Abstract
Substantial progress has been made in our understanding of the risks and benefits of RBC transfusion through the performance of large clinical trials. More than 7000 patients have been enrolled in trials randomly allocating patients to higher transfusion thresholds (∼9-10 g/dL), referred to as liberal transfusion, or lower transfusion thresholds (∼7-8 g/dL), referred to as restrictive transfusion. The results of most of the trials suggest that a restrictive transfusion strategy is safe and, in some cases, superior to a liberal transfusion strategy. However, in patients with myocardial infarction, brain injury, stroke, or hematological disorders, more large trials are needed because preliminary evidence suggests that liberal transfusion might be beneficial or trials have not been performed at all.
Learning Objective
To understand the clinical trial evidence that has evaluated the effect of blood transfusion on mortality and morbidity
Introduction
With the performance of many important clinical trials, there has been significant progress in the understanding of when to transfuse RBCs. However, most high-quality evidence is in the nonhematological setting; with the exception of sickle cell anemia, very little trial evidence exists for RBC transfusion in hematological disorders. Here, we review the key randomized clinical trials evaluating RBC transfusion. We emphasize clinical trial data because observational studies are unreliable and the results can be misleading.1,2 We begin by describing the goals of therapy in the acute and chronic anemia settings. We then review the risks of anemia and summarize the clinical trials in both the nonhematological and the hematological setting. We complete the discussion with a summary of the guidelines.
Goals and risks of RBC transfusion
The often-stated goal of RBC transfusion is to improve oxygen delivery to the tissues. Although, in principle, this is true, it is very hard to measure oxygen delivery directly. Instead, we evaluate the impact of transfusion on symptoms and clinical events. In the acute setting such as surgery, the goal of transfusion is to maximize survival and minimize morbid events such as infection and myocardial infarction (MI). In contrast, the goals of RBC transfusion for chronic forms of anemia such as myelodysplasia are to enhance quality of life and function while trying to minimize the side effects of chronic exposure to RBC transfusion.3 In patients with hemoglobinopathies or other RBC disorders, transfusions may also be administered to suppress the production of abnormal cells. Therefore, in the acute setting, studies generally focus on mortality and morbidity, whereas in the chronic setting, the focus is on symptoms and function.
RBC transfusions transfer biologically active products, induce immunologic responses, and expand vascular volume. Acute complications related to hemolytic transfusion reaction, lung injury, and/or transmission of infectious diseases are uncommon, but circulatory overload and minor immune reactions resulting in the use of additional medications are common and can result in acute toxicity.4 Immune modulation, iron overload, prothrombotic or hemorrhagic effects, and events related to metabolic and biological products (eg, cytokines, lysed cells) in stored blood may have short- and long-term clinical impact. Therefore, RBC transfusion carries with it a spectrum of beneficial and adverse effects that might have variable effects in diverse clinical settings.
Risks from anemia
The risks associated with anemia have been well studied in patients undergoing surgery who refuse blood transfusion. An analysis in 1958 patients of Jehovah's Witness faith who underwent a surgical procedure found that, as the preoperative hemoglobin level fell, the risk of death rose and was especially high when the hemoglobin level was <5-6 g/dL.3 An important finding was that patients with underlying cardiovascular disease and hemoglobin level <10 g/dL had a higher mortality than patients without cardiovascular disease. These results suggest that patients with underlying cardiovascular disease are less tolerant of anemia. An analysis of the same study population examining the risk of death associated with postoperative anemia <8 g/dL found an especially high mortality when the nadir hemoglobin level was <5-6 g/dL.5
Several large, retrospective analyses examined the association between anemia and perioperative morbidity and mortality. One study in >310 000 patients 65 years of age or older undergoing major noncardiac surgery found mild preoperative anemia associated with increased 30-day mortality and cardiovascular morbidity (cardiac arrest or Q-wave MI). There was a rise in mortality and cardiac outcomes when the hematocrit was <39%.6 Similar findings were identified in the American College of Surgeons' National Surgical Quality Improvement Program database. This study evaluated >227 000 patients who underwent major noncardiac surgery and found that even mild anemia (hematocrit 29%–39%) was independently associated with an increase in 30-day morbidity and mortality.7 An important caution is that, in all of these studies, it is unclear whether anemia is just a marker for sicker patients or is itself responsible for increased mortality.
Clinical trials
There have been 20 clinical trials performed in adults comparing liberal (∼9-10 g/dL) and restrictive (∼7-8 g/dL) RBC transfusion in >7000 patients.8 These trials have been performed in many different clinical settings, including intensive care unit (ICU) patients; those undergoing cardiac, orthopedic, and other surgeries; patients suffering from gastrointestinal (GI) bleeding; and other settings.
The big 3
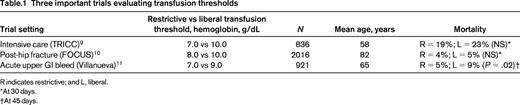
The 3 most important trials to date were performed in ICU patients, hip fracture patients, and patients with upper GI bleeding (Table 1). These trials are the most important because they are among the largest and most rigorously performed trials and each of them broke important new ground.
TRICC trial
The TRICC (Transfusion Requirement in Critical Care) trial is the landmark trial published in 1999.9 TRICC challenged the widely held view that a 10 g/dL threshold was required to recover from acute life-threatening illness. A total of 838 euvolemic intensive unit patients with hemoglobin <9 g/dL were randomly allocated to a 10 g/dL RBC transfusion threshold (liberal) group or a 7 g/dL RBC transfusion threshold (restrictive) group. Thirty-day mortality was lower in patients in the restrictive group (18.7%) than in the liberal group (23.3%) (P = .1). Patients in the restrictive transfusion group had fewer MIs (P = .02), pulmonary edema (P = .01), and acute respiratory distress syndrome (P = .06) than those in the liberal group. However, in the subgroup of patients with ischemic heart disease, the death rate was nonsignificantly (P = .3) higher in the restrictive group (26%) compared with the liberal group (21%). The TRICC trial was the first to suggest that a 7 g/dL threshold was as safe—and perhaps safer than the 10 g/dL threshold.
FOCUS trial
The FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial enrolled patients with underlying cardiovascular disease or risk factors who underwent surgical repair of hip fracture.10 This trial is especially important because it included a high-risk of group of elderly patients with underlying cardiovascular disease. FOCUS compared a 10 g/dL transfusion threshold with an 8 g/dL threshold or symptoms. No difference was found in the primary outcome of death or inability to walk across a room unassisted (35.2% in the liberal group and 34.7% in the restrictive group). There were also no differences in secondary outcomes including mortality at 30 days (5.2% in the liberal group and 4.3% in the restrictive group), infection, function, or length of hospital stay. Furthermore, the composite outcome of acute MI, unstable angina, or in-hospital mortality occurred in 4.3% of the liberal group and 5.2% of the restrictive group (difference, −0.9%; 99% confidence interval, −3.3%-1.6%). FOCUS was the first trial to provide evidence that those patients with preexisting cardiovascular disease or risk factors can be safely managed using a restrictive transfusion strategy.
GI bleeding trial
A total of 921 patients with upper GI bleeding were enrolled in a trial comparing a 7 g/dL threshold (restrictive) with a 9 g/dL threshold (liberal).11 This trial excluded any patient with underlying cardiovascular disease. At 6 weeks, 5% of the patients in the restrictive group died compared with 9% of the patients in the liberal group (hazard ratio = 0.55; 95% confidence interval, 0.33-0.92; P = .02). The restrictive strategy was also associated with less rebleeding and congestive heart failure. Importantly, the portal pressure gradient was significantly higher in the liberal group (P = .03) and was hypothesized to explain the increased rate of rebleeding in the liberal transfusion group. Given the results of this trial, most patients with upper GI bleeding should not be transfused until the hemoglobin drops below 7 g/dL, recognizing that it might be difficult to anticipate how low the hemoglobin will go. This is the first trial that found a statistically lower mortality in patients in the restrictive RBC transfusion group.
Other smaller trials
Cardiac surgery studies.
The TRACS (Transfusion Requirements After Cardiac Surgery) study randomly allocated 502 patients to a liberal transfusion group (hematocrit <30%) or a restrictive transfusion group (hematocrit <24%).12 The primary composite outcome of 30-day all-cause mortality and in-hospital morbidity occurred in 11% of the restrictive group and in 10% in the liberal group. Secondary outcomes were also similar. A second cardiac surgery trial found no difference in 428 patients comparing an 8 g/dL hemoglobin threshold with a 9 g/dL threshold.13 A large trial based in the United Kingdom, the Transfusion Indication Threshold Reduction trial, should report their results in the next 1-2 years.
Acute coronary syndrome studies.
There have been 2 small clinical trials published that enrolled patients with acute coronary syndrome. Both trials compared transfusion triggers of 8 and 10 g/dL. In the CRIT (Conservative versus Liberal Red Cell Transfusion in Acute MI) trial of 45 patients, there was a higher incidence of congestive heart failure in the liberal group.14 In the MINT (Myocardial Infarction and Transfusion) trial in 110 patients, there was a trend toward fewer major cardiac events and deaths in the liberal group (7 deaths in the restrictive group and 1 death in the liberal group; P = .03).15 Combining the 2 trials in acute coronary syndrome, there were 9 deaths in the restrictive group and 2 deaths in the liberal group. These trials are the first to signal that liberal trsfusion might be superior to rerictive transfusion in the setting of acute coronary syndrome. However, these preliminary findings await definitive answers in a large trial.
Sickle cell anemia studies.
There are 3 published trials evaluating preoperative transfusion in patients with sickle cell anemia undergoing surgery and the results are different in each of the trials. A total of 551 patients undergoing 604 procedures were randomly assigned to aggressive transfusion to reduce hemoglobin S concentration to <30% (group 1) or to transfusion to hemoglobin concentration >10 g/dL (group 2).16 Patients in group 1 received more RBC transfusions (3.8 vs 2.5 units). The frequency of serious complications was similar in the 2 groups and there were no differences in acute chest syndrome. However, the development of a new alloantibody occurred more often in patients transfused to hemoglobin concentration <30% (odds ratio = 2.3; 95% confidence interval, 1.21-4.49).
The second trial enrolled 70 patients 1 year or older with sickle cell anemia or sickle thalassemia disease scheduled to undergo low- or medium-risk surgery. Patients were randomly allocated to transfusion to increase the hemoglobin concentration to >10 g/dL or no transfusion.17 Clinically important complications occurred more frequently in patients who were not transfused (39%) than in patients who were transfused (15%). Nearly all of the complications were acute chest syndrome.
The third trial includes 369 patients with sickle cell anemia randomly allocated to no preoperative transfusion and simple or partial exchange transfusion. Patients in the preoperative transfusion group developed more postoperative complications (14%) than patients in the no transfusion group (7%). The report of this study is less detailed.18
Other hematological settings
We identified 2 trials examining transfusion thresholds in hematological malignancies. A pilot trial was performed in 60 patients undergoing induction chemotherapy or stem cell transplantation. Patients were randomly allocated to receive 2 units of RBCs when the hemoglobin concentration was <8 g/dL or 2 units of RBCs when the hemoglobin concentration was <12 g/dL. The main hypothesis was that there would be less bleeding and less need for platelet transfusions in the group with higher transfusion threshold. The pilot demonstrated that such a trial was feasible, but there were too few patients to detect clinical differences in the groups. The second trial, TRIST (Transfusion of Red Cells in Hematopoietic Stem Cell Transplantation), is enrolling patients undergoing hematopoietic stem cell transplantation and comparing transfusion thresholds of at 7 and 9 g/dL.19 This trial is still in progress and no results are available at this time.
Pediatric trials.
There are 3 trials evaluating transfusion thresholds in children. The largest trial was performed in 637 critically ill children cared for in the pediatric ICU. Patients with a hemoglobin level <9.5 g/dL were enrolled and randomly allocated to 7 or 9.5 g/dL transfusions.20 The primary outcome of new or progressive multiple organ dysfunction syndrome was similar between the 2 groups. These results are similar to adult patients, providing support for the 7 g/dL threshold in pediatric ICU patients. Two small trials in premature infants have been published,21,22 but a definitive trial is still under way in this group of patients.
Transfusion alternative: erythropoiesis-stimulating agents.
Recombinant human erythropoietin and other erythropoiesis-stimulating agents (ESAs) have been studied therapeutically to reduce the number of RBC transfusions in a variety of settings. In the United States, the Food and Drug Administration has approved erythropoietin for the treatment of the following: (1) anemia in patients with chronic renal failure, (2) anemia in patients with HIV infection receiving zidovudine, (3) highly selected cancer patients with anemia due to myelosuppressive chemotherapy, and (4) patients with anemia who are at high risk for perioperative blood loss. Recombinant human erythropoietin and ESAs are not likely to be useful for the acute management of anemia in the ICU and other acute care settings because of delayed correction of anemia. Furthermore, many patients with inflammatory and/or infectious states may be refractory to erythropoietin.23 Randomized studies in ICU patients have not demonstrated a uniform reduction in RBC transfusions in patients administered recombinant human erythropoietin and one study indicated an increased risk for thrombosis.24,25 Additional studies testing alternate dosing regimens may provide further information and clarify the potential benefits and toxicities associated with recombinant human erythropoietin and ESAs in the ICU and other acute care settings. Recombinant human erythropietin and ESAs have also been used in some patients who have religious objection to blood product transfusion (eg, Jehovah's Witnesses).
Meta-analyses
We performed a systematic review and meta-analysis of clinical trials evaluating transfusion thresholds.8,26 This analysis included 19 studies published through 2012. The trials were performed in many different settings, including multiple surgical settings (orthopedic, cardiac, vascular), the ICU (adults and pediatrics), in patients with GI bleeding, in patients with MI, and others. The studies show consistently that patients in the restrictive transfusion group receive ∼40% fewer RBC transfusions than patients in the liberal transfusion group.
Clinical outcomes were assessed and, most importantly, there was no evidence that patients were harmed using a restrictive transfusion strategy of 7-8 g/dL in most clinical settings. In contrast, there is some evidence that patients given fewer RBC transfusions may have a superior outcome. Thirty-day mortality was borderline lower (P = .07) in the restrictive group (relative risk = 0.80; 95% confidence interval, 0.63-1.02), although this was not statistically significant. These findings are consistent with a more recently published trial in patients with GI bleeding, which found a significantly lower 45-day mortality in patients in the restrictive (7 g/dL) transfusion arm than in patients in the liberal (9 g/dL) transfusion arm (hazard ratio = 0.55; 95% confidence interval, 0.33-0.92; P = .02). Similarly, the risk of infection was borderline significant in restrictive group. However, these findings have been updated recently to include trials published after 2012 and now show that the risk of infection is significantly lower in the restrictive transfusion group (relative risk = 0.82; 95% confidence interval, 0.72-0.95).27 These findings are borderline significant and could change when the next large trial is published. However, what is important is that these studies show unequivocally that using a liberal transfusion strategy does not improve outcome in these clinical settings. However, there are several clinical settings for which the safety of a restrictive transfusion strategy has not been established. First, in patients with acute MI, there are 2 small trials and in 1, mortality was lower in patients in the liberal transfusion group. Second, trials have not been performed in patients with brain injury or stroke. Some believe that higher transfusion thresholds could improve outcome in these patients with or at high risk for ischemic brain damage. Third, trials are needed in other acute and chronic hematological disorders.
Clinical guidelines
We recently published RBC transfusion guidelines that assessed 4 questions.4 First, in hospitalized, hemodynamically stable patients, at which hemoglobin concentration should a decision to transfuse RBCs be considered? Second, in hospitalized, hemodynamically stable patients with preexisting cardiovascular disease, at what hemoglobin concentration should a decision to transfuse RBCs be considered? Third, in hospitalized, hemodynamically stable patients with acute coronary syndrome, at what hemoglobin concentration should an RBC transfusion be considered? Fourth, in hospitalized, hemodynamically stable patients, should transfusion be guided by symptoms rather than hemoglobin concentration? We based all of the recommendations on a systematic review of clinical trials and grading of the evidence. Most importantly, we only provided advice when there were clinical trials that addressed the questions directly.
In general, the guidelines recommended restrictive transfusion defined as 7-8 g/dL thresholds. The strongest recommendations are in the settings where there is direct clinical trial evidence including adult and pediatric ICU patients and orthopedic surgery patients undergoing hip fracture repair. The guidelines recommend 7 g/dL in ICU patients because that threshold was used in these trials, whereas 8 g/dL was used in surgery trials. The guidelines also recommend an 8 g/dL threshold in patients with underlying cardiovascular disease, but acknowledge that only one trial has addressed this patient group. The guidelines make no specific recommendation in patients with acute MI because there is no high-quality evidence in this group of patients. Finally, the committee also advised using clinical symptoms to guide transfusion decisions, but these recommendations were judged to be based on low-quality evidence and only received a weak recommendation because only one trial incorporated symptoms in the RBC transfusion decision-making process. The ideal trial to evaluate the use of symptoms to guide transfusion would compare patients receiving transfusions based only on symptoms versus hemoglobin level. However, such a trial is unlikely to be performed.
Conclusions
The weight of the evidence strongly supports using a restrictive transfusion strategy (7-8 g/dL) in most patients with nonhematological disorders. However, the optimal threshold in patients with acute MI and hematological disorders awaits further randomized clinical trials.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Jeffrey L. Carson, MD, Division of General Internal Medicine, Rutgers Robert Wood Johnson Medical School, 125 Paterson St, Rm 2302A, New Brunswick, NJ 08901. Phone: 732-235-7122; Fax: 732-235-7144.