Abstract
Thrombotic complications are increasing at a steady and significant rate in children, resulting in the more widespread use of anticoagulation in this population. Anticoagulant drugs in children can be divided into the older multitargeted agents (heparin, low-molecular-weight heparin, and warfarin) and the newer targeted agents (argatroban, bivalirudin, and fondaparinux). This review will compare and contrast the multitargeted and targeted anticoagulants and suggest situations in which it may be appropriate to use argatroban, bivalirudin, and fondaparinux. The various agents differ in their pharmacokinetics, requirements for therapeutic drug monitoring, frequency of administration, efficacy, and adverse effects. The targeted anticoagulants have properties that may make them more attractive for use in specific clinical situations. Prospective clinical trial data are presented supporting the current and future use of these agents in children.
Learning Objectives
To gain knowledge of the properties of anticoagulant drugs that are prescribed in children
To understand when targeted anticoagulants should be considered for use in children
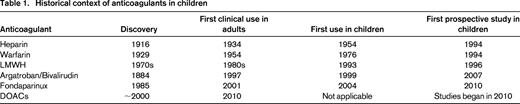
The incidence of venous thromboembolism (VTE) in children has seen a significant and steady increase in recent years.1-3 This is attributable in large part to technological advances in the management of critically ill children, such as sick neonates, children with congenital heart disease, and children with serious and often life-threatening and chronic conditions, such as cancer. In addition and in relation, a sizable proportion of the rising incidence is attributable to the widespread use of central venous catheters in both acutely ill children with poor venous access and for those with chronic disorders that require intravenous medication and frequent laboratory testing.3 As a direct result of the rise in the incidence of VTE, the use of anticoagulant medications has seen a commensurate increase over the past decades.1 There are numerous situations in which anticoagulant medications are administered to children, ranging from short courses (hours to days) for the prevention of thrombosis in children on extracorporeal circulation, such as cardiac bypass and extracorporeal membrane oxygenation, to lifelong anticoagulation for children with recurrent deep vein thrombosis and cardiac valve replacement, for example. Because there are published treatment guidelines, albeit with low levels of evidence, providing dosing regimens for the use of anticoagulants in children in general4 and for children with heart disease,5 this review will focus on the properties of the available anticoagulants and in particular compare and contrast the multitargeted anticoagulants [heparin, low-molecular-weight heparin (LMWH), and warfarin] with the targeted anticoagulants (argatroban, bivalirudin, and fondaparinux). Several recent studies regarding these agents have been published for the treatment of VTE and heparin-induced thrombocytopenia (HIT) and for the prevention of thrombosis in children undergoing cardiac catheterization. Last, although there is an abundance of data on the use of direct oral anticoagulants (DOACs) in adults6 for a variety of indications, there are as of yet no data to support the use of these agents in children, but robust clinical trial programs are underway. As such, only a brief reference to these agents will be made. For a historical context on the anticoagulation in children, see Table 1.
Multitargeted anticoagulants
The multitargeted anticoagulants currently in widespread use in children include unfractionated heparin, LMWHs of which there are several available, and vitamin K antagonists (VKAs), primarily warfarin. Despite the long history of use in pediatrics (Table 1) and the widespread application of these agents in the management of children with VTE, there are remarkably few prospective studies guiding the use of these agents especially compared with the numerous, large studies in adults. Nevertheless, it is clear that these agents have an important role in the management of the conditions described above to both prevent procedure-related thrombi and treat VTE when it occurs. In general, pediatric practitioners rely on a combination of data extrapolated from adult studies, guidelines written by pediatric experts in the field, and personal experience to make clinical decisions regarding the prescription of anticoagulants, recognizing that all of these are fraught with major limitations.
Heparin
Heparin is a polysaccharide compound derived from porcine intestine and functions as an anticoagulant by potentiating the inhibitory effects of antithrombin on thrombin and factor Xa as its primary anticoagulant effect and factors IXa, XIa, and XIIa, as well. It is most often used for the treatment and prevention of thrombosis in critically ill children and is also used to maintain the patency of extracorporeal circuits and venous and arterial catheters. There are only 2 prospective studies of heparin in children with 65 and 38 patients treated both prophylactically and for prevention of thrombosis in patients with congenital heart disease.7,8 Regardless, many pediatric specialists involved in the care of such children have an almost cavalier level of comfort with heparin, despite the fact that it can lead to both serious bleeding8,9 and, rarely, HIT.10-13 This degree of comfort can be attributed to the many years of collected clinical experience. Some advantages of heparin include its short half-life and the presence of an available antidote that would allow rapid reversal should bleeding occur. Conversely, heparin has a number of significant limitations. One of the most crucial issues involves the laboratory monitoring of heparin, which is challenging on a number of levels.14 First, there are several different assays used for therapeutic drug monitoring: (1) the activated partial thromboplastin time (aPTT); (2) the anti-factor Xa level; and (3) the activated clotting time. Second, the degree to which these assays accurately reflect the degree of anticoagulation is not clear.15,16 Furthermore, several studies have also demonstrated discrepancies between the aPTT and the anti-Xa assay.15-17 Assay issues aside, what is clear is that there is a high degree of interpatient and even intrapatient variability in dosing, further complicating management.18 Furthermore, heparin therapy can result in HIT, a serious and, in pediatrics, often under-recognized phenomenon, which has the potential to lead to severe consequences in an already vulnerable population of patients. Several studies evaluated the incidence of HIT in children and reported rates of between 1.3% and 2.3%, which is not dissimilar from the incidence rate in adults.11-13 Despite these limitations, heparin is used widely in children and is still considered the first line therapy for the prevention of thrombosis in patients undergoing cardiac catheterization and cardiopulmonary bypass surgery and for anticoagulation of extracorporeal circuits.
LMWH
The LMWHs are derived from unfractionated heparin, and the shorter length of the polysaccharide chains results in distinct properties. First, LMWHs have a more profound effect on factor Xa than on thrombin.19,20 Second, LMWHs have more stable pharmacokinetics, resulting in a more predictable dose response. Last, these agents have a longer half-life, making them particularly useful in the outpatient setting. This has led to the widespread use of LMWH in children over the past 2 decades, and it has replaced heparin as the agent of choice in the initial treatment of VTE.1 In addition, its lack of drug and food interactions and limited need for drug monitoring has led pediatricians to select LMWH over warfarin for long-term management.1 Several different LMWH preparations are in clinical use throughout the world, and a number of pharmacokinetic and dose-finding studies have been published previously.21-28 Although the longer half-life when compared with unfractionated heparin allows for outpatient use, treatment of VTE requires twice daily dosing as demonstrated by detailed pharmacokinetic studies of enoxaparin.23,24 The LMWHs have some similar drawbacks to heparin, such as risk for HIT but at lower rates than unfractionated heparin. In addition, there is at least theoretical concern regarding the effect of LMWH on bone mineral metabolism. Although there are no studies in children addressing this issue, both in vitro and in vivo animal data indicate that they have a negative effect on bone mineral metabolism.29 Last, the antidote protamine is only partially effective at reversing the anticoagulant effect of LMWH.
VKAs
Compared with heparin and LMWH, the major advantage of the VKAs is their oral route of administration. Despite the long history of use of warfarin in children for a variety of indications, there are several significant limitations that have led pediatricians to slowly decrease their use of this agent.30 Most important is its narrow therapeutic index, leading to a high risk for serious bleeding.31 In addition, warfarin has numerous drug interactions and is affected by the vitamin K content of the diet, further complicating the ability to maintain a therapeutic dose. This problem is exacerbated in children for several reasons. First, the majority of children who require warfarin have serious chronic medical conditions and are often receiving polypharmacy frequently with drugs that interact with warfarin. Second, children especially early in life have a rapidly changing diet altering their vitamin K consumption. Third, even otherwise healthy children receive intermittent antibiotic therapy that can significantly affect the international normalized ratio (INR). Finally, although the oral route of administration is clearly a significant advantage in adults, this is not always the case with children. For example, the use of warfarin in infants is difficult because of their inability to swallow whole tablets and the fact that warfarin cannot be safely compounded into a liquid formulation. Crushing the tablets (a common practice in pediatrics) may lead to inconsistent dosing, which can lead to additional variations in the INR, and is not in general recommended. Furthermore, a well-designed study demonstrated that the target INR is not met on a sufficiently consistent basis in children and in particular in infants.32 Additional issues relative to the use of warfarin include a premium on medication adherence and laboratory adherence to evaluate and maintain therapeutic levels, which can be problematic in children during the teenage years, and the frequent monitoring of the INR can be especially difficult in young children as a result of poor venous access. Thus, there is a clear unmet need for safer and more reliable oral anticoagulants for children.
Targeted anticoagulants
In previous reviews,33,34 anticoagulants other than heparin, LMWH, and VKA were referred to as “novel” anticoagulants when describing their use in children, but because these agents are no longer novel, they will be hereafter referred to as targeted anticoagulants. There are several ways to classify these agents: (1) mechanism of action; (2) route of administration; (3) half-life; and (4) others (Table 2). One approach is to consider the route of administration and to compare the multitargeted anticoagulants with the targeted ones that are administered in the same manner. As such, heparin, a continuous infusion agent, could be compared with lepirudin, bivalirudin, or argatroban, fondaparinux could be compared with LMWH, whereas oral VKA could be compared with DOACs. The following section will describe the available data on the targeted anticoagulants. The pharmacokinetics and pharmacodynamics have been discussed previously in detail.25
Parenteral direct thrombin inhibitors
There are 3 parenteral direct thrombin inhibitors (argatroban, bivalirudin, and lepirudin) for which case reports and case series have been published. More recently, prospective studies evaluating bivalirudin and argatroban in children have been published. There are no prospective studies evaluating lepirudin in children, and this drug appears to cause more bleeding than the others.35,36 Furthermore, it is no longer marketed and thus not a therapeutic option.
Three prospective studies regarding treatment with bivalirudin have been published, 2 for patients with VTE37,38 and one for prophylaxis in patients undergoing cardiac catheterization.39 The 2 VTE studies differed in only 2 ways. First, they enrolled different age groups (<6 months and 6 months to 18 years), and second, the study in the older group collected pharmacokinetic data in addition to the pharmacodynamic data that both studies collected. These studies led to several important findings. First, dosing for children has been established (Table 3). Second, this agent appeared to be very safe because there were no serious bleeding events, albeit in a small number of patients (n = 34). Third, the studies evaluated early clot resolution (at 48-72 hours after drug initiation) because of the unique (relative to heparin) property of this agent to inhibit clot-bound thrombin. The results demonstrated that 15 of the 34 subjects had partial or complete resolution of their thrombus at this early time point, a finding that is not known to occur with heparin, although no direct comparison with heparin has been performed. Of note, another report from a retrospective study40 demonstrated rapid clot resolution in all 10 patients such that, together with the prospective studies, early clot resolution occurred in 57% of patients. Although it would be important to confirm this finding in comparative studies with heparin, it is unlikely that such a study is feasible. Although early clot resolution has not specifically been demonstrated to improve long-term outcome, it makes physiologic sense that it would, and delayed clot resolution (the converse) has been proven to result in worse outcomes in children.41 Another important contribution of this research revolves around the issue of therapeutic drug monitoring. The second bivalirudin study measured both pharmacokinetics (bivalirudin levels) and pharmacodynamics (aPTT) in all subject samples (n = 182 paired samples), and it was noted that the better predictor of bivalirudin drug concentration was the infusion rate rather than the aPTT. In fact, the aPTT often led to dose adjustments that were deemed unnecessary based on the pharmacokinetics.
Suggested dosing and monitoring for targeted anticoagulants
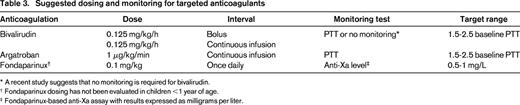
* A recent study suggests that no monitoring is required for bivalirudin.
† Fondaparinux dosing has not been evaluated in children <1 year of age.
‡ Fondaparinux-based anti-Xa assay with results expressed as milligrams per liter.
A third prospective study evaluating bivalirudin for the prevention of thrombosis in children undergoing cardiac catheterization has been published.39 This study was a single-arm, safety, efficacy, and dose-finding study in children from birth to 16 years of age divided into 4 age cohorts and enrolled 110 patients using dosing similar to that for the adult licensed indication of bivalirudin. The results demonstrated a high degree of safety, with only 2 of 110 patients experiencing protocol-defined major bleeding events, which included wound hematomas of >2.5 cm. Eight patients experienced thrombotic events, but only 2 were deemed serious enough to be treated. This rate of thrombosis in this procedure is considered excellent.
The only other prospective study of a direct thrombin inhibitor in children evaluated the use of argatroban in children requiring an alternative to heparin, most of whom had either documented or suspected HIT.42 This study enrolled 18 patients and demonstrated safety and efficacy and also established dosing guidelines that are now included in the prescribing information in the United States (a first for any anticoagulant in pediatrics). A detailed pharmacokinetic analysis was undertaken that resulted in a separate publication43 that supported the dosing schema (Table 3) now approved by the Food and Drug Administration (FDA).
Fondaparinux
Fondaparinux is a synthetic, antithrombin-dependent inhibitor of factor Xa with a substantially longer half-life than LMWH for which 2 pediatric studies have been completed. The first was a single-arm, open-label, dose-finding, pharmacodynamic and safety study and enrolled 24 patients aged 1 to 18 years in 3 age cohorts.44 The study did not enroll patients aged <1 year because of an investigational new drug restriction imposed by the FDA. The second was a follow-up long-term continuation study in which the data were collected retrospectively.45 Both studies demonstrated an excellent safety profile, with a bleeding rate of 0.5 events per 1000 patient-days noted in the continuation study. With respect to dosing, a detailed pharmacologic analysis conducted in the prospective study led to 2 important findings: (1) at a dose of 0.1 mg/kg/d, the pharmacodynamic profile in children mirrored that found in adults, supporting the once-daily dosing regimen; and (2) 22 of 24 patients were therapeutic after the first dose, with the other 2 becoming therapeutic after one dose adjustment. Furthermore, the second study demonstrated that 71% of the patients required no dose adjustments, with a mean duration of therapy of 371 days (median, 152; range, 2 to 1566). Last, although the first study was not designed to assess efficacy, the continuation study demonstrated complete clot resolution in 64% of patients and partial resolution in 27%. Thus, fondaparinux can be considered as an excellent alternative to LMWH given its once-daily dosing and similar safety and efficacy profile.
When applied clinically, several points should be made with reference to using fondaparinux in children. First, unlike in adults, it is recommended that patients have therapeutic drug monitoring using a fondaparinux-based anti-Xa assay. Peak levels should be measured at 3 hours after infusion, targeting a level of 0.5–1 mg/L (units are expressed as a concentration, but this is a unit conversion from the anti-Xa assay). In addition, for patients requiring procedures that are receiving fondaparinux, to the extent possible, procedures should be performed at least 24 hours after the last dose. In my institution, fondaparinux is dosed in the morning, and thus skipping the daily morning dose allows for procedures to be done that day. Last, one of the limitations regarding the use of fondaparinux is the fact that a multidose vial is not available, such that providing doses that are not available in prefilled syringes (2.5, 5, 7.5, and 10 mg) can be problematic. A compounding pharmacy can overcome this limitation, but such a service is not available universally. In my institution, as much as possible, the dose is rounded to the nearest prefilled vial according to Table 4.
Agents in clinical development
Clinical development programs for DOACs in children are currently underway, with the initial results from a phase 1 study of rivaroxaban recently presented.46 New regulations in Europe and stricter adherence to existing regulations in the United States have spurred the manufacturers of these agents to begin drug development programs for pediatrics. Details regarding the clinical trial programs for rivaroxaban, apixaban, edoxaban, and dabigatran can be found at ClinicalTrials.gov. Importantly, these should not be prescribed to children (outside of the context of a clinical trial) until data on safety and dosing are established with some exceptions provided that there is a clear rationale for said exception. For example, a 16-17 year old who is adult-sized and for all intents and purposes physiologically an adult could be considered for a DOAC according to adult treatment guidelines.
Conclusions and recommendations
The multitargeted anticoagulants used in children, heparin, LMWH, and VKAs, all have significant limitations and will most likely eventually be replaced by a wide variety of targeted anticoagulants. Based on the results of the above-described studies of bivalirudin, argatroban, and fondaparinux, several recommendations for the use of targeted anticoagulants can be made (Table 5). First, the only clear indication for the use of one of these anticoagulants is the presence or suspicion of HIT, which requires avoidance of heparin and LMWH and for which warfarin is not appropriate for acute treatment. The only agent studied in children specifically for this indication is argatroban, and it is thus the agent of choice for children with HIT.
Treatment with targeted anticoagulants in pediatric practice
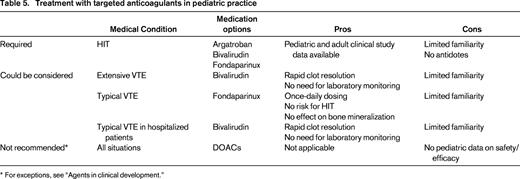
* For exceptions, see “Agents in clinical development.”
The next strongest case for use of a targeted anticoagulant would be for the use of bivalirudin for the prevention of thrombosis in children undergoing cardiac catheterization based on the excellent results of the published clinical trial.
With respect to the management of acute VTE for hospitalized or critically ill patients in whom a continuous infusion medication is indicated, there are 2 points of view. One is to consider the targeted agents as second-line therapy for patients who have a poor response to or are difficult to manage with heparin. An alternative view is to consider these agents, particularly bivalirudin, as the ideal first option. To this end, it should be noted that there is as much (if not more) quality prospective study data for bivalirudin as there is for heparin, but more importantly, bivalirudin has been shown to rapidly resolve (partially and completely) thrombi in nearly 60% of the children evaluated in the clinical trials and the retrospective study. This phenomenon has not been tested in patients receiving heparin. I recommend bivalirudin for critically ill and/or hospitalized children for the acute management of VTE or at least for those with extensive thrombi in whom thrombolysis is considered but not undertaken because of the risk for bleeding.
With respect to long-term anticoagulation, the multitargeted options of LMWH and VKA both have limitations discussed above. In contrast to LMWH, fondaparinux allows for once-daily dosing, does not interfere with bone metabolism, and has no risk for HIT or contamination. In addition, the quantity and quality of prospective clinical trial data available for both agents is similar.
In conclusion, a new era has emerged recently with respect to pediatric anticoagulation. After nearly 20 years since the initial use of LMWH in children began, several targeted anticoagulants are available now that have undergone prospective studies establishing dosing, safety, and efficacy and can be prescribed in the circumstances described above. Furthermore, a wide variety of DOACs are being studied in children currently. Last, with the new regulations requiring pediatric studies for such drugs, a period of fruitful research in this field can be anticipated for many years to come, leading to improved management of pediatric thrombosis.
Correspondence
Guy Young, Hemostasis and Thrombosis Center, Children's Hospital Los Angeles, Saban Research Institute, 4650 Sunset Blvd, Los Angeles, CA 90027; Phone: 323-361-5507; Fax: 323-361-7128; e-mail: gyoung@chla.usc.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: All anticoagulants in children are off-label, and I will be discussing all of the ones in use in the presentation: heparin, warfarin, enoxaparin, dalteparin, argatroban, bivalirudin, fondaparinux, rivaroxaban, apixaban, edoxaban, and dabigatran.