Abstract
Although academic science has always provided a fundamental understanding of the biological and clinical basis of disease, the opportunity and imperative for academics to contribute more directly to the discovery of new medicines continues to grow. Embedding medicinal chemists with cancer biologists creates collaborative opportunities for drug discovery and the design and synthesis of chemical biology tool compounds (chemical probes) to better elucidate the role of specific proteins and pathways in biology and disease. Two case studies are presented here: (1) the discovery of inhibitors of mer kinase to treat acute lymphoblastic leukemia in children and (2) the discovery of chemical probes targeting epigenetic regulators. These case studies provide lessons in target selection strategies, the requirement for iterative optimization of lead compounds (useful drugs/probes rarely come directly from a screen), and the value of mutually dependent collaborations between medicinal chemists and cancer biologists.
Introduction
The aberrant cellular homeostatic processes and biochemical pathways that drive tumor formation are increasingly understood at the molecular level, and potential small-molecule drug targets can be defined within this rational context. Unfortunately, the daunting complexity and temporal instability of each patient's tumor has also been revealed through sequencing of multiple cancer genomes.1 This complexity means that many potential molecular targets need to be considered and triaged based on therapeutic validity and tractability for ligand discovery to increase the oncologist's repertoire of drugs to appropriately target each patient's disease. Although the pharmaceutical industry will undoubtedly continue to develop new cancer therapies, it is imperative that academic scientists contribute more to the early drug discovery phase so that the best targets are rapidly identified and advanced. Industry alone cannot be relied on for the breadth of discovery efforts required for the following reasons. First, decreasing investment: although industrial investment in research and development grew steadily from the 1950s until 2010, approval of new medicines has remained constant.2-4 These conditions have resulted in frequent mergers, reorganizations, and reductions in scientific staff in for-profit drug discovery, with 2011 recording the first decrease in research and development investment in this sector. Second, organizational change: a drug discovery project requires at least 10 years from concept to the market and it is increasingly difficult for a research project or strategy to bear fruit before it is abandoned for nontechnical reasons in industry.5 With every merger or change in leadership, many projects face the political realities of loss of sponsorship and reprioritization irrespective of scientific rationale. Third, loss of expertise: the general instability in the industry has resulted in disease-specific therapeutic groups changing their fundamental focus every 3-4 years, with many scientists departing the industry altogether. It is impossible to endure this degree of disruption and maintain the expertise required for insightful and innovative drug discovery. As a result of these conditions, there is a growing trend for large pharmaceutical companies to outsource and externalize the early phases of drug discovery.
In this context, there is a clear societal need for enhanced stability, innovation, and productivity in oncology drug discovery in order for advances in basic research to result in new medicines. Academia is a critical area where innovation in drug discovery can flourish.6,7 Indeed, a recent review provided strong support for the role of academics and biotechnology companies (often spawned from academia) in contributing key intellectual property to 56% of the new small-molecule and biologic drugs judged by the FDA to warrant priority review in the period spanning 1998 to 2007.3 The research culture of a university, which is based upon fostering innovation and nurturing new ideas, is fertile ground for the hypothesis generation, revision and exploration that characterize early drug discovery. Academic health centers are uniquely poised to bring together basic scientists, medicinal chemists, clinicians, and patients to identify new targets and discover new medicines that are most likely to be relevant to improving patient care. The future of drug discovery must look more diverse, more flexible, more tailored to patient needs, and more efficient than the model of the past 50 years.
Based on the factors discussed above, I joined the faculty of the University of North Carolina at Chapel Hill (UNC) in 2007 after 20 years in the pharmaceutical industry to establish the Center for Integrative Chemical Biology and Drug Discovery (CICBDD). My vision for the CICBDD was 2-fold: to bring dedicated medicinal chemistry expertise to bear on biological targets of therapeutic relevance under investigation by UNC faculty and to initiate a chemical biology effort focused on chromatin regulation to pioneer a new area for chemical probe discovery and target validation. Synthetic chemists and assay development and compound profiling scientists work in the Center and create dedicated, multidisciplinary project teams with other groups on campus to progress targets through the drug discovery process. The Center also collaborates extensively in the area of chromatin regulation and has especially benefited from our relationship with the Structural Genomics Consortium (http://www.thesgc.org).
Target selection
The central paradigm of modern drug discovery is the selection of molecular targets the function of which can be modulated to influence a disease phenotype. Research in academic institutions continues to provide the scientific understanding of biology that underpins this process for the identification of targets. Academia excels at this basic research because the fundamental product is new knowledge and the likelihood that a specific research project will lead to a novel, important, and valid target (ie, one ultimately proven to be a useful disease intervention point) is so small that industry, where the product must be a profitable drug, will not take this risk. Many pathways and targets must be explored to identify a few that are appropriate for drug discovery and even then many targets will not ultimately lead to a successful medicine.2-4 For example, therapies resulting from exploitation of the protein kinase target class represent a culmination of the pre-genome-sequencing era of oncogene biology and drug discovery.8 This successful paradigm is based on a gradual progression over 20 years from understanding the role of kinases as drivers of proliferation and survival (research done mostly in academia) to their establishment as druggable targets (research done mostly in industry) with clinically useful levels of efficacy and selectivity. This foundation has rendered any new potential kinase target in cancer readily accessible for drug discovery with proven technologies. Focusing some academic effort on novel members of tractable target classes is entirely appropriate to ensure timely clinical impact. In addition, there are many kinases with connections to cancer that have not been pharmacologically explored.9 Our efforts toward the discovery of mer kinase inhibitors to treat childhood acute lymphoblastic leukemia (ALL) will be briefly summarized to illustrate academic drug discovery for this target class.
In the postgenomic era, tumor genome sequencing efforts such as the Cancer Genome Atlas (TCGA) are cataloguing genetic events that cause or sustain human cancers and have identified some novel kinase targets for therapy (eg, B-RAF-vemurafinib and Alk-crizotinib). However, such efforts have also shown epigenetic phenomena to be critical for tumor initiation and maintenance.10 Outside of the histone deacetylase area, chromatin modifiers represent a relatively underexplored area for drug discovery and few potent and selective small-molecule ligands for these targets exist.11 Our efforts to discover chemical probes for methyl-lysine readers will be outlined to depict how chemical biology can be used to advance our understanding of target tractability before addressing drug discovery for a novel protein family.12
Mer kinase inhibitors
The Mer receptor tyrosine kinase was originally discovered in the Earp laboratory (University of North Carolina Lineberger Comprehensive Cancer Center) by Doug Graham, now a pediatric oncologist at the University of Colorado, during his doctoral studies.13 The Earp and Graham laboratories have actively collaborated with the CICBDD in the initiation and progression of our drug discovery efforts toward Mer kinase.14,15
Previous studies have indicated a role for the Mer receptor tyrosine kinase (RTK) in ALL. Mer is ectopically expressed in at least 50% of pediatric T-cell ALL samples, as well as in pre-B-ALL samples, particularly those with a (1;19)(q23;p13) translocation encoding an E2A/PBX1 fusion protein.15-17 In contrast, Mer is not expressed in normal mouse and human T and B lymphocytes at any stage of development. In previous studies, transgenic expression of Mer in mouse lymphocytes resulted in the development of T-cell leukemias and lymphomas. In addition, a Mer-expressing human T-ALL cell line was able to produce a lethal malignancy in immunocompromised mice; the development of leukemia was significantly delayed or completely eliminated by Mer shRNA knock-down in these cells. Mer also contributes to lymphoblast chemoresistance. For example, Mer-transgenic lymphocytes have a statistically significant survival advantage relative to wild-type lymphocytes when treated with dexamethasone, a critical component of ALL induction chemotherapy. In human ALL cell lines, Mer expression also mediates resistance to other commonly used ALL chemotherapeutics (vincristine, 6-mercaptopurine, methotrexate, and doxorubicin). These data provide a rationale for the development of Mer kinase inhibitors as selective therapeutics for ALL and other Mer-related diseases.18 In addition, these inhibitors may mediate synergistic antileukemia activity in combination with standard chemotherapy, thereby permitting dose reduction and reducing toxic side effects.19
Mer is a member of the TAM (Tyro3, Axl, Mer) RTK family. Each member of the TAM family contains an extracellular domain, a transmembrane domain, and a conserved intracellular kinase domain.14 Compared with other tyrosine kinases, the intracellular kinase domain of the TAM family is quite dissimilar (the average sequence identity of the Mer kinase domain to other RTK families is 40%). This makes Mer an excellent candidate to be targeted selectively by small molecules.
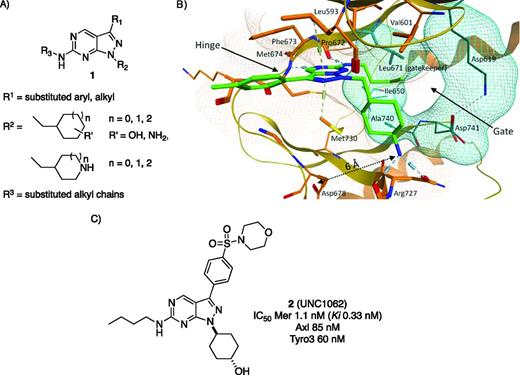
Based on the crystal structure of a weak inhibitor with Mer,20 we proposed the synthesis of pyrazolopyrimidine heterocycles (Figure 1A, compound 1) as potential inhibitors.21 The structural features of 1 also provided at least 3 sites (R1, R2, and R3) for facile synthetic modification. The substituents at the R1, R2, and R3 positions were initially chosen on the basis of their chemical features and the existing x-ray cocrystal data.18 For example, polar substituents of various sizes at the R2 substitution site were expected to form an ionic bond and/or hydrogen bond with Asp678. At the R1 and R3 positions, a variety of groups were tested to explore structure-activity relationships (SARs) driving potency, cellular permeability, solubility, and pharmacokinetics. Iterative cycles of compound design and evaluation led to Mer inhibitors such as UNC56921 and UNC1062 (Figure 1B, x-ray cocrystal structure of UNC569 and Mer kinase; Figure 1C, compound 2, UNC1062), with the latter possessing excellent mer-inhibitory properties in colony formation assays with ALL, rhabdoid, and melanoma cell lines.18,22 Although UNC569 is a potent inhibitor of Mer kinase with good pharmacokinetic properties in mice,21 further profiling for potential pharmacological liabilities revealed that it had undesirable activity (EC50 = 1.7 μM) at the human ether-a-go-go–related potassium ion channel (hERG).22 hERG, distinct from the ETS transcription factor ERG, is a well understood “antitarget” for medicinal chemists because it is frequently bound by lipophilic compounds that have a basic amine.23 Our knowledge of the types of structures likely to interact with hERG led us to profile for this effect early in the optimization process. Further rounds of synthesis and profiling eventually led to UNC1062 and related compounds that are much less active at the hERG channel while also gaining potency in cellular Mer assays compared with UNC569.22 The iterative process of multiobjective compound optimization is familiar ground to experienced medicinal chemists, but can prove daunting to academic groups less accustomed to following multiple SAR trends across diverse assays. An essential understanding in this process is that both the compounds and the assays undergo mutual optimization until a relevant assay is developed that can accurately reflect the activity of a quality compound; at the beginning of the process, neither assay nor compound is necessarily optimal. The general impression that optimized compounds drop right out of screening exercises is a very significant limitation to many academic drug discovery efforts that are not psychologically or financially prepared for the multiyear process of hit to lead to candidate progression. The only way to overcome this barrier is through experience. In the CICBDD, this experience was largely gained using a few medicinal chemists with many years of industrial experience.
Mer kinase inhibitor design. (A) General structure of pyrazolopyrimidine template designed for Mer kinase inhibition. R1, R2, and R3 represent substituents that can be synthetically varied to optimize the biological properties of this template. (B) X-ray cocrystal structure (PDB 3TCP) of UNC569 and the Mer kinase catalytic domain illustrating the binding mode and location of R1, R2, and R3 in the enzyme active site. (C) Structure and inhibitory properties of UNC1062 versus the TAM family kinases.
Mer kinase inhibitor design. (A) General structure of pyrazolopyrimidine template designed for Mer kinase inhibition. R1, R2, and R3 represent substituents that can be synthetically varied to optimize the biological properties of this template. (B) X-ray cocrystal structure (PDB 3TCP) of UNC569 and the Mer kinase catalytic domain illustrating the binding mode and location of R1, R2, and R3 in the enzyme active site. (C) Structure and inhibitory properties of UNC1062 versus the TAM family kinases.
Thanks to significant funding through the National Cancer Institute's chemical biology consortium (http://dctd.cancer.gov/CurrentResearch/ChemicalBioConsortium.htm) and the excellent collaboration between the CICBDD and the Earp and Graham laboratories, the Mer project has progressed to the clinical candidate selection stage. We are focused on comparative mouse efficacy studies with several compounds structurally related to UNC1062 (Figure 1, compound 2) possessing improved solubility, excellent pharmacokinetic properties, and limited hERG liabilities. The ability to explore both compound SAR and assay optimization while working directly with the discoverers of the target who also treat ALL patients exemplifies what can go right with academic drug discovery: a combination of expertise and motivation that maintains momentum when the inevitable scientific hurdles arise.
Methyl-lysine “reader” inhibition
In addition to drug discovery projects such as Mer kinase, the CICBDD has a program directed toward the chemical biology of chromatin regulation.24,25 This focus requires exploration of novel biochemical target classes and ligand design, making this an ideal training ground for students and postdoctoral fellows.
Chromatin represents the complex of histone proteins, DNA, and RNA that efficiently packages the genome in an appropriately accessible state within each cell.26 The state of chromatin, and therefore access to the genetic code, is largely regulated by specific chemical modifications to histone proteins and DNA, as well as the recognition of these marks by other proteins and protein complexes. The chemical modification of chromatin is carried out by families of enzymes that can both “write” (ie, create a posttranslational modification [PTM]) and “erase” (ie, chemically remove) such PTMs.10 These enzymes include druggable targets such as protein kinases and histone deacetylases, and there is also much recent excitement in the area of inhibitor discovery for protein lysine methyl transferases, which is the focus of the Jin laboratory within the CICBDD.27-30 Although enzymes are frequently favored as targets for drug discovery because of the ligand design information inherent in the chemical transformations they perform and the precedent for medicinal chemistry success, these chromatin-modifying enzymes also frequently create a binding site for the recruitment of other proteins.12,31 Targeting the readers of chromatin PTMs represents a novel emerging area of drug discovery focus32-34 that may prove useful in modulating both chromatin state and the activity of epigenetic writers and erasers, which also frequently read existing PTMs to recognize their substrates.35
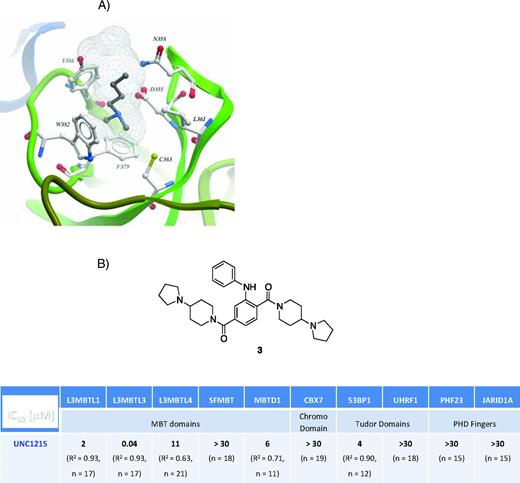
There are more than 200 methyl-lysine (Kme) reader domains described within several protein families: PHDs; the so-called “royal family” made up of the Tudor, Agenet, chromo, PWWP, and MBT domains, and the WD40 repeat proteins consisting of WDR5 and EED.34 Lysine methylation is a subtle modification that preserves the positive charge on lysine and, remarkably, the addition of a single methyl group to a histone tail can have profound effects on gene expression. A unifying feature of these domains is the existence of an aromatic cage that comprises the Kme-binding pocket, facilitating recognition of the methyl-ammonium group via cation–π, hydrogen bond, and van der Waals interactions (Figure 2A).36,37 The cation–π interaction is a noncovalent association between the face of an electron-rich π system (eg, phenyl-alanine, tryptophan, tyrosine) and an adjacent cation (eg, R-NH3-0Me0-3+). This interaction is an example of noncovalent bonding between a monopole (cation) and a quadrupole (π system). Cation–π interaction energies are significant, with solution phase values on the same order of magnitude as hydrogen bonds.38
Inhibitors of methyl-lysine binding proteins. (A) The methyl-lysine-binding pocket of L3MBTL1 illustrating the aromatic cage that surrounds the dimethyl-lysine moiety in the H4K20me2 cocrystal structure (Valérie Campagna-Slater, SGC, PDB 2PQW). (B) Structure and inhibitory properties of UNC1215 versus a panel of methyl-lysine reader domains.
Inhibitors of methyl-lysine binding proteins. (A) The methyl-lysine-binding pocket of L3MBTL1 illustrating the aromatic cage that surrounds the dimethyl-lysine moiety in the H4K20me2 cocrystal structure (Valérie Campagna-Slater, SGC, PDB 2PQW). (B) Structure and inhibitory properties of UNC1215 versus a panel of methyl-lysine reader domains.
Because the recognition of methyl marks is a cornerstone of chromatin regulation, Kme readers are also of interest as potential therapeutic targets.31 For example, Wang et al reported that fusing a histone 3, lysine 4 trimethyl (H3K4me3)-binding PHD finger such as the carboxy-terminal PHD finger of PHF23 or JARID1A to a common fusion partner nucleoporin-98 (NUP98), as also identified in sequencing of human leukemias, generated potent oncoproteins that arrested hematopoietic differentiation and induced acute myeloid leukemia in murine models.39 In these processes, a PHD finger that binds H3K4me3/2 marks was essential for leukemogenesis. Mutations in PHD fingers that abrogated H3K4me3 binding also abolished leukemic transformation, supporting the hypothesis that small-molecule inhibitors of methyl-lysine binding domains might be useful therapeutics.
The unique recognition mode of Kme reader proteins and the lack of known high-affinity ligands prompted us to undertake a target class approach toward the parallel discovery of chemical probes for members of this family. In this approach, all synthetic Kme mimics, designed by using the abundant structural biology information for Kme readers interacting with histone peptides,36 are biochemically screened against a panel of proteins from the MBT domain family and other representative Kme readers. By maximizing the breadth of our Kme reader assay panel, the chances of finding potency and selectivity enhancing features in synthetic ligands is increased via testing each ligand hypothesis against a large number of functionally homologous, but structurally distinct binding sites. This is in contrast to a typical disease-oriented approach (such as our Mer kinase project outlined above) in that chemical tractability is the initial hypothesis to be tested for Kme readers. Our panel is also biased to include Kme reader proteins that have been linked to specific disease states,39 increasing the chances that a potent chemical probe could lead to target validation data that would encourage a drug discovery effort. Thus far, this method of structure-based ligand discovery has led to UNC1215 (Figure 2B, compound 3), a first-in-class chemical probe for the Kme1,2 reading function of L3MBTL3.40 Although the biology of this chromatin reader, L3MBTL3, is beyond the scope of this article, we believe that UNC1215 will contribute to the understanding of the role of L3MBTL3 in cellular differentiation and disease. Our approach of freely sharing chromatin-directed chemical probes with collaborators and the scientific community at large with no requirement for an material transfer agreement or intellectual property constraints should greatly accelerate biological understanding and is the type of precompetitive approach appropriate to this portion of our research (http://www.thesgc.org/scientists/chemical_probes/UNC1215).41-44
Conclusions
If progress in the treatment of cancer is to continue, the innovation, expertise, and commitment of academic biomedical science must be brought to bear more frequently and directly on the discovery of new medicines. Fortunately, there are increasing efforts to embed drug discovery expertise within academic units to drive this progress (http://addconsortium.org/addc/). The lessons I've learned since joining UNC include: (1) select collaborators who are devoted to science and their target hypothesis, willing to learn, and fun to work with; (2) understand academic values and be prepared to meet academic expectations for publication and funding productivity; (3) be patient with collaborators—we all juggle multiple priorities (see #2); and (4) “draw a bigger circle”: collaborate openly and share your knowledge and compounds freely. Drug discovery is a team sport and requires trust, humility, and a willingness to fail. Drug discovery also requires deep expertise in both biology and chemistry, and institutions that successfully create a culture where these disciplines can succeed while working together will have an exciting future. I do not believe that academic institutions will contribute by designing more efficient scientific or business processes; rather, the contribution of academic research should be to explore diverse approaches and high-risk targets and pioneer the understanding of therapeutic relevance with small molecules. Although this effort will undoubtedly lead directly to new medicines, it will more broadly define the tractable frontier for industrial drug discovery in which a focus on process efficiency is both appropriate and achievable.
Acknowledgments
The author acknowledges the University of North Carolina and University of Colorado Mer project team members whose individual names appear in the cited publications. The Mer kinase work discussed was supported by the University of North Carolina Cancer Research Fund and Federal Funds from the National Cancer institute, National Institute of Health, under Contract No. HHSN261200800001E. The methyl-lysine antagonist work research described was supported by the National Institute of General Medical Sciences, the National Institutes of Health (grants RC1GM090732 and R01GM100919), and the Carolina Partnership and the University Cancer Research Fund of the University of North Carolina at Chapel Hill. The Structural Genomics Consortium is also acknowledged for their contributions to the methyl-lysine antagonist project.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for, has received research funding and honoraria from, has consulted for, and has equity ownership in Biotech and Pharma. Off-label drug use: None disclosed.
Correspondence
Stephen V. Frye, UNC Eshelman School of Pharmacy, 2095 Genetics Medicine Bldg, 120 Mason Farm Rd, Box 7363, Chapel Hill, NC 27599-7363; Phone: 919-843-5486; Fax: 919-843-8465; e-mail: svfrye@email.unc.edu.