Abstract
A better understanding of risks associated with allogeneic blood transfusions (ABTs), along with a growing population of patients who do not accept transfusions, have led to the emergence of new treatment paradigms with “bloodless medicine.” In this chapter, we review prior studies describing management and outcomes in patients who refuse transfusion (referred to as “bloodless patients” herein) and summarize the approaches used at our institution. Bloodless management for surgical patients includes treatment of preoperative anemia, use of autologous blood salvage, and minimizing blood loss with procedures. Other adjuncts for both medical and surgical patients include minimizing blood loss from laboratory testing using pediatric phlebotomy tubes and conservative testing. Anemia can be treated with erythropoiesis-stimulating agents, as well as iron, folate, and B12 when indicated. Although there are limited retrospective studies and no prospective studies to guide management, prior reports suggest that outcomes for surgical patients managed without ABTs are comparable to historic controls. A recent risk-adjusted, propensity-matched, case-control study of outcomes of all hospitalized patients who refused ABT at a large academic health center showed that bloodless management was not an independent predictor of adverse outcomes. Surprisingly, there was a lower overall mortality in the bloodless group and discharge hemoglobin levels were similar for both bloodless and control groups. Further research is now needed to optimize therapy and identify novel interventions to manage bloodless patients. Lessons learned from bloodless patients are likely to benefit all patients given recent evidence suggesting that patients who avoid ABTs do as well, if not better, than those who accept transfusions.
Learning Objectives
To describe patient populations for whom transfusions are not an option
To discuss prior studies with outcomes and “bloodless medicine”
To outline currently available approaches to manage anemia or bleeding in “bloodless patients”
To discuss areas in need of further research to advance bloodless medicine
Introduction
Although blood transfusions are among the most common medical procedures performed in hospitalized patients, a better understanding of transfusion risks, together with a growing population of patients who wish to avoid transfusions, have led to the emergence of new treatment paradigms for anemia.1-3 In fact, several academic health centers are establishing “Bloodless Medicine & Surgery Programs” that specialize in treating patients who do not accept allogeneic blood transfusions (ABTs).4-16 A multidisciplinary approach is frequently required to optimize clinical outcomes for these patients, particularly in the setting of multiple comorbidities or high-risk surgical procedures.
At most centers, the majority of patients who request bloodless medicine are members of the Jehovah's Witness (JW) faith.4-16 Based on religious beliefs, these individuals do not accept blood products considered to be “primary components,” which includes RBCs, WBCs, platelets, or plasma.4 In addition, JW patients do not typically accept autologous blood donated preoperatively, although most will accept autologous blood that is considered to be physically contiguous with one's body.4 For example, surgical practices such as blood salvage and intraoperative autologous hemodilution can be used.4 Fractions of blood products, such as cryoprecipitate, albumin, clotting factors, or hemostatic agents (thrombin), can also be used in some cases because the decision to accept these products is left to the discretion of each individual. The majority of JW patients will agree to accept these products because they are considered to be “minor blood fractions” according to their religious doctrine. Importantly, the JW faith currently includes >8 million people worldwide, with 1.2 million in the United States alone, and the number is increasing.4 Therefore, there is a growing population of patients who require bloodless medicine. As discussed here, these patients provide a unique challenge to health care providers, particularly when presenting for surgeries or invasive procedures that can be associated with significant blood loss or for complex medical illnesses.
Despite the significant population of patients who decline ABTs, there are few studies that compare patient outcomes between those receiving bloodless care with a matched control group receiving standard care.4-17 Moreover, there are no standard, established guidelines to manage cytopenias in these patients, nor are there many studies to inform optimal treatment approaches. Here, we outline prior studies on bloodless management and discuss the approaches used at our institution to care for patients who refuse transfusions (hereafter referred to as “bloodless patients”). We close with suggestions for further studies to guide management of bloodless patients. Because recent evidence suggests that bloodless patients do as well, if not better than other patients,4-16 advances in bloodless medicine are likely to benefit all patients with anemia. Given the significant expense associated with transfusion therapy, practices to limit transfusion therapy should also significantly reduce health care costs.
General principles of bloodless medicine
The primary goals in managing patients who do not accept ABTs are to diagnose and treat anemia and minimize blood loss.18 Surgical patients who present for preoperative evaluations should be screened for both anemia and bleeding diatheses. The prevalence of anemia in patients who present for elective surgery is highly variable, ranging from 5% to 75% depending on the patient population, underlying pathology, and definition of anemia.18 The World Health Organization defines anemia as a hemoglobin <12 g/dL for women and <13 g/dL for men, which provides useful guidelines for most patient populations.18 Ideally, the diagnosis of anemia should be made at least 4-8 weeks before an elective procedure to ensure adequate time for evaluation, therapy, and correction of the anemia.4,8 This proves to be a challenge for many institutions, because preoperative evaluations are often done less than 1 week before surgery. Iron deficiency is the most common form of anemia worldwide and is often associated with renal insufficiency and inadequate erythropoietin production, particularly in elderly patients.18 For all patients, the preoperative diagnosis of anemia is important because it is associated with increased morbidity, length of hospital stay, and mortality.18 Not surprisingly, anemia is a significant predictor of RBC transfusion in patients who accept ABT.18 Intriguingly, recent evidence suggests that RBC transfusions to treat anemia is an additive risk factor for adverse outcomes independent of the anemia itself.18 Bleeding diatheses such as VWD are relatively common and can be associated with both anemia and excessive bleeding with surgical procedures.19 Patients with VWD can be managed with desmopressin (DDAVP) or VWF concentrates (Humate-P or cryoprecipitate) if acceptable to the patient.19 In addition, many patients take drugs or supplements that interfere with platelet function and can cause excessive bleeding, such as cyclooxygenase inhibitors (aspirin, celecoxib, etc) or platelet adenosine diphosphate receptor (P2Y12) inhibitors (clopidogrel, prasugrel, etc). Therefore, patients on these agents are instructed to stop them before surgical procedures. Similarly, elderly patients with atrial fibrillation are frequently on anticoagulation therapy such as warfarin, and need to receive appropriate counselling regarding when to stop these agents and whether they will require shorter-acting, bridging agents such as low-molecular-weight or standard heparin.
Limiting phlebotomy for laboratory testing is important because this is frequently a source of significant blood loss, particularly in patients hospitalized in intensive care units (ICUs). In fact, it is estimated that ICU patients can lose up to 1% of their blood volume each day from phlebotomy alone, which becomes significant with prolonged ICU stays.20 Pediatric or low-volume microtainer tubes can be used to minimize the volume of blood needed for laboratory testing, although they need to be manually inserted into the laboratory instruments, which requires more effort by laboratory technicians.
Previous studies of bloodless medicine
Although there are previous reports of management and outcomes for patients who do not accept ABT,4-17 most are series of cases of surgical patients with simple outcome measures, such as length of stay and mortality, without matched control groups for comparison.5-17 A study from 2002 showed that the risk of death in surgical patients with a postoperative hemoglobin level of 7.1-8.0 was low, although a morbid event (defined as a myocardial infarction, arrhythmia, congestive heart failure, or infection) occurred in 9.4% of patients. However, in patients with a hemoglobin of 4.1-5.0, there was an extremely high risk of death (34.4% chance of death; 95% confidence interval, 18.6%–53.2%) and a 57.7% chance of a morbid event or death (95% confidence interval, 36.9%–76.6%).17 A few subsequent studies used a propensity-matched control group, which is a statistical matching technique that attempts to account for confounding variables and decrease bias associated with these variables.9,11,15 The majority of studies with propensity-matched controls, however, have focused on cardiac surgery.9,11,15 Interestingly, 2 of these studies reported similar outcomes between patients who accept ABT compared with those who do not.11,15 Moreover, a recent study of cardiac surgery patients and a propensity-matched control group actually showed a lower incidence of myocardial infarction and reoperation for bleeding in the bloodless patient group.9 There were also shorter durations of mechanical ventilation, shorter length of both ICU and total hospital stays, and better 1-year survival in the bloodless patients. These intriguing findings suggest that bloodless management can yield similar, and possibly better, outcomes for a subset of patients.
One relatively large study of bloodless patients (n = 91) undergoing cardiac surgery over a 10-year period (2000-2010) at a large academic health center found similar outcomes in bloodless patients compared with expected outcomes based on Society of Thoracic Surgeons risk models and historical controls.8 The favorable outcomes were observed for both elective and urgent cardiac surgeries. Although the average age for all patients in this study was slightly younger (63.5 ± 9.2 years) than some cardiac surgery studies,21 this report describes a practical approach to patients undergoing bloodless cardiac surgeries.8 All patients were evaluated by a “bloodless team” and consented for an itemized list of blood products they would accept or refuse. Details of laboratory evaluations were not described, but anemia was managed preoperatively with intravenous iron supplementation and subcutaneous erythropoietin (40 000-60 000 IU/week) to achieve a target hemoglobin of 14-16 g/dL or a measure that correlates with RBC volume (RCV) (Hb × body weight in kilograms) of 1200.8 The target value of 1200 was chosen because the investigators noted an increased risk for ABT in patients with a value <800 who were hospitalized at their institution. In addition, lower hemoglobin levels were tolerated in larger patients, for whom this value was ≥1200. Lower hemoglobin thresholds were also accepted for patients with renal failure or cancer. If elective cardiac catheterizations were done, they were performed 3 weeks before surgery to allow recovery from procedure-related anemia when possible.8 For surgeries needed within 48 hours of cardiac catheterization, a vascular closure device was recommended to reduce blood loss. Acute coronary syndromes were managed medically, followed by coronary artery stent placement. Patients were evaluated for bleeding risk factors and advised to discontinue use of alcohol and medications or supplements associated with an increased bleeding. Before surgery, aspirin (3-5 days) and clopidogrel (7 days) were withheld. Patients on warfarin had the international normalized ratio corrected and some were converted to low-molecular-weight or unfractionated heparin.8
Intraoperative management included acute normovolemic hemodilution, use of a single sponge, and hemostatic adjuncts. Postoperative care included warming patients to normothermia and avoiding hypertension. If tolerated hemodynamically, positive end-expiratory pressures were maintained at 7-10 cm H2O, which is thought to reduce bleeding by exerting mechanical pressure on the surface of heart and thus the vascular anastomoses. Crystalloids (if acceptable to a patient) were used to maintain euvolemia. Desmopressin (0.3 μg/kg) was considered for patients who received aspirin preoperatively or those with renal failure. Cryoprecipitate or recombinant factor VIIa was used for bleeding unless patients were undergoing coronary artery bypass, in which case factor VII was avoided. A low threshold was recommended for reexploration if surgical bleeding was suspected. Phlebotomy was minimized with low-volume tubes for laboratory blood testing, point of care testing, and in-line blood draw systems. Coagulation profiles were avoided unless patients were suspected of active bleeding. Erythropoiesis-stimulating agents (ESAs) were administered in some cases, although more specific details were not provided.8 The comorbidities in JW patients were similar to a large 2012 study of cardiac surgery patients, except for renal failure, which was less common in the bloodless patients.20 Nonetheless, the JW patients undergoing either elective or urgent cardiac surgery had similar outcomes to historical controls.5-15
Importantly, obstetrics appears to be a clinical setting with significantly increased mortality in patients who do not accept ABTs, although data in this patient population are limited. A study comparing concurrent, non-risk-adjusted pregnant women showed a 44-fold greater maternal mortality for patients who refused ABT compared with patients who accept ABT.22 Although limited to a single institution, these findings indicate the importance of vigilant care for patients in labor and delivery, including the use of autologous blood salvage and a high suspicion for postpartum hemorrhage when symptoms of bleeding arise. Further studies are needed to determine whether the estimated increase in mortality is relevant to a more diverse patient population.
Most of the prior studies on outcomes with bloodless management lacked sufficient numbers of patients for adequate statistical power and did not include a well-matched control group.5-15 After recently establishing a Bloodless Medicine and Surgery Program to care for such patients at our institution, we reported a risk-adjusted, propensity-score-matched retrospective case-control study of clinical outcomes for hospitalized patients who did not accept ABT (bloodless patients; n = 294) compared with a control group of patients (control patients, n = 1157).4 Of the 294 bloodless patients, 98 underwent a surgical procedure and were therefore considered surgical patients and 196 were medical patients. Each bloodless patient was matched by associated risks to ∼4 control patients to increase the sample size and power to detect differences. Risk assessment in both the bloodless and control patients were estimated using 3 well-established indices, including the Charlson index (which estimates risks based on comorbidities and other factors),23 the APR-DRG complexity score (which estimates disease severity),24 and, for surgical patients, the American Society of Anesthesiologists classification (which estimates operative risk based on systemic disease).25 After risk adjustment, bloodless care was not an independent predictor of the composite adverse outcome (death or any morbid event; P = .92; odds ratio = 1.02; 95% confidence interval, 0.68-1.53). Surprisingly, overall mortality was lower in the bloodless group (0.7%) versus the control group (2.7%; P = .046), primarily attributed to the surgical subgroup. In addition, the mean discharge hemoglobin concentrations were also similar in the bloodless and control groups (10.8 ± 2.7 g/dL vs 10.9 ± 2.3 g/dL, respectively; P = .42). Total and direct hospital costs were 12% (P = .02) and 18% (P = .02) less in the bloodless patients, a difference attributed to the surgical group. A caveat to this and other studies comparing bloodless patients with controls, however, is that patients who decline transfusion therapy may not be offered surgery due to perceived risks, which could select for healthier patients and contribute to favorable outcomes associated with bloodless surgical care. Even patients who accept blood products may not be considered surgical candidates if premorbid conditions are thought to be associated with unacceptable surgical risks. Therefore, future studies are needed to compare patients for whom surgery was not offered from both bloodless and control groups to determine whether this contributes to favorable outcomes in bloodless patients.
Our recommendations for bloodless medicine
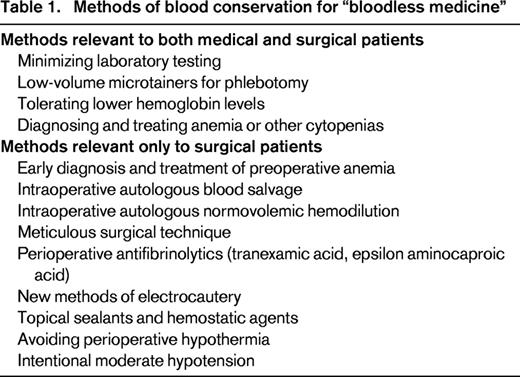
Based on prior reports and our experience thus far, we follow 5 tenets of patient blood management in caring for bloodless patients at our institution1-17,26-28 : (1) minimizing iatrogenic blood loss for laboratory testing, (2) tolerating lower hemoglobins, (3) diagnosing and treating preoperative anemia, (4) salvaging intraoperative blood, and (5) optimizing surgical hemostasis (Table 1). We request that all surgical patients seeking bloodless care obtain a preoperative complete blood count as soon as possible, preferably at least 4-8 weeks before the surgery. We typically recommend oral iron (325 mg ferrous sulfate; 2-3 doses/day as tolerated) and a multivitamin supplemented with B12 and folate after we identify a patient seeking bloodless surgery because it is relatively innocuous and results of a complete blood count may not be readily available. If microcytic or normocytic anemia is present, iron studies are recommended (ferritin, transferrin saturation, total iron binding capacity), whereas serum B12 and RBC folate are performed if RBCs are macrocytic. For patients with microcytosis, an elevated RBC count, and minimally elevated RBC distribution width, a hemoglobinopathy variant screen is recommended (hemoglobin variant with quantitative hemoglobin A2 and F). For patients undergoing cardiac surgery, our typical goal is to increase the hemoglobin to the 14-16 gm/dL range when possible based on a prior study of cardiac surgical patients.8 Intravenous iron (generally 200 mg of iron sucrose administered 2-3 times weekly) and occasionally ESAs are administered for patients with hemoglobins <13-14 who are undergoing cardiac surgery after consultation with the cardiac surgeon to determine the lowest acceptable hemoglobin. Typically, standard erythropoietin (∼20 000-30 000 IU) is given 3 times before scheduled surgeries (usually administered 2-3 times weekly), but occasionally up to 8 times, particularly for a subset of smaller patients for whom a higher preoperative hemoglobin is targeted to approximate a correlate of RCV goal of ∼1200. For outpatient ESA therapy, standard erythropoietin (20 000-30 000 IU) is generally given subcutaneously due to the ease of administration. For patients undergoing hemodialysis, standard erythropoietin (20 000-30 000 IU) is generally administered intravenously after dialysis (usually 3 times weekly). When possible, we favor intravenous administration because absorption is not an issue and there have been no reports of anti-erythropoietin antibodies after intravenous administration, which would be a devastating complication for JW patients.29
For other surgeries such as hip replacement, nephrectomies, or tumor resections, we consult the surgeon and together ascertain an acceptable preoperative hemoglobin. We also consider the RCV and expected blood loss when estimating an acceptable preoperative hemoglobin value. Ideally, we prefer bloodless patients undergoing higher blood loss procedures to have an RCV correlate of ≥1200.8 For some patients whose hemoglobin did not fall within levels considered to be anemia, insurance companies decline to cover the costs for preoperative erythropoietin therapy and therefore it is not administered. A subset of patients are willing to pay on their own for erythropoietin therapy.
We recommend that all surgical patients be evaluated for a bleeding diathesis by detailed histories and, when indicated, undergo further laboratory testing. Patients diagnosed with Type 1 VWD are screened for response to desmopressin. We also advise patients to discontinue supplements associated with an increased bleeding risk. Patients on warfarin are evaluated in our anticoagulation clinic for correction of the international normalized ratio and conversion to bridging anticoagulation, as recommended by the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines.30 The final preoperative dose of low-molecular-weight heparin is typically given 48 hours before surgery.
Blood salvage is paramount to patient safety when ABT is not an option during surgical procedures associated with moderate to high blood loss, and has been life-saving in some cases.4 Blood salvage refers to a method of collecting and reinfusing blood that leaves the intravascular space during surgery. Blood is suctioned from the surgical site into a device (or cell saver) that includes an anticoagulated reservoir, after which the blood is filtered, washed, and centrifuged before it is reinfused back to the patient. The end product, however, consists of only RBCs and saline, because plasma, clotting factors (factors XII, XI, X, IX, VIII, V, II, I), WBCs, and platelets are removed in the process. Therefore, a dilutional coagulopathy often results after ∼1/2 of a patient's circulating blood volume is processed through the cell saver, which is the primary limitation of this technique. Newer methods of electrocautery that more effectively achieve hemostasis, along with topical hemostatic agents and sealants, are also effective in reducing blood loss.31 Autologous normovolemic hemodilution typically involves phlebotomy of 2-4 units of the patient's whole blood into citrated bags at the beginning of surgery, replacing the intravascular volume with crystalloid or colloid solutions, and creating a dilutional anemia during the part of the surgery most likely to be associated with blood loss. This approach results in relative decreases in the total RBCs lost with bleeding during the procedure. The patient's own fresh whole blood is reinfused near the end of surgery. In addition to RBCs, the patient also receives clotting factors and platelets with reinfusion of their whole blood. Other methods to reduce bleeding include avoiding hypothermia, intentionally lowering arterial blood pressure, and limiting laboratory testing. Not to be overlooked is what has been termed meticulous surgical technique. We have observed that JW patients often lose less blood during surgery compared with other patients, possibly because the surgical team is more focused on achieving hemostasis and limiting blood loss. To our knowledge, whether the procedures take longer has never been reported. We also consider antifibrinolytic therapy under certain circumstances for intraoperative or postoperative bleeding.32
From our population of hospitalized patients, we identify bloodless patients as those who reported to be members of the JW faith on admission, which is recorded on the inpatient census and reviewed daily by our bloodless nurse or patient coordinator. We are also occasionally notified of patients who are not of the JW faith, but who decline to accept ABTs. In addition, we receive consultations from services that prefer to avoid blood transfusions in specific patients if possible, such as renal transplantation patients or those who are severely alloimmunized. Most patients are seen by at least one member of our team, which includes a patient coordinator (who is also a JW congregation elder), 2 nurse coordinators, our bloodless program director (an anesthesiologist), or our hematology consultant. Low-volume, pediatric phlebotomy tubes are always recommended and placed at the bedside for all patients. A sign is posted on the patient doors reminding nurses and phlebotomists to use low-volume tubes. We routinely limit laboratory testing to essential tests only. We also use an in-line reinfusion device (SafeSet) to eliminate blood wastage during sampling from arterial and central venous catheters.4,20 Previous studies have shown that the use of this device can reduce total blood loss by 50% in ICU patients.20 For patients with anemia and iron deficiency, oral iron is recommended if patients were tolerating oral intake (or through a gastric or nasogastric tube when appropriate). Intravenous iron is given for patients with more severe anemia that is unresponsive to oral iron or for patients who are not taking or who cannot tolerate oral iron due to gastrointestinal side effects. For patients with severe anemia and ongoing blood loss, we frequently recommended both intravenous iron (iron sucrose, 200 mg IV daily for 3-5 days contiguously or 3 times a week) and intravenous erythropoietin (20 000-30 000 IU intravenously daily to every other day). If patients have a significant infection, we attempt to limit or avoid iron therapy when possible given the potential risk of exacerbating infection with iron administration.33 In patients with ongoing bleeding on anticoagulation therapy, the anticoagulation therapy is stopped if bleeding is significant and the benefits of stopping therapy appeared to outweigh the risks.
All patients who wish to receive bloodless care are provided with a checklist of available blood products by a member of our bloodless team. Each patient identifies products that they are willing to receive and those that they will not accept. Information about how each product is derived is also provided when requested so that each patient can make an informed and personalized decision regarding each product. Patients sign a consent form listing the products that they will accept or refuse. Once this list is completed, the patient's directives are entered into their electronic record. Patients are free to modify this list at any time, after which their electronic record is updated.
Special considerations for pediatric patients
Although pediatric patients or their parents frequently identify themselves as members of the JW faith, we provide all minors with the standard of care to ensure the best possible outcomes, particularly when withholding blood could lead to substantial harm or death. This approach is consistent with the 1944 US Supreme Court decision (Prince vs Massachusetts) and advocated by the American Academy of Pediatrics. We include pediatric patients among our list of bloodless patients and counsel our young patients and their families that we will do everything possible to minimize blood loss and avoid unnecessary transfusions, although we will be unable to honor the parents' wishes in life-threatening situations. In our experience, parents, patients, and their families support this approach.
There are a few published trials demonstrating benefit from selected bloodless management practices in pediatric patients.34-36 For example, erythropoietin administration preoperatively (3 times per week for 3 weeks) was found to decrease the transfusion requirement in pediatric patients undergoing craniofacial surgery (n = 30 for both treatment and control groups).36 Similarly, a small study of infants undergoing surgery for craniosynostosis (n = 14 for treatment group; n = 15 for controls) also showed an increase in hemoglobins and decrease in transfusion requirements with preoperative ESA therapy.35 A more recent study showed a decrease in transfusion requirements for infants undergoing surgery for craniosynostosis who were treated with ESA therapy and the institution of preoperative normovolemic hemodilution.34 In contrast, studies in pediatric patients undergoing orthopedic surgeries showed no benefit to ESA therapy.37,38 For example, 2 studies in adolescent patients undergoing spinal surgery showed no significant decrease in transfusion requirements or cost benefit using preoperative ESA therapy.37,38 The low rate of transfusions, together with the high cost of ESA therapy, were cited as the basis for the negative studies. Although these studies were relatively small, no adverse outcomes were reported.34-38 Interestingly, the high cost of ESAs was also noted as a significant limitation to their use in critically ill adult patients with anemia.39 Further studies are needed to determine whether these approaches are safe and effective for selected pediatric and adult patient populations.
Summary and recommendations for future studies
Prior studies from our center and others indicate that bloodless patients have favorable outcomes when managed by an experienced team.4-16 Emerging evidence also suggests that transfusions themselves are associated with adverse outcomes that are independent of anemia.18 Therefore, further studies are needed to evaluate approaches for bloodless patients, including comparison of surgical patients from bloodless or control groups who were not considered surgical candidates. In addition, trials evaluating various treatment regimens with erythropoietin and iron are needed to optimize these interventions. Thrombopoietin mimetic therapy has also been used in a JW patient on veno-venous extracorporeal membrane oxygenation after respiratory failure for pneumonia, and this approach could also be tested in selected patients with thrombocytopenia who do not accept platelet transfusions.40 Therapy with hemoglobin substitutes41 and other agents that stimulate blood cell production, including RBCs, WBCs, or platelets, would be beneficial for bloodless medicine and could potentially benefit all patients. Clinical trials to identify safe and effective antifibrinolytic and hemostatic therapies are also needed. We anticipate that future research in this arena will advance care and may limit costs for all patients and should also lead to improved outcomes for the growing number of patients requesting bloodless medicine.
Acknowledgments
The authors thank The New York Community Trust for their generous support of our Bloodless Medicine and Surgery Program; our entire “bloodless team” for their meticulous care and dedication to our patients, including Andrew and Joan Pippa, Liz Dackiw, Ish'shah Sherd, Elizabeth Wick, and Paul Ness; and all of our patients and their families for their support and appreciation, without whom this work would not be possible.
Disclosures
Conflict-of-interest disclosures: L.M.S.R. declares no competing financial interests. S.M.F. has received research funding from Haemonetics and has consulted for and received honoraria from Haemonetics and Medtronic. Off-label drug use: Erythropoietin to treat anemia or increase hemoglobins preoperatively for major surgical procedures in patients who do not accept allogeneic blood transfusions.
Correspondence
Linda M.S. Resar, MD, Departments of Medicine, Oncology, and Institute for Cellular Medicine, The Johns Hopkins University School of Medicine, Ross Research Building, Room 1025, 720 Rutland Ave., Baltimore, MD 21205; Phone: (410)614-0712; Fax: (410)955-0185; e-mail: lresar@jhmi.edu.