Abstract
The increasing use of immunophenotypic and molecular analysis in the routine evaluation of patients with lymphocytosis, lymphadenopathy, or other hematologic disorders has led to the identification of unexpected small clonal lymphoid populations. These clones, sometimes with disease-specific markers, such as the t(14;18), are especially challenging for the clinician because of their unknown biologic potential and uncertain clinical behavior. Study of these early lymphoid lesions is providing important clues to the process of lymphomagenesis, and may provide the rationale for preemptive therapy in the future. More and more, the hematologist/oncologist is consulted regarding otherwise healthy individuals with lymphadenopathy and/or lymphocytosis, and pathology reports that confound the referring internist or surgeon. The report does not name a malignant lymphoproliferative disorder, but is not completely “normal”. Does the patient have a benign or malignant condition? How should they be evaluated? Is treatment indicated? These patients prove challenging for the consulting hematologist as well as the referring physician. In this review, we will focus on some of these scenarios and attempt to provide guidance for their management.
Learning Objectives
To manage appropriately patients with incidentally identified clonal lymphoid proliferations
To recognize the broad spectrum of lymphoid disorders associated with the t(14;18) and its implications for lymphomagenesis
Lymphadenopathy
In most instances, it is the general internist or primary care physician who is confronted with the patient with lymphadenopathy and the question of whether or not to biopsy. Infectious, inflammatory, and autoimmune causes are among the most common disorders associated with lymphadenopathy. The clinical presentation is the best guide to determining when to biopsy an easily accessible peripheral lymph node (LN). For symptomatic patients, an early biopsy may provide the desperately needed answer to an otherwise puzzling case but may add to the confusion if a straightforward diagnosis is not apparent. An excisional rather than a fine needle aspiration or a needle core biopsy is most likely to provide a diagnosis. Face-to-face discussions between the clinician and pathologist are the best approach to solving the most challenging cases. For asymptomatic patients there is less urgency. In this review, we will focus on the otherwise healthy appearing adult with lymphocytosis, lymphadenopathy, or challenging laboratory results.
Imaging
Palpable peripheral adenopathy may be monitored and/or biopsied, whereas incidental intra-abdominal adenopathy detected on imaging presents a greater challenge. Modern imaging with multi-detector CT and high-resolution MRI now routinely identifies subcentimeter lymph nodes, and the radiologist is charged with guiding the clinician regarding the significance of the radiologic findings. The term “lymphadenopathy” conveys a message of abnormality to the patient and clinician. A White Paper by the American College of Radiology's “Incidental Findings Committee II” addressed the challenge faced by radiologists who must advise clinicians on the significance of “incidentally noted” lymph nodes in the abdomen and pelvis.1 Their “White Paper” is intended to provide guidance only. They emphasize the need for integrating the clinical history and patient's risk for development of lymphadenopathy into the assessment. Comparison of any prior imaging studies is integral to the evaluation, and often can eliminate concern if the findings are longstanding. The Committee identified several imaging features consistent with a “normal” lymph node, namely: (1) short-axis diameter <1 cm in the retroperitoneum, (2) normal architecture (elongated, fatty hilum), (3) normal enhancement, and (4) normal number of nodes. In contrast, imaging features suggestive of an “abnormal” lymph node(s), include the following: (1) short axis diameter ≥ 1 cm in the retroperitoneum, (2) abnormal architecture (round, indistinct hilum), (3) presence of enhancement (necrosis; hypervascular), and (4) increased number of nodes defined as a cluster of ≥3 LNs in a single nodal station or ≥2 LNs in ≥2 regions. The Committee recommends biopsy if nodes are suspicious and advises against FDG-PET scanning as being nonspecific, more expensive, and less efficient. For lymphoproliferative disorders, FDG-PET may be negative in the presence of some low-grade lymphomas, including marginal zone lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and positive in many inflammatory, infectious, and autoimmune disorders. If there is no underlying disorder, follow-up imaging in 3 months is suggested by the radiologists, although most lymphoma experts would recommend repeat imaging in no less than 6 months. According to the Committee, if unchanged for one year, the “abnormal” lymph nodes are considered benign, although the hematologist/oncologist should recognize that indolent lymphoproliferative disorders may be present for longer than 1 year, and wax, wane, or stay the same.
Results of immunophenotypic or molecular analysis
Once the decision has been made to biopsy a lymph node and/or perform immunophenotypic and/or molecular studies on the peripheral blood or bone marrow, the presence of abnormal results, eg, clonality or a disease-specific molecular abnormality, such as the t(14;18), in an otherwise normal appearing tissue specimen proves challenging for the physician and patient. Flow cytometric analysis is now often considered to be part of the routine evaluation of a bone marrow or lymph node biopsy, despite the fact that we would not routinely order the test had we known the morphology was “normal”. Increasingly, we are faced with having uncovered what are now being called “early lymphoid lesions” with unknown biologic potential and uncertain clinical behavior. Whereas clonal populations of both B and T cells may be detected in many inflammatory or infectious disorders, and molecular markers, such as the t(14;18) are detectable at low levels in a significant proportion of healthy individuals, distinguishing which findings to pursue and which to ignore is a growing challenge.
A recent workshop convened by the European Association of Hematopathology and the Society of Hematopathology to address this issue, underscored the difficulties in categorizing these cases and recommended renaming some clonal disorders detected by chance to reflect the uncertainty regarding their long-term risk of neoplasia.2
Small monoclonal B-cell populations
Peripheral blood
Highly sensitive flow cytometric analysis has made it possible to detect small monoclonal B-cell populations in blood, bone marrow, or lymph nodes that show no other evidence of a possible lymphoproliferative disorder. The identification of fewer than 5 × 109/L monoclonal B cells with a CLL-like immunophenotype (CD5+, CD20 dim, CD23+) present for at least 3 months in the peripheral blood of otherwise healthy individuals fulfills criteria for “monoclonal B-cell lymphocytosis” (MBL).3,4 The frequency of this finding in the population ranges from 0.6% to as high as 20% of individuals studied depending on the sensitivity of the techniques used for analysis and the population, with males, the elderly, and family members of patients with CLL being at higher risk.5,6 Patients with lower levels of MBL (<0.5 × 109/L) are now identified as “low-count” MBL, whereas those with 0.5-5.0 × 109/L are distinguished as “high-count” MBL. This distinction is made because those with “high-count” MBL more commonly have IGHV mutations with a repertoire similar to CLL and a rate of progression to CLL of 1%-2%, whereas patients with “low-count” MBL have the same risk of developing CLL as the general population.7 Among normal blood donors, the majority of MBL are low count, occurring in as many as 7% of normal blood donors. Whereas MBL has been detected in previous blood samples from patients with CLL, MBL is considered to be its precursor. The majority of patients with MBL will not develop CLL, however, with the clonal B-cell count as the best predictor of progression.8,9
Although the risk of progression of MBL with a typical CD5+ CLL− phenotype has been well studied, the risk of progression to clinical lymphoproliferative disorders for patients with atypical CD5+ [CD20+ (bright), CD23−] or CD5− phenotypes is unknown. These clonal proliferations are rare, occurring in 1%-2.5% of the population in one study,10 and may be transient. Because they may be associated with underlying lymphoproliferative disorders, such as mantle cell lymphoma, clinical judgement will guide the evaluation and follow-up. In one of the few reports addressing outcomes among individuals with clonal B-cell lymphocytosis and a phenotype consistent with a marginal zone origin, the majority remained stable with a median follow-up of 5 years, despite morphologic evidence of marrow involvement in all cases and lymphocyte counts greater than 4.0 × 109/L in the majority.11 A small subset developed splenomegaly, but no features could be identified to predict progression. The impact of early detection of these clonal proliferations is unknown. Today, asymptomatic indolent lymphoproliferative disorders are not treated but a “watchful waiting” strategy is recommended, even for those with mantle cell lymphoma and a CLL-like presentation. However, should early treatment be shown to benefit patients, our approach to the detection of small clonal proliferations will likely require adjustment.
Bone marrow
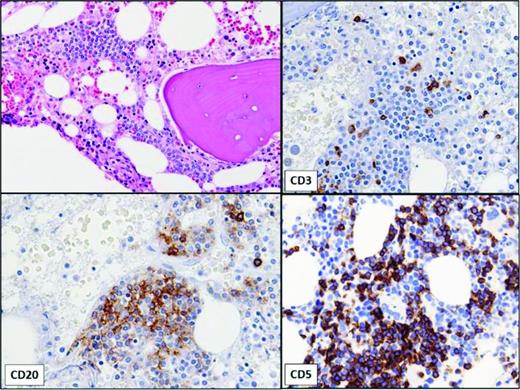
Small monoclonal B-cell populations can also be detected incidentally in bone marrow aspirates (Figure 1).12 In one study, monoclonal B-cell populations were incidentally identified by flow cytometric analysis in 1% of bone marrow aspirates performed in patients without a history of lymphoma; these patients either had no history of lymphoma or the phenotype of the B-cell clone differed from that of the lymphoma. Although some exhibited CLL-like phenotypes similar to MBL in the blood, the majority were found to have non-CLL-like phenotypes. More than one-half had no evidence of lymphoid aggregates, despite immunohistochemical staining. This is in contrast to MBL, in which the majority have lymphoid infiltrates on bone marrow biopsy.13 Whether small non-CLL monoclonal clones detected in bone marrow will progress to clinically significant lymphoproliferative disorders is unknown.
Bone marrow showing small, non-paratrabecular lymphoid aggregate in a patient with a very small B-cell clone with a CLL-like immunophenotype identified by flow cytometry of aspirate. No peripheral lymphocytosis was present. The immunohistochemical stains show that the aggregate is composed of a mixture of CD20+, CD5+ B lymphocytes and CD3+ T lymphocytes.
Bone marrow showing small, non-paratrabecular lymphoid aggregate in a patient with a very small B-cell clone with a CLL-like immunophenotype identified by flow cytometry of aspirate. No peripheral lymphocytosis was present. The immunohistochemical stains show that the aggregate is composed of a mixture of CD20+, CD5+ B lymphocytes and CD3+ T lymphocytes.
Lymph nodes
Little is known about the natural history of patients with small clonal B-cell populations in otherwise normal or reactive appearing lymph nodes, including follicular hyperplasia.14,15 Flow cytometric analysis of the peripheral blood of these patients may reveal an overlooked diagnosis of CLL with greater than 5 × 109/L monoclonal B cells. Alternatively, a monoclonal B-cell population that meets criteria for MBL, high- or low-count, may be detected. Criteria for MBL, however, exclude patients with palpable lymphadenopathy and/or splenomegaly. On the other hand, histologic evidence of CLL/SLL is required for the diagnosis of SLL by the WHO, and is preferred by the IWCLL. Based on a series of lymph node biopsies in which the majority showed morphologic evidence of CLL/SLL but had low volume disease, a new category of “tissue involvement by CLL/SLL-like cells of uncertain significance” has been proposed for cases without proliferation centers and a favorable prognosis. The outcome for cases without morphologic evidence of CLL/SLL in the biopsied lymph node, but a monoclonal B-cell population detected by flow cytometric analysis, is likely to be favorable, but long-term follow-up of these kinds of cases is not available. Non-CLL clones have also been reported in reactive lymph nodes including follicular hyperplasia, without progression to lymphoma.14
Detection of t(14;18)
The t(14;18)(q32;q21) is the hallmark of follicular lymphoma (FL), occurring in more than 85% of cases. Translocation of the B-cell lymphoma 2 proto-oncogene (BCL2) on chromosome 18 and the non-expressed IgH allele on chromosome 14 places BCL2 under the control of IGH enhancers, leading to constitutive expression of BCL2, an anti-apoptotic protein. This genetic event occurs early on in B-cell development in bone marrow pre-B cells and has been attributed to a repair error occurring in the V(D)J recombination process. This is the earliest event in FL lymphomagenesis, but is not the definitive event as evidenced by the detection of circulating cells with the t(14;18) in as many as 50%-70% of apparently normal individuals, the vast majority of whom never develop clinically evident FL. Similarly, the t(14;18) may be detected in hyperplastic lymph nodes or other benign-appearing lymphoid tissues in healthy persons.
Peripheral blood
Much like MBL, the level of t(14;18) cells detectable in peripheral blood has been divided into low and high levels with 10−4 as the cutoff, distinguishing high and low levels of t(14;18). Recently, retrospective analysis of blood samples collected from healthy individuals who subsequently developed FL as long as 15 years later, identified the t(14;18) as a significant risk factor.16 For individuals with a t(14;18) frequency greater than 10−4, the risk of developing FL was as high as 23-fold over normal controls. Of interest, there was no significant correlation between the t(14;18) frequency and time to diagnosis. Furthermore, clonal analysis of paired specimens demonstrated that t(14;18) clones identified in healthy individuals represent precursors that subsequently evolve into overt, clinically evident FL. Report of the synchronous development of FL with identical BCL2/IGH rearrangements in a bone marrow donor and recipient 7 years after donor lymphocyte infusion supports the hypothesis that commitment to malignant transformation may occur years prior to the clinical onset of disease.17 The long but similar latency in the donor and recipient is of interest.
Lymph nodes
Development of FL, therefore, requires more than the t(14;18) and ectopic expression of BCL2.18 19,20 Additional oncogenic events are acquired in the germinal center, resulting in expansion of clonal populations of memory B cells with the genotypic and phenotypic characteristics of FL cells. These cells have been called FL-like cells (FLLCs), and have been identified in the peripheral blood or found scattered in the germinal centers of reactive lymph nodes without overt FL. Unlike normal germinal center B cells, FL is characteristically BCL2–positive allowing for the identification of FLLC's in the germinal center by immunohistochemistry. In one study, t(14;18) was detectable by quantitative PCR in 14% of reactive lymph nodes with follicular hyperplasia.21 In this subset of reactive lymph nodes, scattered cells with the FL phenotype (CD20+, BCL2+, CD10+) were identified within the germinal center, despite normal morphology. The poorly proliferative B cells detected in the germinal centers of reactive lymph nodes in this series likely represent an earlier precursor stage in FL lymphomagenesis than follicular lymphoma in situ (FLIS).21,22
FLIS is a relatively recently described diagnostic category with a very low risk of progression to FL in which cells with a FL phenotype are confined to the follicle center.20,22-24 It is best identified by immunohistochemical staining of a reactive, hyperplastic lymph node, and generally is made incidentally. The architecture remains intact; the involved follicles are normal in size, widely scattered within the lymph node, and composed of centrocytes with very strong expression of BCL2 and CD10. The abnormal B cells with an FL phenotype are confined to the germinal center. FLIS has been detected in 2.3% of hyperplastic lymph nodes in one series.25 Concurrent FL occurring in either the same lymph node or at another site, may occur but is very uncommon. The t(14;18) is present as well as clonal IGH rearrangements. Genetically, FLIS shares only a relatively small subset of additional genetic alterations with FL, supporting the concept of a continuum, with FLIS at an early point in evolution in which the genetic changes required for a commitment to malignant transformation have not yet occurred.19 The risk for evolution to clinically overt FL is low, but the few reports with clinical information are limited by relatively short follow-up. Given the very low risk of progression to FL, the nomenclature may be revised in the upcoming WHO classification to reflect the uncertain nature of this entity.2,26,27 Staging to identify a coexisting FL is recommended. Whereas the true risk of long-term FL is unknown, close monitoring is recommended.
In contrast to FLIS, partial involvement by FL (PFL) may be detected by routine histologic examination because the architecture is disrupted with expanded follicle centers, generally grouped together in the lymph node. PFL is composed of centrocytes with less intense or more variable expression of the FL-characteristic phenotype (BCL2+, CD10+), admixed with small numbers of centroblasts, some of which may be found outside the germinal center. The border between the GC and the remainder of the follicle is less well demarcated than in FLIS. Like FLIS, PFL may be associated with concurrent FL, and has a much higher rate of subsequent development of FL, with a third of patients, in one small series, being diagnosed either concomitantly or within 6 months with FL.23 This entity requires staging at the time of identification to exclude the presence of a concomitant FL or lymphoma of another histologic subtype. There are no guidelines for surveillance, but close follow-up is recommended and at most should be similar to those of low burden advanced stage FL, with imaging no more frequently than every 6 months for the first 2 years, only, or only when there are clinical indications such as new signs or symptoms of disease1,24,28
Detection of cyclin D1–positive B cells
Similar to FLIS, “in situ” mantle cell lymphoma (MCL) is an infrequent incidental finding in lymph nodes or extranodal lymphoid tissue characterized by involvement restricted to the inner mantle zone of reactive follicles.22,29,30 The Cyclin D1–positive B cells identified by immunohistochemical staining are positive for the t(11;14) (CCND1-IGH) by fluorescence in situ hybridization (FISH). This entity appears to be less common than FLIS, and the clinical behavior less predictable, consistent with the wide-range of clinical behavior seen in MCL.
Progressive transformation of germinal centers (PTGCs)
Most often seen in children or young males, but sometimes in adults, PTGC is associated with reactive follicular hyperplasia in which the reactive follicles are infiltrated by small lymphocytes with blurring of the mantle zone. First described by Lennert, this entity has been associated with nodular lymphocyte predominant Hodgkin lymphoma, and may be identified prior to, concurrently, or subsequent to LPHL or in the setting of autoimmune disease.31 It does not necessarily lead to HL, and hence represents a challenge to the clinician, especially when occurring subsequent to therapy. The optimal surveillance strategy is unknown, but a conservative approach is recommended.
Future directions
We do not yet have the capacity to distinguish those indolent lymphoid cases destined to progress to clinical lymphoma from those that will persist indefinitely as “worrisome” but not clinically malignant. The process of progression is as yet poorly understood, but with our new molecular tools, we will soon have a better understanding of the events that underlie the later steps in lymphomagenesis. A role for early treatment of selected patients with “early lymphoid” lesions may soon emerge and cause us to rethink whether screening or surveillance makes sense.
Correspondence
Jane Winter, Division of Hematology/Oncology, Department of Medicine and the Robert H. Lurie Comprehensive Cancer Center, Feinberg School of Medicine, Northwestern University, Suite 850, Arkes Pavilion, 676 N. St. Clair Street, Chicago, IL 60611; Phone: 312-695-4538; Fax: 312-695-4770; e-mail: Jwinter@nm.org.
References
Competing Interests
Conflict-of-interest disclosure: J.N.W. has received research funding from Glaxo-Smith-Kline, Seattle Genetics, Spectrum, Janssen, and Pharmacyclics; and L.C.P. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.