Abstract
Mechanical circulatory support (MCS) provides a bridge to heart transplant in children and adults with life-threatening heart failure and sustains patients ineligible for transplant. Extracorporeal membrane oxygenation (ECMO) provides temporary support for patients in cardiac or pulmonary failure through external gas exchange and continuous flow of blood. Because the median time to heart transplant exceeds event-free time on ECMO, pulsatile left ventricular assist devices (LVADs) are used to support infants and children. Continuous flow LVADs are preferred in adolescents and adults due to increased pump durability and improved overall survival. The shear stress created by the mechanical pumps cause changes in the hematologic system; acquired von Willebrand syndrome occurs in almost all patients treated with MCS. Despite the improvements in survival, major bleeding occurs in one-third of patients with a LVAD and ischemic stroke and LVAD thrombosis can affect 12% of adults and 29% of children. An antithrombotic strategy to mitigate LVAD bleeding and thrombotic complications has been tested in a randomized trial in children, but intensity of antithrombotic therapy in adults varies widely. Consensus guidelines for antithrombotic therapy during ECMO were created due to significant differences in management across centers. Because of the high risk for both bleeding and thrombotic complications, experts in hemostasis can significantly impact care of patients requiring mechanical circulatory support and are a necessary part of the management team.
Learning Objectives
Understand available options for mechanical circulatory support and the impact of mechanical circulatory support on the hematologic system
Differentiate the bleeding and thrombotic risks between children and adults requiring mechanical circulatory support
Recognize possible treatment options for mechanical circulatory support thrombosis
Heart failure affects >5 million adults in the United States (US) and 23 million people globally.1 The incidence of heart failure in children is significantly less than adults with ∼18 admissions per 100 000 children per year; however, there is significant economic impact of pediatric heart failure given the frequent need for surgical intervention and the loss of productive years in the event of a child's death.2 The heart failure etiology also differs with age as most children have congenital heart disease and adults experience cardiomyopathy or coronary artery disease.1
Heart transplantation offers a cure for heart failure, but availability of organs is limited. The International Society of Heart and Lung Transplantation (ISHLT) registry represents ∼66% of the thoracic transplants worldwide. In 2012, 567 pediatric and 3529 adult heart transplants were reported, compared with 93 pediatric and 3719 adult lung transplants.1,3 Only 36% of patients on the heart transplant list received an organ.1,3 The median time to heart transplantation for patients at highest urgency is 78 days in adults and 85 days in children.1 A multicenter cohort study analyzed 3098 children in the US listed for a heart transplant between 1999 and 2006. The waiting list mortality was 17%. Mechanical circulatory support (MCS) including extracorporeal membrane oxygenation (ECMO), a total artificial heart (TAH), or ventricular assist devices (VADs) can be used to bridge the gap between life-threatening heart failure and heart transplantation, or support patients ineligible for transplant (Table 1).
Mechanical circulatory support options for children and adults35
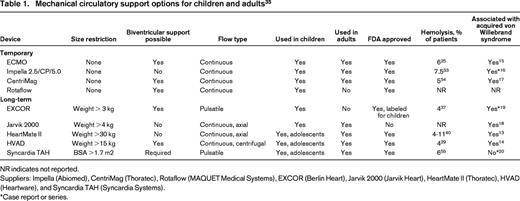
NR indicates not reported.
Suppliers: Impella (Abiomed), CentriMag (Thoratec), Rotaflow (MAQUET Medical Systems), EXCOR (Berlin Heart), Jarvik 2000 (Jarvik Heart), HeartMate II (Thoratec), HVAD (Heartware), and Syncardia TAH (Syncardia Systems).
*Case report or series.
ECMO provides nonpulmonary gas exchange through passage of blood over a membrane that removes carbon dioxide and oxygenates the blood. If cannulated from the venous to arterial system (VA ECMO) a pump can augment or replace heart function.4 VA ECMO is the most common method to support infants in heart failure; 60% of infants who received a heart transplant were supported with ECMO in 2012.3 The proportion of patients receiving ECMO declined dramatically with age; only 18% of transplant recipients aged 11-17 years and 1.1% of adults were supported with ECMO prior to heart transplant.1,3 Technologic advances and improved mortality outcomes during the 2009 H1N1 influenza outbreak has increased the use of venovenous (VV) ECMO for patients with acute respiratory failure and around the time of lung transplant.5 After 10-20 days of support though, ECMO is associated with serious complications including vasomotor instability, capillary leak syndrome, bleeding, and multisystem organ failure. In both children and adults, the average wait time for organ transplantation is much longer than the event-free time using ECMO.
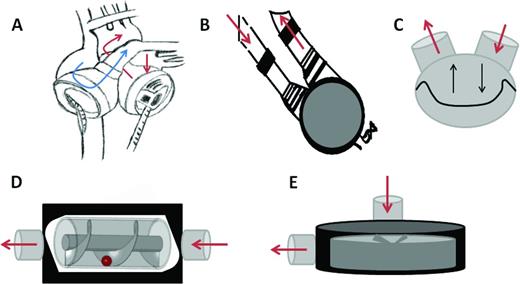
More durable options for mechanical circulatory support for heart failure include a TAH and LVAD. A TAH will provide biventricular support using one device. The Syncardia TAH functions by blood flowing from the atria, passing through the pulsatile pump, and returning into the aorta to provide systemic circulation and pulmonary artery to replace right ventricular function (Figure 1). Pulsatile left ventricular assist devices (LVADs; eg, Berlin EXCOR; Berlin Heart) are available in sizes from 10-60 cm3 and can support children as small as 3 kg (Table 1).6 The Syncardia TAH and pulsatile VADs pump blood through pneumatically driven diaphragms (Figure 1).6 In children >1 year, VAD or TAH support is used more often than ECMO prior to transplantation.3 Because the oscillating membranes in pulsatile LVADs lack durability, continuous flow (CF) LVADs were developed. Axial LVADs (eg, HeartMate II; Thoratec) create flow through a rotor containing helical blades that spin between 7000-12 000 rotations per minute (rpm). Third-generation centrifugal LVADs (HeartWare HVAD; Heartware) were developed to eliminate the need for mechanical bearings. Because the rotor diameter for centrifugal devices is larger, flow is created at lower rotational speeds (2000-3000 rpm), thus creating less shear stress and hemolysis.7 MCS support of adults prior to transplant has increased over the last decade to 37%, primarily because of increased LVAD use.1 In addition, LVADs can be implanted in adults ineligible for heart transplantation to alleviate heart failure symptoms. The CF VADs have also been extended to use in older children with similar outcomes (ie, 10%-20% mortality),8 but the affect of non-pulsatile flow on maturing vasculature is unknown.
Schematic representation of mechanical circulatory support devices. (A) Total artificial heart. Blue arrow represents the path of deoxygenated blood and red arrows represent the path of oxygenated blood. (B) Pulsatile LVAD. (C) Oscillating membrane of pulsatile devices. (D) Continuous flow of axial LVAD. (E) Continuous flow of centrifugal LVAD.
Schematic representation of mechanical circulatory support devices. (A) Total artificial heart. Blue arrow represents the path of deoxygenated blood and red arrows represent the path of oxygenated blood. (B) Pulsatile LVAD. (C) Oscillating membrane of pulsatile devices. (D) Continuous flow of axial LVAD. (E) Continuous flow of centrifugal LVAD.
Effects of MCS on the hematologic system
The shear stress created by MCS can cause damage to circulating blood cells and hemostatic proteins. Testing during device development is proprietary information. The degree of blood damage caused by the device can be inferred from the number of patients in the clinical trials that develop hemolysis, but hemolysis is also used as a marker of device thrombosis. Among the temporary and long-term devices, hemolysis episodes ranged from 4%-11% (Table 1). Baseline levels of hemolysis are higher in patients with the HeartMate II compared with the HVAD CF LVADs.7 Decreased contact pathway proteins (factors XI and XII) were found in patients implanted with older devices,9 but how contemporary devices affect contact proteins is unknown. Older devices also caused significant platelet activation but the degree of platelet activation with CF LVADs is debated depending upon the method used to detect activation.10 Elevated levels of prothrombin fragment 1.2 and D-dimer are most pronounced post-operatively, and levels return to near-normal within 6-12 months.11 Increased levels of tissue factor, E-selectin, and intercellular adhesion molecule on circulating endothelial cells of LVAD patients suggests persistence of endothelial cell activation after LVAD implantation.12
Nearly all patients treated with MCS develop acquired von Willebrand syndrome with loss of high molecular weight von Willebrand factor multimers (Table 1).13-19 The exception may be the Syncardia TAH, as three patients with normal von Willebrand multimers after TAH implantation have been reported.20 The severity of acquired von Willebrand syndrome is similar between the HeartMate II and HVAD devices.14 The association between GI bleeding and acquired von Willebrand syndrome is debated as the severity of the acquired von Willebrand syndrome is not associated with an increased risk for GI bleeding.14 Additional investigation is underway to understand the pathophysiology of GI bleeding in LVAD patients and the role of von Willebrand factor in angiogenesis.
Differences in hemostasis and anticoagulation in infants and children
Anticoagulation management in infants and children cannot be extrapolated from adult guidelines. Age related differences in levels of hemostatic proteins exist [proteins involved in coagulation (FXII, XI, X, IX, VII, II), fibrinolysis (plasminogen, PAI, uPA, and tPA), and inhibitors of coagulation (antithrombin [AT] proteins C and S)].21,22 The pharmacokinetics and pharmacodynamics of antithrombotic therapy result in dosing differences.22 In addition, children normally generate less thrombin than adults when hemostasis is activated23 ; however, MCS generates large amounts of thrombin because of the presence of biomaterials and generated shear forces, necessitating antithrombotic therapy to prevent thrombosis in all patients.
Indications and outcomes for pediatric ECMO
ECMO is used for temporary support in children with cardiac or respiratory failure and during extra-corporeal cardiopulmonary resuscitation (ECPR). Technologic advances have broadened the indications for ECMO to include prematurity (>30 weeks and birth weight >1 kg) and prolonged mechanical ventilation.24,25
There are a limited number of large prospective studies of ECMO for infants and children. One trial of neonates with severe respiratory failure randomized 185 neonates to medical care or ECMO (64% received VA ECMO, 29% VV ECMO). The study closed early due to a significant survival advantage in the ECMO group versus medical therapy (67.5% vs 41.3%, respectively; p = 0.0005).26 Evaluation of these children at 4 yrs of age demonstrated 37% mortality or severe disability in the ECMO group versus 59% in the conventional treatment group (p = 0.004).26
ECMO patients are represented in multiple databases including the Extracorporeal Life Support Organization (ELSO) database, Interagency Registry for Mechanically Assisted Circulatory Support Database (INTERMACS) and large collective groups. However, merging data from these sources is challenging as the collected data elements differ, and definitions of diagnoses, complications, and outcomes are not standardized.
The ELSO database reported that as of January 2015, 65 171 patients have been treated with ECMO; 53% were neonates and 25% were pediatric. In neonates and children, survival to discharge is highest for respiratory indications, 74% and 57%, respectively, compared with 41% and 50% survival for cardiac failure.25 Infants and children bridged to transplant with ECMO versus VAD had decreased survival (64% vs 84%, respectively; p < 0.0001).27
Most hemorrhagic complications associated with ECMO in neonates and children are related to cannulation and surgical site bleeding (Table 2). Mortality of cardiac patients with hemorrhage is significantly higher than patients without hemorrhage (38% vs 21%; p < 0.0001).28 Hemorrhagic stroke rates were 8.7% and 4.1% in cardiac ECMO versus 7.4% and 6.2% in respiratory ECMO in neonates and children, respectively.28
Bleeding and thrombotic complications during mechanical circulatory support

NR indicates not reported.
*Surgical site bleeding.
†Percentage of reported events.
‡GI and intracranial hemorrhage.
§Chest re-exploration and intracranial hemorrhage.
‖Combined number of thrombosis events at any circuit site.
Indications and outcomes for adult ECMO
Similar to children, ECMO can be used as a rescue therapy for adults in refractory cardiac arrest, hypoxic respiratory failure, or cardiogenic shock unresponsive to inotrope treatment. Over 14 000 adults undergoing ECMO have been included in the ELSO registry. The ELSO 2015 report noted the indication for adult ECMO was respiratory failure in 49% of patients, cardiac failure in 39%, and ECPR in 12% of patients.25 A recent Cochrane Database systematic review summarized four randomized control trials of adults with respiratory failure treated with VV ECMO. Contemporary studies reported all-cause mortality rates ranging between 18%-37% in patients treated with ECMO and there was no survival benefit of ECMO compared with best supportive care. However, 1 study showed improved functional status in patients treated with ECMO (disability free survival 63% ECMO vs 47% control; RR 0.69; 95% CI 0.05-0.97). Older studies noted a higher incidence of alveolar hemorrhage, but protective lung ventilation strategies had been not developed. Later trials reported increased blood transfusion requirements in patients treated with ECMO, though, and hemorrhage led to discontinuation of EMCO in one-half of patients.29 There are no randomized trials of ECMO in patients with cardiac failure but a review of 533 patients with cardiogenic shock reported a 52% survival to discharge.29 The ELSO registry reported a lower survival rate to discharge of 41% in patients treated with ECMO for cardiac failure.25 Additional randomized studies are ongoing to understand if ECMO would be beneficial in patients experiencing cardiac arrest outside of the hospital.
ECMO antithrombotic therapy
Standardized thromboprotective strategies and monitoring methods during ECMO have not been systematically studied. A recent international survey of 187 ELSO centers (67% pediatric, 30% mixed adult, and pediatric and 3% adult only) demonstrated significant variability in practice between centers.30 Unfractionated heparin (UFH) was used in 100% of ECMO patients. The parenteral direct thrombin inhibitors were available in 53% of centers, but were used rarely (only 8% of centers). In addition, there was limited use of antiplatelet agents including aspirin and dipyridamole. AT supplementation, using AT concentrate or plasma, occurred in 91% of centers with the goal AT level varying between 30% and 100%. Retrospective studies published in children treated with ECMO have used various AT concentrate dosing regimens including bolus dose and continuous infusions.31 Anticoagulation was monitored in 97% of centers using activated clotting time (ACT) with variable target ranges; the minimum and maximum goals were 183 (range, 140-220) and 210 (range, 170-240) seconds, respectively. In addition, the activated partial thromboplastin time (aPTT) and anti-factor Xa were monitored in 96% and 65% of centers, respectively, with centers using variable target ranges for the anti-factor Xa levels, but with 0.3-0.7 units/ml being the most common. Thromboelastography provided additional information in 43% of centers, but test systems differed between centers. Testing to monitor antiplatelet therapy in those centers using aspirin or dipyridamole was not discussed.30
The variability between treatment centers lead to the development of the ELSO anticoagulation management guidelines which are based on expert consensus and review of multiple anticoagulation practices.28,32 UFH is recommended with a 50-100 unit/kg bolus at cannulation followed by an infusion starting at 7.5-20 units/kg/hr and adjusted based on an ACT of 180 to 220 seconds. Typically 20-50 units/kg/hr is required to achieve the target ACT range. The ACT correlates poorly with the heparin dose.33 Therefore, some centers use an anti-factor Xa level or aPTT to manage heparin. Whole blood assays including thromboelastography (TEG) and thromboelastometry (ROTEM) are used by some centers, but further studies to standardize these techniques are required.
Occasionally, low AT precludes obtaining a therapeutic heparin effect. AT complexes with administered UFH (∼1/3 of saccharide chains bind to AT) potentiating the inhibition of thrombin and factor Xa by 2000- to 3000-fold. Increasing AT levels during UFH therapy might achieve a greater antithrombotic effect, thereby minimizing the unwanted pharmacologic effects of excessive UFH.31 In children, physiologic AT levels are lower than adults, resulting in the administration of AT concentrate by some centers during ECMO, despite the lack of safety and efficacy data.30,34
Challenges with UFH exist including development of heparin induced thrombocytopenia and an inability to achieve therapeutic anticoagulation. In both clinical scenarios, alternate anticoagulant agents are required. Parenteral direct thrombin inhibitors, most commonly argatroban or bivalirudin, are being administered to patients as an alternative to UFH, despite the paucity of safety and efficacy data in ECMO and the lack of a reversal agent.32
Pediatric VAD
Outcomes.
Until recently, outcome event definitions were not standardized between centers making comparison of data challenging. The PEDIMACS Database (US)35 (NCT00119834) and the German Heart Database36 systematically collect data using standard outcome definitions. The most common adverse events in the EXCOR study were major bleeding (50%), infection (63%), stroke (29%), and hypertension (50%; Table 2).37 Median survival time in children <0.7m2 and ≥0.7 m2 implanted with the EXCOR was greater than 174 days and 144 days, respectively, and 13 and 11 days in the matched ECMO controls. Retrospective studies of the EXCOR have reported a decreased incidence of major bleeding and thrombosis, but different outcome definitions were used.38
Antithrombotic therapy management.
The optimal intensity of antithrombotic therapy in children with VADs is unknown, but is probably device and patient specific. Currently, children with VADs are most commonly treated with the same anticoagulant and antiplatelet agents used in adults.39 A retrospective review of children with VADs (n = 466) between 2000 and 2011 demonstrated variability in antithrombotic therapy use. Anticoagulation was administered in 98.3% of patients and UFH was used in all patients. Enoxaparin usage increased (38% of patients), whereas warfarin usage was stable (26% patients) over the time period. AT administration increased (44% patients) over time reflecting the clinical practice to minimize the intensity of UFH therapy. Alteplase was used in 31.8% of patients who required LVAD thrombolysis or unblocking of a central line. Direct thrombin inhibitors were used in <1% of children.34 Antiplatelet medication use over time increased to 67% of children, with aspirin being the most common (52%), followed by dipyridamole (32%), pentoxifylline (2%), omega 3 fatty acids (1%), and abciximab (0.2%).34
Pediatric and device specific antithrombotic guidelines (Table 3) were developed and prospectively studied during the EXCOR trial.37,38 AT supplementation was recommended if the level was <70%,37 but no dosing guidance was given. For long term therapy, low molecular weight heparin (LMWH) and warfarin were recommended in children <12 and ≥12 months of age, respectively. UFH and LMWH therapies were monitored using aPTT and anti-factor Xa levels, respectively. The targeted INR range for patients treated with warfarin was 2.7-3.5. Additional information regarding hemostasis was provided by thromboelastography using TEG. In the EXCOR study,37 aspirin and dipyridamole were used with dosing based on the level of platelet inhibition as established in the TEG/platelet mapping testing. Older children receiving a CF VAD are treated similarly to adults with anticoagulant and antiplatelet agents.39
Summary of Edmonton Antithrombotic Guidelines for Management of Children with the Berlin Heart EXCOR Pediatric VAD48

TEG indicates thomboelastogram (Haemanetics); MA, maximum amplitude; MAcKH, maximum amplitude citrate kaolin heparinase; MAcK, maximum amplitude citrate kaolin; INR, International Normalized Ratio; AA, arachidonic acid inhibition; ADP, adenosine diphosphate; and Net ADP G, [(100 − %ADP inhibition) × GCKH]/100.
Adult VAD
Outcomes.
In contrast to children with heart failure, CF LVADs (eg, HeartMate II and HVAD) are the standard of care for adults due to improved survival and pump durability. In the HeartMate II trial of patients ineligible for heart transplant, the patients randomized to the HeartMate II implantation had more than a doubling of 2 year survival compared to patients implanted with a pulsatile LVAD (58% vs 24%; p < 0.01).40 Significantly fewer patients with the HeartMate II required LVAD repair or replacement compared to the pulsatile pump (10% HeartMate II vs 36% pulsatile LVAD; p < 0.01), proving durability.40 Both the HeartMate II and HVAD devices are FDA approved for patients eligible for heart transplant (Table 1). The HVAD was compared in a prospective cohort study to a contemporary INTERMACS cohort (95% continuous flow devices, presumed HeartMate II) and showed similar outcomes (survival, explantation, or transplantation 91% in HVAD vs 90% in INTERMACS cohort; p < 0.001, non-inferiority).29 A steady improvement in survival of LVAD patients has been noted over time (currently >80% 1 year survival), likely because of improvements in surgical techniques, patient selection, and medical therapy.
Despite the improved survival with CF LVADs, bleeding events are frequent (Table 2). In the HeartMate II randomized trial, 81% of patients required transfusion and 30% required reoperation due to bleeding.40 A recent systematic review of antithrombotic therapy for LVAD patients reported a major bleeding incidence of 30% (697/2294 patients) in the 24 included prospective and retrospective cohort studies. Postoperative bleeding complications occurred in 6%-69% of patients. The definition of major bleeding in the literature varies widely from transfusion of >2 units of blood to hemorrhage at a critical site [gastrointestinal (GI) or intracranial (ICH)] or surgical management, which limited the ability to compare bleeding risks between devices or antithrombotic regimens.41
Thromboembolic complications occur less frequently than bleeding complications in patients with LVADs, but have devastating complications. Ischemic stroke occurred in 6%-8% of patients in trials leading to device approval by the FDA.29,40 LVAD thrombosis can cause ischemic stroke, systemic embolism, or pump failure and subsequent cardiogenic shock. LVAD thrombosis has been reported on average in 5% of patients in a recent systematic review, but another report suggested a recent increase in incidence of HeartMate II LVAD thrombosis to 12% at 2 years.41,42 An etiology of the increased thrombosis incidence has not been identified.
Antithrombotic therapy management.
To prevent thromboembolic complications, patients requiring LVAD support are treated with anticoagulation. UFH is the most common perioperative anticoagulant and 50%-60% of patients in clinical trials were treated with UFH prior to LVAD placement.29,40 After cardiopulmonary bypass, protamine is used to reverse the anticoagulant effect of UFH. Once hemostasis is achieved, typically within the first postoperative day, patients are treated with low-dose UFH therapy. Consensus guidelines from the International Society for Heart and Lung Transplantation recommend a gradual increase in the level of anticoagulation over the subsequent 2-3 days.43 Vitamin K antagonist (VKA) therapy provides long-term anticoagulation and is started after a patient's chest tubes have been removed. Intensity of VKA treatment is typically targeted to an INR between 2.0 and 3.0.43 Variable INR goals in the literature have been reported based on institutional experience.41 Complications also lead an institution to alter their regimens; several centers reported a decrease in their patients' INR goal <2.0 because of increased incidence of hemorrhage, whereas another center increased their patients' INR goal above 2.0 because of thromboembolic events.41 An ongoing randomized trial is comparing the effectiveness of dabigatran to VKA therapy in LVAD patients (EudraCT 2010-024534-38). Outside of a clinical protocol, though, direct oral anticoagulants are not recommended due to the increased bleeding and thromboembolic complications reported in patients with mechanical heart valves.44
Consensus guidelines recommend aspirin therapy in patients with the HeartMate II and HVAD devices.43 Despite these recommendations, antiplatelet therapy varies dramatically in the literature. A multicenter registry of reduced antiplatelet therapy in HeartMate II patients is ongoing in the US (NCT01477528). In the EU-TRACE study, 91% of patients have been treated without antiplatelet therapy because of institutional practice; the 2 year rates of freedom from bleeding, ischemic stroke, and pump thrombosis were 86 ± 5%, 93 ± 3%, and 93 ± 3%, respectively.45 In a systematic review of LVAD patients, thromboembolic events were significantly lower in patients with axial devices who were treated with aspirin and dipyridamole compared with aspirin alone (10% vs 19%, RR 0.50; 95% CI 0.36-0.68) and patients treated without aspirin reported similar thromboembolism rates as those reporting aspirin use.41 None of the studies compared anticoagulant regimens, and differences in demographics, devices, and anticoagulant regimens were present. Therefore, the most effective antiplatelet regimen for adults with LVADs has not been established.
Management of hemorrhagic complications
Hemorrhage in patients requiring MCS can be life threatening. The bleeding source should be identified and treated with aggressive local measures including endoscopy as appropriate. Perioperative bleeding may be due to inadequate heparin neutralization after coronary artery bypass therapy, surgical bleeding, or hemostatic abnormalities due to liver or kidney dysfunction associated with advanced heart failure. Treatment should be targeted at the etiology of the bleeding: protamine for inadequate heparin neutralization, surgical correction of anatomical bleeding, or blood product replacement for hemostatic abnormalities (ie, cryoprecipitate for low fibrinogen, platelets for thrombocytopenia). The need for holding versus reversal of anticoagulation depends on the severity of the hemorrhage. Transfusion of blood products must be weighed against the risk of alloimmunization in candidates for heart transplant. In adults, reversal of VKA therapy with plasma or vitamin K has not been associated with an increase in thrombotic events.46 Given the decreased time to normalization of INR and decreased infusion volume required, 4-factor prothrombin complex concentrate administration should be considered if reversal of anticoagulation is desired.
If life threatening bleeding continues despite local therapy and correction of coagulation abnormalities, administration of procoagulant therapy can be considered. Moffet et al reported an increase in use of procoagulant and antifibrinolytic agents over time in children with VAD associated hemorrhage.34 Recombinant FVIIa was administered in 23% of children compared to factor VIII (0.2%) and factor IX (4.7%). Aminocaproic acid and tranexamic acid were used in 34% and 13% of children, respectively, but the safety and efficacy of anti-fibrinolytic therapy has not been systematically studied. It is unknown how the acquired von Willebrand syndrome seen during MCS contributes to the risk of hemorrhage. Case reports have suggested improvement in hemostasis with infusion of von Willebrand factor concentrate, but thrombotic events have also been reported.47 In adults with refractory gastrointestinal hemorrhage, case series have suggested a benefit with octreotide or thalidomide therapy.47 Lastly, hemorrhage refractory to medical management is an indication for urgent heart transplantation and upgrading to United Network for Organ Sharing status 1A should be considered.
Management of thrombotic complications
Development of thrombosis necessitates prompt determination of appropriate intensity of antithrombotic therapy. Adherence to anticoagulant and antiplatelet treatment should be assessed. UFH is the initial treatment for suspected thrombosis especially if warfarin dosing is subtherapeutic. The incidence and potential implications of resistance to antiplatelet therapy is debated in the literature. Goal antiplatelet therapy based on the TEG with platelet mapping has been used in children, but limited reports exist in the adult literature.48 Without understanding the typical response to antiplatelet agents using standardized platelet assays, it is difficult to determine whether ineffective antiplatelet therapy plays a role in a thrombotic episode.
Thrombosis during ECMO
The most common sites of thrombosis within the EMCO circuit are the oxygenator and bridge tubing. When locations within the ECMO circuit are combined, pediatric EMCO has a 34% device thrombosis event rate in cardiac patients and 42% for respiratory indications (Table 2). The circuit thrombosis rate in adults is less than in children (18% cardiac ECMO and 25% respiratory ECMO).25 At times of thrombosis, replacement of the affected parts of the ECMO circuit and optimization of anticoagulation is required. Alternate coating materials are being tested to decrease the circuit thrombosis events. Confirmed ischemic stroke has been reported in 4%-6.5% of children treated with ECMO and 2%-4% of adults (Table 2).25
Thrombosis of pulsatile VADs in children
In a child with the EXCOR, the pump must be examined regularly for thrombosis using a high-intensity flash light.37 If there is a small thrombus, optimizing anticoagulation may be effective. However, if the thrombus is large, replacing the pump may be required. If the child experiences a stroke, the size of the infarct should be established and discussion should occur as to whether antithrombotic therapy should continue, be decreased, or temporarily held for a period of time depending on the risk for hemorrhagic transformation of the infarct. In the EXCOR study, most strokes occurred within 2 weeks after implantation.37
Thrombosis of continuous flow VADs in adolescents and adults
Because CF VADs are intracorporeal, indirect markers must be used to diagnose VAD thrombosis. Hemolysis occurs in the setting of LVAD thrombosis due to changes in shear forces; thus, lactate dehydrogenase (LDH) and plasma-free hemoglobin are routinely measured. Other etiologies for an increased LDH should be considered including glucose-6-phophate dehydrogenase deficiency, hepatic injury, infections, or neoplasm. Power spikes or other LVAD malfunction can occur because the presence of a thrombus requires additional power to create the same amount of blood flow. If LVAD thrombosis is diagnosed, various medical therapies have been tried, but are supported by only by case series (Table 4). Thrombolysis has been attempted in HVAD thrombosis with adequate success rates, but major hemorrhage occurred in ∼1/3 of patients.49 Similar rates of thrombus resolution have been reported using direct thrombin inhibitor treatment as with thrombolysis. Eptifibatide resolved thrombosis in only one-quarter of cases and major bleeding rates are unacceptably high with eptifibatide treatment.49-51 In the setting of inflow or outflow graft malposition or LDH levels >1000, surgical replacement of the LVAD is recommended because of a 50% mortality associated with medical management.52
Conclusions
MCS can be a lifesaving intervention for patients with respiratory and cardiac failure, but all modalities are associated with high rates of hemorrhagic and thrombotic events. Management of ECMO varies across centers but guidelines based on expert opinion are available. An optimal antithrombotic treatment regimen to minimize VAD adverse events for adults has not been established. A regimen has been tested in one pediatric VAD study, but it may not be optimal because of high bleeding and stroke rates. Properly designed clinical trials focused on anticoagulation in MCS are urgently needed in adults and children. Experts in hemostasis can significantly impact care of patients requiring MCS and are a necessary part of the management team.
Correspondence
Lisa Baumann Kreuziger, BloodCenter of Wisconsin; Division of Hematology and Oncology, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; Phone: 414-937-6826; Fax: 414-937-6580; e-mail: Lisa.BaumannKreuziger@bcw.edu.
References
Competing Interests
Conflict-of-interest disclosures: L.B.K. declares no competing financial interests; and M.P.M. has consulted for NIH/NHLBI PumpKIN study (Pumps in children, infants, and neonates).
Author notes
Off-label drug use: Aspirin, dipyridamole, LMWH, warfarin, and argatroban for thromboprophylaxis of VADs; FVIIa and prothrombin complex for life threatening bleeding; and direct thrombin inhibitors, eptifibatide, and fibrinolytic therapy for device thrombosis.