Abstract
VWF is a multimeric plasma glycoprotein that specifically recruits platelets to sites of vessel injury. VWF multimeric size is central to this function, with larger multimers being more hemostatically active. Regulation of VWF multimeric size is mediated by the plasma metalloprotease ADAMTS13 (A Disintegrin And Metalloproteinase with ThromboSpondin type 1 motifs, member 13). This enzyme can only recognize and cleave VWF when it is unraveled by rheological shear forces of the flowing blood. After the exposure of cryptic exosites, VWF recognition by ADAMTS13 involves multiple interactions that enable the protease to cleave VWF. Loss of VWF multimer size regulation caused by severe ADAMTS13 deficiency (either inherited or acquired) is associated with the microvascular thrombotic disorder thrombotic thrombocytopenic purpura (TTP). The sequelae associated with TTP are widely thought to be linked to hyperreactive circulating VWF that cause unwanted platelet aggregation in the high shear environment of the microvasculature. Diagnosis of TTP is primarily made through a combination of symptoms, analysis of plasma ADAMTS13 activity, and detection of inhibitory anti-ADAMTS13 antibodies. Current frontline treatments for TTP include plasma exchange, which serves to remove inhibitory antibodies (in acquired TTP) and provide a source of functional ADAMTS13, and steroids to treat the autoimmune component of acquired TTP. The use of anti-CD20 therapy has also exhibited encouraging results in the treatment of acquired TTP. Newer therapeutic strategies that are currently being explored or are in development include recombinant ADAMTS13, a hyperreactive ADAMTS13 variant, and anti-VWF therapy. This review discusses the basic biochemistry of VWF and ADAMTS13, their dysfunction in TTP, and therapeutic approaches for the amelioration of TTP.
VWF
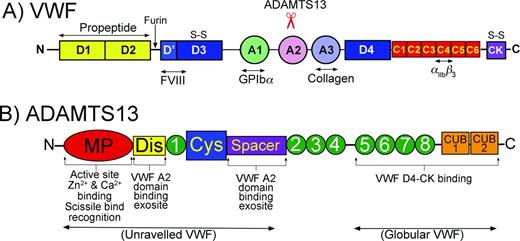
VWF is a multimeric plasma glycoprotein that is critical for platelet tethering at sites of blood vessel damage and therefore is essential for normal hemostasis.1 VWF expression is limited to endothelial cells and megakaryocytes. The nascent translated VWF polypeptide contains a signal peptide, a propeptide (D1-D2 domains) and a recently reassigned domain organization consisting of D′-D3-A1-A2-A3-D4-C1-C2-C3-C4-C5-C6-CK that contains several specific ligand-binding sites (Figure 1A).2 These include binding sites for factor VIII in D′-D3, platelet glycoprotein (GP) Ibα in the A1 domain, ADAMTS13 (A Disintegrin And Metalloproteinase with ThromboSpondin type 1 motifs, member 13) in the A2 domain, collagen in the A3 domain, and platelet integrin αIIbβ3 in the (now termed) C4 domain. VWF monomers dimerize in the endoplasmic reticulum through intermolecular disulfide bonds between CK domains, which then multimerize covalently via N-terminal disulfide bonds in the Golgi apparatus.1 After extensive glycosylation and proteolytic VWF propeptide removal, mature multimeric VWF is specifically trafficked to Weibel-Palade bodies of endothelial cells or α-granules of platelets3 in a spectrum of multimeric sizes ranging from dimers to “ultra-large” (UL) multimeric forms that can exceed 200-mers. VWF multimers can be released constitutively from endothelial cells into the bloodstream4 or upon demand from either endothelial cells or platelets in response to specific activation. Once released from the endothelium into the circulation, VWF folds into a globular conformation in which the A3 domain high-affinity collagen-binding site is exposed. These sites enable recruitment of VWF to subendothelial extracellular matrices exposed at sites of vessel damage. Conversely, the GpIbα-binding site in the VWF A1 domain is partially concealed, preventing any interaction between circulating platelets and VWF while in its circulating globular conformation. Similarly, the VWF A2 domain, which contains ADAMTS13 binding and a cleavage site(s) and separates the A1 and A3 domains, is folded such that the cleavage site is completely buried within the domain, rendering globular VWF resistant to proteolysis.5
Domain organizations of VWF and ADAMTS13. (A) Domain organization of VWF. Bottom: Major ligand-binding sites in VWF are highlighted. Top: Location of the ADAMTS13 cleavage site in the A2 domain is highlighted. (B) Domain organization of ADAMTS13. Bottom: Highlighted functional aspects of ADAMTS13 domains. The N-terminal domains recognize/bind to unraveled VWF, whereas the C-terminal domains interact with both globular and unraveled VWF.
Domain organizations of VWF and ADAMTS13. (A) Domain organization of VWF. Bottom: Major ligand-binding sites in VWF are highlighted. Top: Location of the ADAMTS13 cleavage site in the A2 domain is highlighted. (B) Domain organization of ADAMTS13. Bottom: Highlighted functional aspects of ADAMTS13 domains. The N-terminal domains recognize/bind to unraveled VWF, whereas the C-terminal domains interact with both globular and unraveled VWF.
VWF function in the circulation is dependent upon its multimeric composition and its ability to undergo a dynamic structural transition from a globular to a filamentous conformation. The circulating globular form allows VWF to survey the intact vasculature without unnecessary binding to platelets.6 This changes rapidly in response to vessel damage, which exposes collagen-rich matrices normally hidden from the blood by the endothelium. Globular VWF binds this collagen through its exposed A3 domain collagen-binding site. Once recruited, VWF undergoes a large structural transition in response to the local shear forces exerted on the tethered molecule by the flowing blood.7 These forces cause the folded VWF to unravel into an elongated string-like conformation, which exposes the previously hidden platelet-binding sites in the A1 domains. It is this exposure that now confers the ability of VWF to specifically capture circulating platelets to the site of injury.
The ability of VWF to fulfill this platelet-tethering function is highly dependent upon its multimeric size/composition. The larger VWF multimers are the most hemostatically competent, not only because they contain more collagen and platelet-binding sites, but also because they more readily unravel in response to rheological shear.6
Although VWF is synthesized as a multimeric protein, which is essential for its physiological role, the multimeric composition of plasma VWF is not regulated solely at the level of synthesis. Indeed, an appreciable proportion of the VWF produced by endothelial cells and megakaryocytes is synthesized in hyperreactive UL forms. The VWF plasma multimeric size, and thus its platelet-tethering function, is therefore further regulated after secretion into the blood by processing of UL-VWF by the plasma metalloprotease ADAMTS13.8
ADAMTS13
Intriguingly, the same shear-dependent unfolding of VWF that imparts its platelet-tethering function is also a major determinant of its proteolysis by ADAMTS13 that regulates its function.6 When mechanical shear forces induce the VWF to reveal its A1 domain platelet-binding sites, there is a concomitant unraveling of the VWF A2 domain that exposes cryptic ADAMTS13-binding sites as well as the target cleavage site (Tyr1605-Met1606).5,9
ADAMTS13 is an ∼180 kDa multidomain plasma metalloprotease (Figure 1B). It comprises a metalloprotease, disintegrin-like, thrombospondin type 1 (TSP) repeat and cysteine-rich and spacer domains. Thereafter, there are 7 further TSP repeats and 2 C-terminal CUB domains.8 The regulation of VWF multimer size by ADAMTS13 is particularly complex and requires multiple interactions between the 2 proteins. The C-terminal domains of ADAMTS13, involving the TSP domains 2-8 and CUB domains, appear to be important for ADAMTS13 to bind globular VWF.10,11 This has led to the finding that a small proportion of ADAMTS13 circulates in plasma in complex with its substrate.
The ADAMTS13 spacer domain contains a high-affinity binding site for the C-terminal residues in the VWF A2 domain. This spacer domain site involves residues Arg659, Arg660, Tyr661, and Tyr665 in ADAMTS13 and is responsible for much of the tight binding between ADAMTS13 and unraveled VWF.12,13 The adjacent cysteine-rich domain of ADAMTS13 is essential for ADAMTS13 function, very likely by supporting the functional conformation of the spacer domain.14
The role of the TSP repeat 1 domain that separates the cysteine-rich domain and the disintegrin-like domain is currently unclear, but is structurally important. The disintegrin-like domain is of critical importance in the recognition and proteolysis of VWF because it harbors an exosite involving Arg349 and Leu350 that interacts specifically with the unraveled VWF A2 domain (involving Asp1614) in proximity to the cleavage site.15 This interaction likely contributes to the positioning of the scissile bond into the active site cleft contained within the metalloprotease domain.
The ADAMTS13 metalloprotease domain contains a characteristic Zn2+-binding motif (HEXXHXXGXXHD) involving 3 His residues and a Glu residue that form the active site. In addition to the active site Zn2+ ion, Ca2+ ions are also necessary for ADAMTS13 function. The ADAMTS13 metalloprotease domain contains a double Ca2+-binding site involving Glu83, Asp173, Cys281, and Asp28416 and a highly important single Ca2+-binding site involving Asp182, Asp187, and Glu212 that likely aids in the conformation of the active site cleft.16 The metalloprotease domain also contains specific points of interaction with VWF that are important for recognition and specificity of the enzyme. ADAMTS13 residues Leu198, Leu232, and Leu274 together likely make up a subsite that bind Leu1603 in VWF. This interaction is of particular importance in guiding the cleavage site over the active site.17 Thereafter, specific subsites on either side of the catalytic Zn2+ specifically accommodate the P1 (Tyr1605) and P1′ (Met1606) residues in VWF and dictate cleavage site specificity of ADAMTS13.18
Based on our understanding of the importance of distinct functional sites in ADAMTS13 and the nature of VWF unraveling, a model of VWF regulation by ADAMTS13 has been proposed that involves multiple conformation-dependent interactions that dictate both the specificity of proteolysis as well as the location/timing of proteolytic regulation.19 Normally, VWF circulates in plasma in its globular conformation. In this form, the VWF A2 domain is folded and the cleavage site is hidden. Binding between ADAMTS13 TSP5-CUB domains and the VWF D4-CK domains enable a small proportion of ADAMTS13 to circulate bound to VWF. When shear forces induce the VWF to adopt its string-like conformation (ie, when secreted, when tethered to the site of vessel injury by its A3 domain, or during passage through the microvasculature under high shear), only then are cryptic exosite-binding sites in the VWF A2 domain revealed. The ADAMTS13 spacer domain first recognizes VWF residues Glu1660-Arg1668, which appreciably increases the affinity of ADAMTS13 for VWF. The ADAMTS13 disintegrin-like domain exosite (Arg349/Leu350) then forms a low-affinity interaction with Asp1614 in VWF. Thereafter, essential binding between VWF Leu1603 and a subsite in ADAMTS13 metalloprotease domain takes place that positions the Tyr1605-Met1606 scissile bond over the ADAMTS13 active site and allows these residues to engage with their respective subsite pockets on either side of the active site. Only then can proteolysis occur. This so called “molecular zipper” model is rather unique, and this multistep process goes some way to explaining the unprecedented specificity of ADAMTS13 for nothing other than unfolded VWF.
Sites of ADAMTS13 function
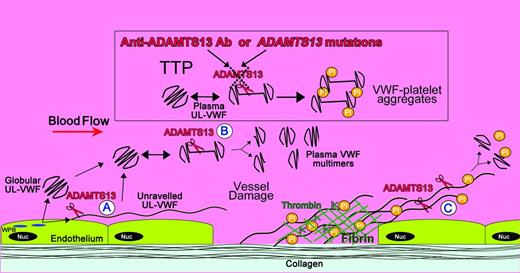
There seem to be 3 distinct situations when ADAMTS13-mediated VWF proteolysis occurs: (1) during secretion of VWF from endothelial cells, (2) in free circulation, and (3) during unraveling of VWF at sites of vessel damage (Figure 2). Although proteolysis in each location probably fulfills a different role, all rely on shear-dependent unfolding of VWF.
Locations of ADAMTS13 cleavage of VWF and its deficiency in TTP. Shown is a diagram illustrating the sites of VWF proteolysis by ADAMTS13. UL-VWF is synthesized by the endothelium and stored within Weibel-Palade bodies (WPB). VWF multimers of various sizes, including UL-VWF, can be secreted directly into the circulation. (A) Alternatively, a proportion of UL-VWF may attach to the endothelial surface during secretion and unravel in response to shear forces. Under these circumstances, the VWF A2 domain unfolds to enable ADAMTS13 (scissors) to cleave VWF and release the VWF string. VWF adopts a globular fold in plasma. However, during passage through the microvasculature, globular UL-VWF in the free circulation may partially/transiently unravel. (B) This permits the processing of the largest, most hemostatically active forms of VWF, resulting in their conversion to smaller plasma VWF multimers. At sites of vessel damage, endothelial damage results in exposure of subendothelial collagen; plasma VWF binds to this, unravels, and recruits platelets. The presence of collagen and thrombin induce rapid platelet activation, which, along with fibrin, consolidates the platelet plug. (C) Downstream of the site of injury (ie, in the absence of collagen and thrombin), VWF-platelet strings may still be proteolyzed by ADAMTS13, which in turn limits/regulates platelet plug formation. ADAMTS13 deficiency either through anti-ADAMTS13 antibodies or through inherited deficiency in the ADAMTS13 gene results in the loss of VWF processing. Under these circumstances, platelets (Pl) can bind unraveled VWF, leading to the accumulation of VWF-platelet aggregates that occlude the microvasculature, as seen in patients presenting with TTP.
Locations of ADAMTS13 cleavage of VWF and its deficiency in TTP. Shown is a diagram illustrating the sites of VWF proteolysis by ADAMTS13. UL-VWF is synthesized by the endothelium and stored within Weibel-Palade bodies (WPB). VWF multimers of various sizes, including UL-VWF, can be secreted directly into the circulation. (A) Alternatively, a proportion of UL-VWF may attach to the endothelial surface during secretion and unravel in response to shear forces. Under these circumstances, the VWF A2 domain unfolds to enable ADAMTS13 (scissors) to cleave VWF and release the VWF string. VWF adopts a globular fold in plasma. However, during passage through the microvasculature, globular UL-VWF in the free circulation may partially/transiently unravel. (B) This permits the processing of the largest, most hemostatically active forms of VWF, resulting in their conversion to smaller plasma VWF multimers. At sites of vessel damage, endothelial damage results in exposure of subendothelial collagen; plasma VWF binds to this, unravels, and recruits platelets. The presence of collagen and thrombin induce rapid platelet activation, which, along with fibrin, consolidates the platelet plug. (C) Downstream of the site of injury (ie, in the absence of collagen and thrombin), VWF-platelet strings may still be proteolyzed by ADAMTS13, which in turn limits/regulates platelet plug formation. ADAMTS13 deficiency either through anti-ADAMTS13 antibodies or through inherited deficiency in the ADAMTS13 gene results in the loss of VWF processing. Under these circumstances, platelets (Pl) can bind unraveled VWF, leading to the accumulation of VWF-platelet aggregates that occlude the microvasculature, as seen in patients presenting with TTP.
Endothelial VWF is synthesized in various sizes, but an appreciable proportion of this is in UL-VWF forms that are hyperreactive and, if left unprocessed, potentially pathogenic. However, as VWF is secreted from the endothelium, the Weibel-Palade bodies containing VWF fuse with the plasma membrane to release the stored VWF. During this release, it seems that VWF unravels through a small aperture, resulting in its transient tethering to the endothelial surface. This process enables the shear forces of the blood to unravel VWF into the string-like form that is permissive to proteolysis.20 Once proteolyzed at a single site, the VWF that is no longer tethered is released from the endothelial surface and adopts a globular, proteolysis-resistant conformation.
It is unlikely that all of the VWF that is secreted by the endothelium is proteolytically processed in this way. Therefore, despite this mechanism, some UL-VWF still gets released into the circulation. However, due to its size, UL-VWF is susceptible to unraveling during transit through the vasculature, particularly the high shear environment of the microvasculature.6 Whereas this could lead to unwanted platelet binding, it also facilitates processing of the larger VWF species by ADAMTS13 in the free circulation. In this way, just the UL-VWF is processed into multimer sizes that are less susceptible to spontaneous unraveling in the absence of collagen binding. Proteolysis of VWF by ADAMTS13 in these first 2 locations probably represents normal VWF homeostasis, which serves to ensure that the multimer size of the circulating VWF pool is regulated.
The third location of ADAMTS13 function is at sites of vessel damage. It is perhaps paradoxical that the unfolding that is necessary to enable platelet tethering is also the very process that renders VWF susceptible to ADAMTS13 proteolysis, which reduces its platelet-binding function. Therefore, there is a balance between pro- and anti-platelet-tethering mechanisms. Vascular damage exposes collagen, to which circulating VWF binds and undergoes its structural transition in response to shear forces, which allows circulating platelets to bind. The binding of platelet GPIbα to VWF occurs rapidly at a rate that likely exceeds that of VWF proteolysis by ADAMTS13.9 Moreover, at sites of vessel damage, the presence of both collagen and thrombin can act as potent local activators of recruited platelets that prompt further VWF-independent adhesion and aggregation (and consolidation of the hemostatic plug by fibrin). These platelets can thus become resistant to the consequences of ADAMTS13-mediated VWF proteolysis. However, as a platelet plug extends beyond the site of injury (and therefore has little/no exposure to collagen and thrombin), VWF-tethered platelets are no longer consolidated by activation or fibrin, enabling ADAMTS13 to cleave VWF and limit platelet plug formation to the site of vessel damage.
The importance of the modulation of VWF multimeric size is exemplified clinically by disorders associated with an imbalance in ADAMTS13-mediated regulation. Patients with type 2A VWD classically harbor mutations in the VWF A2 domain that promote the destabilization and unfolding of the domain in circulation.21 Therefore, excessive proteolysis of VWF in plasma occurs that transforms much of the plasma VWF pool into hemostatically incompetent forms. This leads to the bleeding phenotype associated with these patients. Conversely, individuals with severe (< 5%) ADAMTS13 deficiency (either inherited due to mutations in the ADAMTS13 gene or, more commonly, due to acquired deficiency associated with the development of inhibitory autoantibodies against ADAMTS13) lack the ability to control VWF multimer size, resulting in the presence and persistence of hyperreactive UL-VWF species in plasma. The conformational instability of UL-VWF causes its spontaneous unraveling in plasma, which imparts its platelet-binding function (Figure 2). However, in the absence of ADAMTS13 regulation to counteract such unwanted platelet tethering, excessive platelet aggregation and disseminated microvascular VWF- and platelet-rich thrombus formation can ensue, which is a hallmark of TTP.8
TTP
Patients with TTP were originally classified by a pentad of symptoms including thrombocytopenia, microangiopathic hemolytic anemia, fluctuating neurological signs, renal impairment, and fever. However, TTP patients frequently present without the full pentad. For example, ∼35% of patients do not exhibit signs of neurological dysfunction (ie, confusion, headache, paresis, aphasia, dysarthria, visual problems, encephalopathy) at presentation. Furthermore, renal impairment and fever are not necessarily prominent clinical features. TTP patients generally exhibit appreciable thrombocytopenia (platelet count 10-30 × 109/L) due to the sequestration of platelets (and UL-VWF) in microvascular thrombi. Microangiopathic hemolytic anemia likely arises due to fragmentation of erythrocytes during passage through partially occluded microvessels (resulting in low hemoglobin levels of 80-100 g/L, the presence of circulating schistocytes, and elevated lactate dehydrogenase).
TTP is rare, with a reported incidence of ∼6 cases per million per year, and the majority of cases are acquired, antibody mediated, and not associated with an obvious precipitant.22 Although the use of certain drugs appear to be associated with TTP, this represents < 15% of cases. Such drugs include quinine, thienopyridine in association with ticlodipine, simvastatin, trimethoprim, and PEGylated interferon.
TTP is diagnosed through a combination of clinical history, examination of the patient, and a blood film.23 ADAMTS13 activity assays assist in confirming the diagnosis. In TTP, ADAMTS13 activity is typically < 5% of normal. Additional inhibitor assays/detection of anti-ADAMTS13 autoantibodies are useful in distinguishing between inherited and acquired forms of TTP. These assays are also particularly useful for monitoring patient response to treatments.24
Inherited/congenital TTP is the least common form of the disease and arises due to mutations (generally compound heterozygous mutations) in the ADAMTS13 gene.8 Usually, to cause severe ADAMTS13 deficiency (< 5% ADAMTS13 activity), both alleles must be affected. Heterozygous carriers of an ADAMTS13 mutation on a single allele are generally asymptomatic and free of TTP-like symptoms. A large number of mutations, single nucleotide polymorphisms, and sequence variations have been reported in the ADAMTS13 gene.25 The majority of causative mutations that have been analyzed cause severe deficiency due to disruption of ADAMTS13 folding during synthesis, leading to severe intracellular retention. Congenital TTP patients, therefore, generally exhibit markedly reduced ADAMTS13 antigen levels in plasma in combination with the consequent reduction in activity levels. The inherited form of TTP can have a varied phenotype and can present at any age. In general, those with more severe deficiency present early, during the neonatal period or childhood. However, some congenital TTP patients may not present until later in life and may do so only in conjunction with an additional trigger (eg, pregnancy).
Acute idiopathic TTP is the most common form of TTP and is an autoimmune disease that is usually manifest by the development of by inhibitory autoantibodies, most commonly IgG and, less frequently, IgM and/or IgA classes, which recognize ADAMTS13.26,27 Several studies examining the autoantibody repertoire in TTP patients have suggested that the ADAMTS13 cysteine-rich/spacer domains contain epitopes that are most frequently targeted by autoantibodies.13,28,29 Although antibodies that recognize other domains in ADAMTS13 can be variably detected in acquired TTP patients, this tends to be in addition to anti-cysteine-rich/spacer domain antibodies. Given the highly important role of the ADAMTS13 spacer domain in mediating tight binding of ADAMTS13 to unraveled VWF, it is not unreasonable to suspect that autoantibodies against this domain may be the primary pathogenic antibodies. Autoantibodies against the C-terminal domains of ADAMTS13 may have limited or no inhibitory effects. This, however, does not rule out a role for such antibodies in possibly promoting clearance of ADAMTS13 from circulation.
Relapse in TTP patients is not uncommon (20%-50% of cases) and is defined as the recurrence of acute TTP symptoms 30 days after achieving remission. The likelihood of relapse is elevated in those patients, who, despite entering remission, still have low plasma ADAMTS13 activity (ie, < 10%) or the persistence of anti-ADAMTS13 antibodies.30 For example, nearly 40% of patients with < 5% ADAMTS13 in remission relapsed, whereas in those whom ADAMTS13 activity was > 15%, this figure was ∼ 5%.31 Relapse of acquired, antibody-mediated disease can arise through the continual development of the autoimmune mechanisms during remission, resulting in representation of TTP but with potentially different antibody repertoire (ie, antibodies recognizing different epitopes on ADAMTS13).
Animal models of ADAMTS13 deficiency
Several attempts have been made to create animal models of TTP. Two groups generated Adamts13-knockout mice in the hope that these might model congenital TTP.32,33 Perhaps surprisingly, these mice were viable and exhibited no overt phenotype. In further attempts to provoke TTP-like symptoms, these mice were crossed onto a high-VWF-expressing mouse background and also administered Shigatoxin.32 Only under these conditions could some of the hallmarks of TTP (eg, microvascular platelet/VWF-rich thrombi) start to be observed. Clearly, either species differences exist in the VWF/ADAMTS13 axes of humans and mice or there are additional mechanisms in mice that protect them from the more severe phenotype associated with ADAMTS13 deficiency in humans. ADAMTS13 deficiency in mice certainly results in loss of VWF proteolysis, but the effect of plasma UL-VWF does not, by itself, manifest pathologically.
ADAMTS13 deficiency alone is insufficient to precipitate TTP-like symptoms in mice, making it a difficult model with which to study novel therapeutic approaches. To circumvent this, and to better mimic the human scenario, Feys et al generated a mAb that recognizes human ADAMTS13 and that potently inhibits its ability to cleave VWF. This mAb was administered to baboons, resulting in essentially complete inhibition of plasma ADAMTS13.34 This caused elevated levels of plasma UL-VWF, some platelet-rich thrombi in the microvasculature resulting in thrombocytopenia, and evidence of hemolytic anemia. However, although this approach seems to nicely model “early-stage” human TTP, baboons fail to develop more severe symptoms or life-threatening disease or to exhibit any signs of neurological/renal dysfunction that can be associated with the human disease.
Potential triggers for TTP
The failure of these models to closely model human TTP has raised some interesting questions, one of which is whether ADAMTS13 deficiency alone is sufficient to cause TTP or if additional triggers are necessary to precipitate a clinical episode. There are various additional lines of clinical evidence that may support this contention. Not only do some congenital ADAMTS13-deficient patients not present overtly or do so only later in life, but acquired TTP patients may enter remission despite severely reduced plasma ADAMTS13 activity and the persistence of anti-ADAMTS13 autoantibodies. Moreover, TTP can be associated with a variety of other conditions including HIV infection, pregnancy, and the use of certain drugs. Whether these conditions serve to promote the development of ADAMTS13 deficiency themselves and provide additional triggers that precipitate a TTP episode is difficult to test. Hypothetically, in the setting of partial (rather than severe) ADAMTS13 deficiency, there are potential mechanisms that may contribute to the onset of TTP. Although ADAMTS13 does not have a natural inhibitor, it is susceptible to inhibition by free hemoglobin, interleukin-6, and also by proteolytic degradation by plasmin (and thrombin).35-37 Indeed, this later mechanism was suggested by a TTP patient with rare α2-antiplasmin deficiency that promoted excessive plasmin activity leading to proteolytic inactivation of ADAMTS13.38 Whether any of these potential modes of ADAMTS13 activation might contribute to the precipitation of TTP in some patients requires further investigation. A further contributing factor to TTP may be the actions of the complement system. Atypical hemolytic uremic syndrome presents clinically with symptoms that are similar to TTP and can also be associated with partial, rather than severe, deficiency in ADAMTS13. This has at times led to difficulty in distinguishing these diseases. Recently, a mutation in factor H has been associated with thrombotic microangiopathy associated with ADAMTS13 deficiency.39 Moreover, a report of amelioration of a refractory TTP patient with the use of anticomplement therapy (eculuzimab)40 has added to the potential involvement of complement in TTP. However, further work is clearly required to determine the link between complement activation and perturbation of the VWF-ADAMTS13 axis.
Treatment of TTP
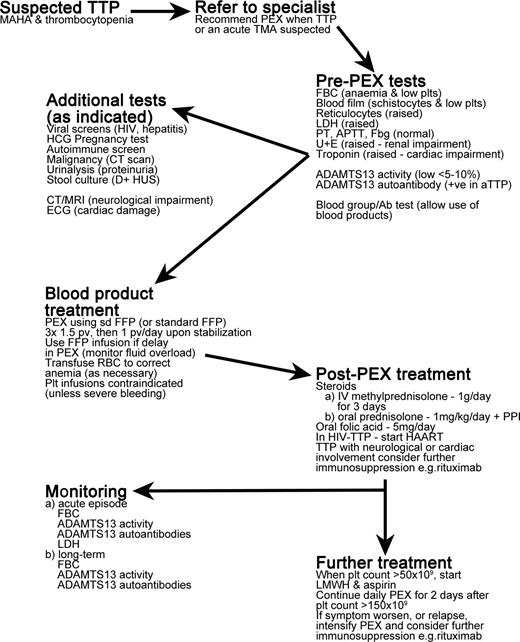
TTP is an acute, life-threatening illness that is a medical emergency requiring prompt treatment. A summary of the treatment of TTP patients is provided in Figure 3.
Overview of treatment of TTP patients. MAHA indicates microangiopathic hemolytic anemia; PEX, plasma exchange; TMA, thrombotic microangiopathy; FBC, full blood count; plts, platelets; LDH, lactate dehydrogenase; PT, prothrombin time; APTT, activated partial thromboplastin time; Fbg, fibrinogen; U+E, urea and electrolytes test; HCG, human chorionic gonadotropin; D+HUS, diarrhea-associated hemolytic uremic syndrome; CT, computerized tomography; MRI, magnetic resonance imaging; ECG, electrocardiogram; sd FFP, single-donor fresh-frozen plasma; pv, plasma volume; PPI, proton pump inhibitor; and HAART, highly active antiretroviral therapy.
Overview of treatment of TTP patients. MAHA indicates microangiopathic hemolytic anemia; PEX, plasma exchange; TMA, thrombotic microangiopathy; FBC, full blood count; plts, platelets; LDH, lactate dehydrogenase; PT, prothrombin time; APTT, activated partial thromboplastin time; Fbg, fibrinogen; U+E, urea and electrolytes test; HCG, human chorionic gonadotropin; D+HUS, diarrhea-associated hemolytic uremic syndrome; CT, computerized tomography; MRI, magnetic resonance imaging; ECG, electrocardiogram; sd FFP, single-donor fresh-frozen plasma; pv, plasma volume; PPI, proton pump inhibitor; and HAART, highly active antiretroviral therapy.
Plasma exchange therapy
Daily plasma exchange therapy represents the standard treatment of TTP and has led to a reduction in mortality from ∼ 90% to between 10% and 20%. Plasma exchange serves to remove circulating anti-ADAMTS13 autoantibodies (in acquired TTP) and also to provide a fresh source of ADAMTS13. Plasma exchange is more efficacious than plasma infusion in acquired TTP. In general, daily 1.5 plasma volume exchange is initiated as soon as possible after diagnosis.41 The number of exchanges necessary to achieve remission is highly variable and is generally longer in acquired, antibody-mediated TTP. Twice-daily plasma exchange may be advantageous in resistant cases and/or if there is the development of additional symptoms (eg, neurological or cardiac). Plasma exchange volume can be reduced to single volume when symptoms and laboratory tests begin to stabilize. A regimen of daily plasma exchange is administered for at least 2 days after platelet counts normalize (ie, > 150 × 109/L).
For treatment of inherited TTP, plasma infusion is usually sufficient in providing a source of ADAMTS13. Due to the lack of a natural inhibitor, ADAMTS13 has a long plasma half-life (particularly for an active enzyme). This fact, in conjunction with the relatively low levels that are required to alleviate the presence/persistence of pathogenic plasma UL-VWF, means that plasma infusion need not be performed that often. The frequency of treatments is naturally dependent on patient symptoms/blood counts, but is typically required every 3 to 4 weeks.
Immunosuppression
For acquired TTP, immunosuppression is widely used to combat the autoimmune component of the disease. Administration of IV methylprednisolone or oral prednisolone can help to counter the production of autoantibodies and are generally used as part of frontline therapy in acquired TTP.42 In recent years, anti-CD20 therapy (eg, rituximab) has been demonstrated to be both efficacious and safe in the treatment of acquired TTP.43 Prospective studies have revealed that rituximab represents an effective treatment for those acquired TTP patients who do not respond to plasma exchange and steroids or in those who relapse. Indeed, the use of rituximab appears to reduce the likelihood of relapse.
Acquired TTP patients in remission usually exhibit markedly reduced anti-ADAMTS13 antibody titer with a consequent recovery in plasma ADAMTS13 activity. This, however, is not always the case. Some individuals may exhibit persistence of anti-ADAMTS13 autoantibodies despite normalizing ADAMTS13 activity. Therefore, these persisting antibodies may not be inhibitory or pathogenic. Due to the risks of relapse, TTP patients require follow-up with ADAMTS 13 monitoring and elective therapy can be considered.
Other/emerging treatments for TTP
Recombinant ADAMTS13
Despite the appreciable reduction in mortality associated with plasma exchange, treatment is specialized, time consuming, and can be associated with further risks/complications (allergic/anaphylactic reactions, thrombosis, infection). Therefore, new safer and simpler strategies to treat TTP are desirable. At this time, therapeutic recombinant ADAMTS13 is not available, but hopefully its availability is just a matter of time. In inherited TTP patients, the provision of a recombinant ADAMTS13 concentrate will very likely simplify treatment of a TTP episode and/or provide a convenient prophylactic option. Certainly in murine models, recombinant human ADAMTS13 appears to be fully corrective of complete genetic ADAMTS13 deficiency.44 However, inherited TTP remains just a small proportion of all TTP patients.
In acquired TTP, the success of plasma exchange is linked to both the provision of ADAMTS13 and the removal of inhibitory antibodies. Therefore, to remove the need for plasma exchange, recombinant ADAMTS13 would need to be provided at levels that exceed patient antibody titers to restore ADAMTS13 activity to nonpathological levels.45 Whether this might represent a viable option in the future remains to be seen.
An alternative for acquired TTP patients may be the provision of an ADAMTS13 variant. Recent studies have shown that the spacer domain is a major antigenic target for autoantibodies. Indeed, there appears to be some overlap between a functional exosite on ADAMTS13 and a core antigenic region recognized by inhibitory antibodies in TTP patients.13,29 Studies have reported that conservative substitution of key antigenic residues in this region not only reduced binding and inhibition of this ADAMTS13 variant by TTP patient autoantibodies, but also resulted in a protease with enhanced VWF-cleaving function.46 This variant may, therefore, provide the opportunity to administer ADAMTS13 that is both resistant to the inhibitory effects of pathogenic autoantibodies and capable of processing VWF more efficiently.
Anti-VWF therapy
Strategies that target VWF have been explored recently for the alleviation of TTP symptoms. Because the clinical features of TTP are primarily linked to elevated plasma UL-VWF and hyperreactive platelet-tethering function, targeting the GpIbα-binding site in the VWF A1 domain may specifically prevent formation of platelet-rich microvascular thrombi seen in TTP. To date, 3 strategies have been explored to accomplish this: an aptamer (termed ARC1779),47 a humanized mAb (termed GBR600),48 and a bivalent nanobody (termed ALX-0681),49 all of which bind the VWF A1 domain and specifically block VWF binding to platelet GpIbα. Using the same baboon model of acquired TTP, both GBR600 and ALX-0681 caused a rapid normalization of platelet count after 3 days, which continued to rise further over the following 3 to 4 days, suggesting that further platelet thrombi development and platelet consumption had been effectively inhibited. Improvement of hemolytic anemia was evidenced by the gradual reduction in schistocytes and signs of increases in plasma haptoglobin. Blocking the VWF A1 domain did not appear to influence the risk of severe bleeding in treated baboons. Targeting VWF may therefore represent an effective strategy to prevent further development of symptoms in TTP in humans. However, whether blocking VWF is as effective as plasma exchange in ameliorating TTP in humans with more severe disease remains to be seen. Moreover, whether, in conjunction with immunosuppression, anti-VWF therapy has the potential to replace plasma exchange will require further studies. Plasma exchange helps to remove autoantibodies and UL-VWF and replenishes plasma ADAMTS13, enabling platelet count recovery. The restoration of ADAMTS13 activity is a likely important therapeutic component of plasma exchange for TTP patients. If given without plasma exchange, anti-VWF therapy would allow the anti-ADAMTS13 antibodies to persist (for at least as long as any immunosuppression may take to take full effect). In addition, because anti-VWF therapies are not capable of disaggregating preformed VWF-platelet complexes, this may represent a potential limitation in treating TTP patients with more severe symptoms. ADAMTS13 appears to exhibit thrombolytic activity, suggesting that restoration of ADAMTS13 activity can be important for the dissolution of existing platelet-rich thrombi in TTP patients,50 which can ameliorate the effects of microvascular occlusion. It will be interesting to determine whether inhibition of VWF may complement plasma exchange in TTP patients, particularly with respect to the time to platelet recovery and the number of required plasma exchanges. However, these therapies are adjuncts and dealing with the underlying pathogenic mechanism of the disease is imperative.
Summary
TTP has long been recognized as a complex and life-threatening disease. In recent years, our understanding of the basic biochemistry of the VWF-ADAMTS13 axis has provided valuable insights into the pathogenesis of TTP, as well as the investigation and development of new therapeutic strategies. Although the mortality associated with TTP has been appreciably reduced, much yet remains to be learned to more effectively treat and better understand this disease, particularly in those patients who have more severe symptoms or are refractory to current treatments.
Disclosures
Conflict-of-interest disclosure: J.T.B.C. declares no competing financial interests. M.A.S. has received research funding and honoraria and has been affiliated with the speakers' bureaus for Octapharma, GSK, and Alexion. Off-label drug use: rituximab.
Correspondence
Dr James T. B. Crawley, Centre for Haematology, Imperial College London, 5th Floor Commonwealth Building, Hammersmith Hospital Campus, Du Cane Road, London, W12 0NN, United Kingdom; Phone: 44-20-8383-2297; Fax: 44-20-8383-2296; e-mail: j.crawley@imperial.ac.uk.