benefit in combination with chemotherapy.34 At present, there are ongoing trials combining CBT with other monoclonal antibodies such as rituximab, but no trials of chemotherapy and CBT, although it is likely only a matter of time before such trials get underway.
Finally, CBT may be combined with other types of immunotherapy. Several immune-based approaches have already demonstrated exciting activity in lymphoma including vaccine therapy, chimeric-antigen receptor (CAR)-T cells, and bi-specific antibodies. The anti-tumor activity of those treatments may be profitably enhanced by combining them with checkpoint blockade, and it is likely that such combinations will soon enter clinical trials.
CBT in the setting of stem cell transplantation
The immunologic context present after stem cell transplantation is very different from that in patients treated with conventional therapy. Early on after ASCT, there is usually a minimal disease burden and a deep remodeling of the immune response. After allogeneic HSCT, there is an adoptive immune system with higher-than-normal antigen disparity with host cells, which in many cases can by itself provides salutary anti-tumor activity. In both cases, though for different reasons, CBT could provide an effective adjunct to the transplantation itself. There are already promising results of PD-1 blockade with pidilizumab after ASCT for patients with DLBCL,13 especially in the high-risk group of patients with persistent FDG-avid disease after salvage therapy. There are also exciting results with CTLA-4 blockade after allogeneic HSCT, suggesting that this therapy may provide effective anti-tumor effect without prohibitive exacerbation of graft-versus-host disease.35,36 A full discussion of those studies is beyond the scope of this review, but they are likely to provide foundational evidence on which future transplantation studies will be built.
Future directions
The combination of practice-changing results in melanoma and other solid tumors and the preliminary but striking findings in lymphoma is fueling great enthusiasm for CBT in lymphoma. With several possible checkpoint receptors to target, many potential tumors to select, and a wealth of possible combination of checkpoint blocking agents, conventional therapies, targeted therapies, and other immunotherapies, there are many more possible interesting trials than can be supported by our current research infrastructure. Ultimately, the success of the clinical research enterprise in this field may depend on our ability to rationally select the most promising trials among the plethora of possible ones. To do so, the following issues may be helpful to consider. First, we have the benefit of a large existing experience with CBT in solid tumors, which has already generated critical scientific insights and questions. These can be incorporated into and adapted for the study and treatment of HM. Second, we must recognize that the patterns of tumor response, including prolonged disease stability and pseudo-progression, may be different enough with CBT compared with conventional treatments to warrant adapting response criteria to this type of treatment, as has been done for solid tumors.37 There is already anecdotal evidence that those phenomena may happen in lymphoma, but more work is needed to systematically collate and analyze the data from completed and ongoing studies. Third, we should carefully plan correlative studies performed in CBT clinical trials to obtain the right type and amount of tumor material and to maximize the yield of this most precious commodity, because so much remains to be known about the determinants and mechanisms of response and of resistance. Fourth, we can acknowledge that in some cases, such as HL, the scientific results can predate and predict the clinical ones; in other cases, such as with FL, the clinical results can themselves provide impetus for further study and through correlative analyses enhance our understanding of results we did not predict. We will therefore need to pursue both scientific and clinical results and strive to enhance their synergy by rapidly disseminating early findings in both the laboratory and the clinic. Finally, as various academic centers and pharmaceutical companies develop novel reagents and assays to perform those studies, we should refine and expand the paradigms for multicenter and academic/industry partnerships in order to leverage each participant's expertise in a way to benefit the entire research community, and ultimately to benefit the patients we serve.
Correspondence
Philippe Armand, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; Phone: 617-632-2305; Fax: 617-632-4422; e-mail: parmand@partners.org.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Bristol-Myers Squibb and Merck, and has consulted for Merck and Infinity Pharmaceuticals.
Author notes
Off-label drug use: Nivolumab, pembrolizumab, and pidilizumab in lymphoma.