Abstract
A 5-year-old boy presents with platelet count of 2 × 109/L and clinical and laboratory evidence of immune thrombocytopenia. He has epistaxis and oral mucosal bleeding. Complete blood count reveals isolated thrombocytopenia without any decline in hemoglobin and he is Rh+. You are asked if anti-D immunoglobulin is an appropriate initial therapy for this child given the 2010 Food and Drug Administration “black-box” warning.
Immune thrombocytopenia (ITP) in children is usually self-limited and can often be managed by cautious observation. However, the presence of “wet” bleeding, such as the oral mucosal bleeding experienced by this child, is considered an indication for treatment aimed at increasing the platelet count. Corticosteroids, IVIG, and anti-D immunoglobulin (anti-D) are all considered appropriate frontline treatments for acute bleeding in both adults and children. Anti-D has been shown to have comparable efficacy rates to IVIG (∼ 70%)1,2 and an overall favorable side effect profile.3,4 In 2010, a specific warning was issued by the Food and Drug Administration (FDA) highlighting the risk of intravascular hemolysis, acute renal failure, and disseminated intravascular coagulation (DIC) after administration of anti-D to patients with ITP.5 In the years that have followed, anecdotal evidence suggests that providers are less comfortable with using anti-D and the data support that the use of the agent for frontline therapy has significantly decreased in the postwarning period, although the decline may have begun before the warning.6,7
To examine the current best evidence for anti-D as frontline treatment of newly diagnosed ITP in adults and children, we conducted a PubMed search using the terms “anti-D,” “Rh,” “RhIg,” and “Rhesus immunoglobulin” along with “ITP.” In addition, the references of included studies were reviewed to identify any additional publications. Of the 33 results that were categorized as clinical trials, 22 were excluded because they did not involve anti-D (n = 5), were not a therapeutic trial (n = 5), used nonstandard administration (n = 1), primarily evaluated response in patients with chronic ITP (n = 9), or duplicated patients reported in another study (n = 2). Three additional trials not categorized as a clinical trials but reporting efficacy data on anti-D were added. The included studies reporting efficacy data are found in Table 1. In summary, multiple clinical trials in both adults and children have shown that anti-D effectively raises the platelet count in the majority of treated patients and is associated with an acceptable safety profile (Table 1). Duration of effect appears to be comparable to IVIG, with some reports of longer duration of effect in anti-D–treated patients.8
Studies reporting anti-D efficacy in adults and children including patients with newly diagnosed ITP
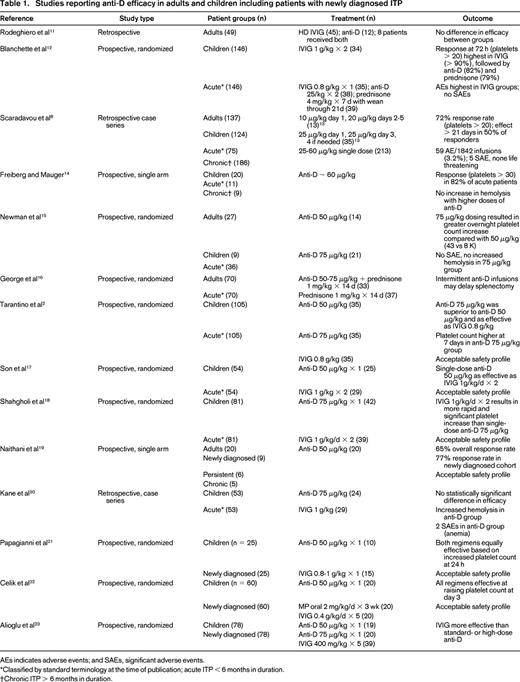
AEs indicates adverse events; and SAEs, significant adverse events.
*Classified by standard terminology at the time of publication; acute ITP < 6 months in duration.
†Chronic ITP > 6 months in duration.
With the increasing use of anti-D after licensure in 1995, rare cases of exaggerated hemolysis and DIC have been reported,9,10 ultimately resulting in the release of a “black-box” warning by the FDA highlighting the risks of intravascular hemolyis, acute renal failure, and DIC after IV administration of anti-D for ITP. Monitoring recommendations were provided.5 The adverse events reported to the FDA and to Cangene BioPharma, the maker of WinRho SDF, the most widely used anti-D preparation, have been comprehensively reviewed in a recent publication and the data are summarized in Table 2.3 The incidence of the severe hemolytic reactions is estimated at 1 in 1115 patients and has not significantly changed since licensure (Table 2).3 Severe hemolytic reactions occurred within 4 hours in 94% of cases. The risk of development of acute hemolytic reactions appears to be highest in adults > 65 years of age, patients with baseline hemolysis or renal function abnormalities, and those with current or recent significant infection, especially EBV. In patients with increased risk based on the above factors, an alternative therapy for ITP is recommended.
Reported acute hemolytic reactions and total infusions per year since licensure
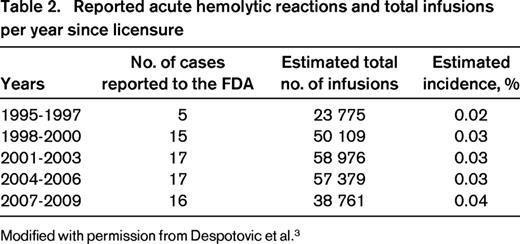
Modified with permission from Despotovic et al.3
For patients who do not have identified risk factors for a severe hemolytic event, we conclude that there is sufficient evidence to support the use of anti-D as a frontline treatment option in adults and children with ITP (Grade 2B recommendation). During the postinfusion period, clinicians should monitor for any evidence of severe hemolysis by obtaining a complete blood count and urinalysis and should perform further testing as necessary.3 After discharge, patients and families should be educated about signs and symptoms that warrant evaluation. Future studies should address possible alternative administration strategies (including subcutaneous administration), the application of monitoring recommendations in routine clinical practice, the effect that the recommended monitoring has had on reducing the number of serious hemolytic reactions, and the role of routine premedication strategies (eg, corticosteroids, diphenhydramine, acetaminophen) to decrease infusion reactions.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Jenny Despotovic, Hematology Service, Texas Children's Cancer Center, 6701 Fannin Street, 14th Floor, Houston, TX 77030; Phone: 832-822-4302; Fax: 832-825-0285; e-mail: jmdespot@txch.org.
