Abstract
Despite the use of less toxic chemotherapy and more limited doses and fields of radiation, the prognosis for patients with all stages of classical Hodgkin lymphoma (HL) has continued to improve over the last 20 years. The challenge today is better identification of prognostic markers that will allow even further reduction of therapy in the most favorable subsets and new approaches for those who have a high risk of failure with current approaches. Most ongoing clinical trials for newly diagnosed HL base therapy decisions on the result of an interim restaging PET/CT, de-escalating for early responders and escalating for patients with a suboptimal response. While awaiting the results of these important trials, the debates rage on regarding the use of consolidative radiotherapy in early stage HL and the use of escalated BEACOPP in advanced stage disease. Unfortunately, we still face the very difficult decision with nearly every patient with HL of “too much,” risking long-term consequences, or “too little,” risking relapse and the need for additional toxic therapy. At present, we need to make these very difficult initial treatment decisions with inadequate data, but reassured by the excellent outcomes for most patients and encouraged by the new agents available for those who fail first-line therapy.
Despite the overall excellent prognosis of the majority of patients with classical Hodgkin lymphoma (cHL), the optimal treatment for both early- and advanced-stage disease remains controversial. Until long-term survival and toxicity data are available from randomized trials conducted and reported in the last decade, the ideal management of cHL will remain uncertain. Successful treatment of cHL must balance the highest cure rate with primary therapy and the fewest treatment-related complications. Due to the unique efficacy of salvage therapy for cHL, a slightly less effective, but less toxic approach as initial therapy, may ultimately optimize survivorship. Clinical trials aimed at tailoring therapy based on results of an interim PET/CT (positron emission tomography/computed tomography) scan performed after 1 to 3 cycles of chemotherapy are in progress around the world, and participation in these studies probably represents the optimal approach for most patients. If current trials confirm the usefulness of midtreatment PET/CT assessments, perhaps we can resolve the debate regarding “too much” or “too little” therapy, even before the gold standard long-term survival and toxicity data become available. Importantly, the approaches discussed herein for cHL are not applicable or appropriate for nodular lymphocyte predominant HL. Current therapy recommendations for nodular lymphocyte predominant HL are not addressed in this review.
Early-Stage cHL
In early-stage disease, arguments regarding the optimal intensity of treatment focus primarily on the role of consolidative radiotherapy (RT) and to a lesser extent on the details of the chemotherapy regimen. Despite imperfect data, we must make an effort to answer the following questions for patients with early-stage cHL:
Is chemotherapy alone adequate therapy?
Should unfavorable and favorable subsets be approached differently?
What is the role of interim PET outside the setting of a clinical trial?
Is Chemotherapy Alone Adequate?
Despite outstanding 5- and 10-year event-free survival (EFS) and overall survival (OS) rates of 90% to 99% with combined modality therapy in early-stage disease, many physicians continue to have reservations about this approach due to concerns of serious late toxicities related primarily to RT.1–5 In patients treated for cHL, RT increases the risk of second cancers, cardiovascular disease, and stroke by 2- to 7-fold.6–9 Although most of the data on late effects are based on patients treated with “outdated” chemotherapy and radiation approaches, it is difficult to ignore this disturbing data and simply wait for 25-year follow-up of studies using lower doses and smaller fields of RT. In a study by Hodgson et al,6 second cancers were evaluated among 32,591 Hodgkin disease (HD) patients reported in 16 population-based cancer registries in North America from 1935 to 1994. The relative risk of cancers was 2.3, with an actuarial risk of 22% at 25 years. Importantly, after 14 years of follow-up, there was no difference in the incidence of solid tumors in patients treated between 1965 and 1979 and from 1980 to 1994, despite the likelihood that patients received more limited doses and fields in the second era. Brusamolino et al10 reported the 15-year follow-up of 120 patients with nonbulky stage I/II cHL treated with four cycles of ABVD (adriamycin [doxorubicin], bleomycin, vinblastine, and dacarbazine) and 30 to 36 Gy involved-field RT (IFRT). The 15-year EFS and OS rates were 78% and 86%, respectively, with a tumor mortality of 3%. Cause of death in nine patients included progressive cHL (n = 3), second cancers (n = 4), cardiovascular (n = 2), suicide (n = 1), and car accident (n = 1). In a preliminary report of the German Hodgkin Study Group (GHSG) HD10 and HD11 trials, which compared limited chemotherapy combined with 20 or 30 Gy IFRT in early-stage cHL, there was no difference in the incidence of second malignancies or deaths due to second cancers at a median follow-up of 7 years.4,5 Encouragingly, DeBruin et al11 recently reported a significant reduction in the incidence of second breast cancers in women treated with mediastinal RT, compared with mantle RT for cHL and a meta-analysis of cHL trials by Franklin et al12 showed a relative risk of second cancers of 3, comparing extended field to IFRT. Newer techniques, including involved nodal RT and intensity-modulated RT are currently under investigation as ways to reduce RT field and protect normal tissues. However, efficacy and toxicity of these approaches will not be known for at least a decade.13,14
With the aim of eliminating the late effects of RT, many advocate chemotherapy alone for early-stage disease. As shown in several randomized trials (Table 1), this approach likely results in an absolute increase in the failure rate of about 8%.15–19 However, with an intermediate duration of follow-up of 4 to 5 years, no trial comparing chemotherapy alone to combined modality therapy (CMT) in early-stage disease has ever shown a survival difference, likely because second-line strategies, often including RT, result in durable disease control in most patients with relapsed disease.20,21 Most pertinent in the modern era are chemotherapy only trials utilizing ABVD, which show 4- to 8-year EFS rates ranging from 81% to 94% (Table 2). Importantly, these results may not reflect the optimal outcome of this approach in favorable or selected subgroups of early-stage cHL, because eligibility criteria for these studies were broad. In addition, during the time these trials were conducted, dose delays and reductions were common with ABVD, compromising dose intensity. Based on retrospective studies, current recommendations favor administration of full-dose ABVD on schedule regardless of the neutrophil count.22,23 Maintaining the dose intensity of ABVD may prove to be most important in the setting where consolidative RT is not administered, but this is speculative.
Randomized trials of chemotherapy vs. combined modality therapy in early stage cHL
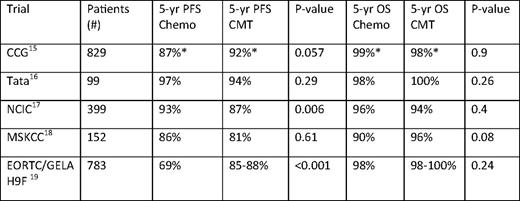
Chemo indicates chemotherapy; CCG, Children's Cancer Group; NCIC, National Cancer Institute of Cancer; MSKCC, Memorial Sloan Kettering Cancer Center; GELA, Groupe d'Etude des Lymphomes de l'Adulte.
*Three-year results
Results of ABVD chemotherapy in non-bulky stage I-II cHL
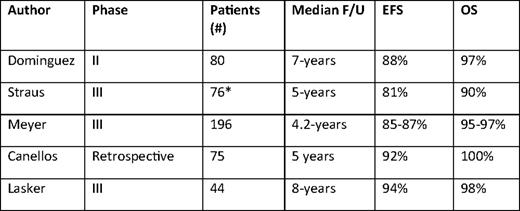
F/U indicates follow-up.
*Includes 11 patients with stage IIIA disease.
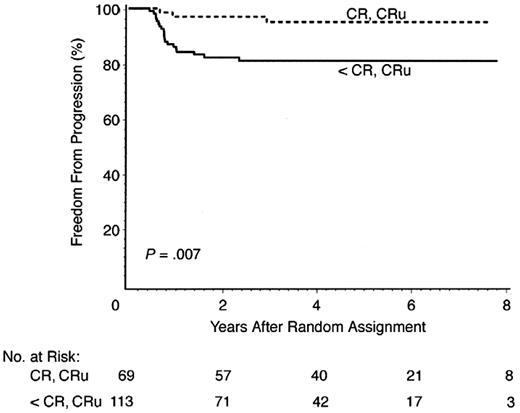
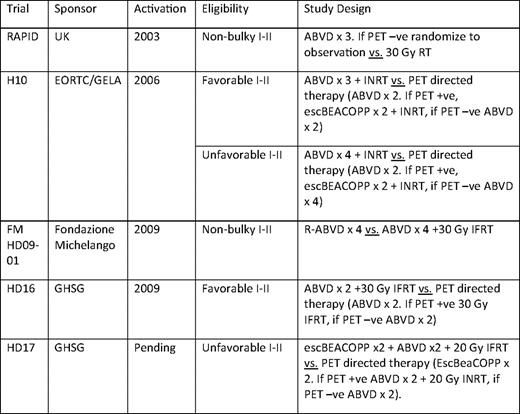
The optimum number of cycles of chemotherapy is not defined for patients receiving chemotherapy alone. With the exception of the National Cancer Institute of Canada (NCIC)/Eastern Cooperative Oncology Group (ECOG) study, all reported series of ABVD alone administered six cycles.17 In the NCIC/ECOG study, 69 of 196 (35%) patients treated with ABVD alone achieved a complete response (CR) by CT criteria following two cycles of ABVD. Of these 69 CR patients, 57 received a total of four cycles of ABVD (per protocol) and 12 received six cycles (physician decision), with a 5-year freedom from disease progression of 95%, compared with 81% for the 113 patients not in radiographic CR after two cycles (Figure 1). In the small subset of patients with a CR by CT following two cycles of ABVD, a total of four cycles appears adequate. As discussed below, it is probable, but not proven, that these results with limited chemotherapy will also apply to the much larger percentage of early-stage patients who achieve an early metabolic CR by PET/CT, as opposed to the anatomic CR in the NCIC/ECOG study. Five phase III trials designed to answer the question of the effectiveness of chemotherapy alone compared with CMT in nonbulky cHL are ongoing or near activation in Europe (Table 3). As described in more detail, four of the five trials include PET-directed therapy, with three to four cycles of ABVD prescribed in the chemotherapy-only arms. Efforts to minimize the number of cycles of ABVD are relevant due to reported short- and long-term cardiopulmonary toxicities associated with bleomycin and doxorubicin.7,8 Limiting the toxicity of ABVD by entirely eliminating B, V, or D from the regimen has been tested by the GHSG in the setting of combined modality therapy, but preliminary results have not been published. These results will not be directly applicable to patients treated with chemotherapy alone, because RT may preserve effectiveness when chemotherapy agents are removed from the regimen.
Freedom from progression for stage I/II patients achieving CR versus less than CR after two cycles ABVD in NCIC/EORTC Trial. Modified from Meyer et al.17
Freedom from progression for stage I/II patients achieving CR versus less than CR after two cycles ABVD in NCIC/EORTC Trial. Modified from Meyer et al.17
Unfavorable Versus Favorable Subsets
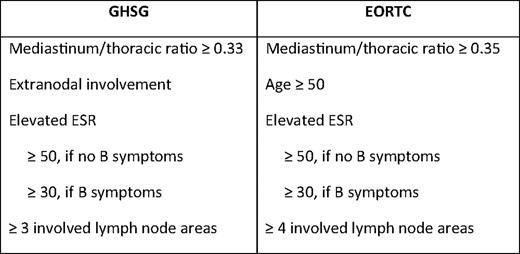
Use of prognostic factors in early-stage cHL is much less uniform than in advanced-stage disease. Studies and recommendations in the United States and the United Kingdom often distinguish between nonbulky and bulky early-stage disease, as defined by a mediastinum/thoracic ratio ≥ one-third or a single mass ≥ 10 cm, but do not utilize other risk factors. Both the GHSG and the European Organization for Research and Treatment of Cancer (EORTC) define distinct subsets as favorable or unfavorable, with patients having any high-risk features as defined in Table 4 assigned to more intensive treatment approaches. As new treatment approaches evolve, which minimize chemotherapy or RT, it will be important to determine if less aggressive approaches apply to all patients with early-stage disease or only those with the most favorable features. In a subset analysis of the NCIC/ECOG trial comparing ABVD to RT (plus ABVD for unfavorable patients), there was no difference in 5-year EFS (87% vs 85%) or OS (97% vs 95%) for favorable versus unfavorable patients treated with chemotherapy only.17 In the NCIC study, unfavorable was defined as age ≥ 40 years, erythrocyte sedimentation rate (ESR) ≥ 50, mixed cellularity or lymphocyte depleted histology, or more than four nodal sites. Patients with bulky disease were excluded. Both the GHSG HD10 (favorable) and HD 11 (unfavorable) trials for early-stage cHL, included ABVD × 4 + 20 Gy IFRT and ABVD × 4 + 30 Gy IFRT arms. In contrast to the NCIC/ECOG study, 20% of patients in the unfavorable group had bulky mediastinal disease. The 5-year freedom from treatment failure (FFTF) for favorable early-stage patients treated with ABVD × two or four cycles plus 20 or 30 Gy IFRT was equivalent in all four arms at 93%.4 In contrast, in the DH11 trial for unfavorable patients, the 5-year FFTF was 86% for ABVD × 4 + 30 Gy and 81% for ABVD × 4 + 20 Gy.5 Although not a direct comparison of unfavorable and favorable subsets, results from GHSG HD10 and HD11 suggest that, with similar approaches, patients with high-risk features have a modestly worse outcome compared with the most favorable patients. Importantly, as efforts are made to reduce therapy by decreasing cycles of chemotherapy, eliminating drugs, or limiting doses and fields of RT, the significance of prognostic factors will likely increase. Based on current data, treatment with ABVD × 2 + 20 Gy RT is only applicable to the most favorable subset of early-stage cHL patients and cannot be generalized to all early-stage patients receiving CMT. Similarly, in patients with any high-risk features, ABVD × 4 + 20 Gy IFRT is inferior to ABVD × 4 + 30 Gy RT (P = .05).5 The most recent GHSG trial for unfavorable cHL, HD14, showed a significant 3-year FFTF advantage to escalated (esc) BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) × 2 + ABVD × 2 + 30 Gy IFRT (97%), compared with ABVD × 4 + 30 Gy IFRT (91%).24 In the HD11 and HD14 trials, patients with one to two high-risk features did better than patients with three or more risk factors; however, there was still a slight advantage to escBEACOPP × 2 + ABVD × 2, compared with ABVD × 4 (P. Borchmann, oral communication, May 2010). Whether escBEACOPP represents “too much” therapy for early-stage patients requires longer follow-up. As described later, perhaps the use of interim PET will improve on our ability to tailor therapy based on early response to therapy instead of factors present at diagnosis. Again, when applying study results to practice, care should be taken to ensure that patients' risk factors reflect those of the study population.
The Role of Interim PET
Based on compelling data from Gallamini et al25 showing a 2-year progression-free survival (PFS) of 12% for cHL patients with a positive PET scan after two cycles of ABVD, compared with 95% for patients with a negative interim PET, the use of interim PET has become widespread in standard practice and is the focus of most current clinical trials in both early and advanced cHL. The Gallamini report included 210 patients, 70 of whom had stage II disease, and implied, but not explicitly stated in that report, is that the dramatic predictive value of a positive interim PET applies to patients with all stages. However, early data by this same group suggested that patients with stage I or stage II cHL who had a positive PET after two cycles of ABVD, still had an approximately 75% chance of durable remission with additional ABVD chemotherapy and consolidative IFRT.26,27 Investigators at Dana Farber Cancer Institute also reported very encouraging results for 73 patients with cHL referred for consolidative RT, including 20/43 patients with a positive interim PET scan and 13/73 patients with a positive PET scan at completion of chemotherapy. The majority of patients (86%) had stage I or stage II disease. Following IFRT, the 2-year failure-free survival (FFS) was 85% for patients with a positive interim PET and 69% versus 95% for patients with a positive versus negative PET at the completion of all chemotherapy, implying the majority of early-stage PET+ patients can be cured with consolidative RT.28
Whether a PET-directed approach will allow us to safely minimize chemotherapy cycles and avoid RT is the subject of several ongoing large randomized trials. The treatment arms of these studies are listed in Table 3. As these trials complete accrual and results mature, we hope to have a definitive answer as to whether three to three to four cycles of ABVD alone will be sufficient therapy for the majority of patients with early-stage cHL and whether ABVD + RT or alternatively escBEACOPP + RT will be sufficient therapy for patients with less than a complete metabolic response after two cycles of ABVD. Preliminary results of the UK RAPID (Randomized Trial of Accelerated Partial Breast Irradiation) trial were presented after 369 patients of a planned 525 patients were accrued.29 Eligibility for the RAPID trial included nonbulky cHL without regard to other risk factors. All patients received three cycles of ABVD and were restaged with a PET/CT scan. Patients with a negative PET were then randomized to receive IFRT versus observation. In the initial report of the study, 79% of patients had a negative PET scan following three cycles of ABVD. With a median follow-up of 13 months, 96% of patients (245/255) are alive and progression-free, 6 patients progressed, 1 patient died of cHL, 1 patient died from treatment-related toxicity, and there were 2 unrelated deaths. This study is powered to detect a 7% difference in EFS between the observation and IFRT arm. The outstanding early overall results are quite encouraging and support the concept that therapy can be safely minimized in a majority of patients.
Optimal Approach to Therapy in Early-Stage Disease
Until the question of the utility of interim PET is confirmed, participation in one of the clinical trials designed to answer this question remains the optimal therapy for early-stage patients. Outside the setting of a clinical trial, the following options should be considered.
Nonbulky stage I/II with favorable features (young age, low ESR, and limited nodal sites).
o ABVD × 2 + 20 Gy IFRT.
o ABVD × 2 followed by an interim PET/CT.
If PET/CT negative, two to four additional cycles of ABVD.
If PET/CT positive, two additional cycles of ABVD + IFRT or alternate chemotherapy + IFRT.
Nonbulky stage I/II with unfavorable features (age > 40–50, elevated ESR, more than two to three nodal sites).
o ABVD × 4 + 30 Gy IFRT.
o ABVD × 2 followed by an interim PET/CT.
If PET/CT negative, two to four additional cycles of ABVD.
If PET/CT positive, two additional cycles of ABVD + IFRT or alternate chemotherapy + IFRT.
Bulky stage I/II.
o ABVD × 4–6 + 30 Gy IFRT.
Advanced-Stage cHL
Controversy continues to surround the choice of optimal first-line therapy for advanced-stage cHL. Initially reported in 2003, a large randomized trial comparing escBEACOPP to COPP (cyclophosphamide, vincristine, procarbazine, and prednisone)/ABVD showed that escBEACOPP resulted in better tumor control and OS.30 Subset analysis, according to the International Prognostic Score (IPS), showed improved 5-year FFTF and OS in all risk groups, with the most significant survival differences in the highest risk patients. Ten-year follow-up of the trial was recently published with FFTF and OS rates of 64% and 75% for COPP/ABVD, compared with 82% and 86% for escBEACOPP (P < .0001).31 The OS advantage of escBEACOPP was only significant in the IPS 2–3 group. When analyzed by age, there was not a significant difference in FFTF or OS for patients age 60 to 65, and escBEACOPP is not recommended in patients older than age 60 secondary to unacceptable acute toxicities. Despite the improved FFTF, and perhaps even a survival advantage, physicians hesitate to recommend escBEACOPP because of toxicity concerns. Acute hematologic toxicity is high with escBEACOPP, with grade 3/4 infections occurring in 22% of patients.31 Acute myeloid leukemia (AML) developed in 3% of patients on the escBEACOPP arm. The escBEACOPP results in azospermia and infertility in the majority of male patients and induces premature menopause in 40% of women younger than age 30 and in 70% of women older than age 30.32,33 Neither AML nor infertility is associated with ABVD therapy.34,35
In two other randomized trials of escBEACOPP versus ABVD, escBEACOPP was not associated with a survival advantage, despite a lower rate of relapse.36,37 Importantly, both trials, in an effort to reduce both acute and long-term toxicity, administered a slightly less intensive BEACOPP regimen than the GHSG. In the Gruppo Italiano per lo Studio dei Linformi trial, patients received four cycles of escBEACOPP and two cycles of standard BEACOPP; in the second Italian cooperative group trial by Gianni et al,36 patients received four cycles of escBEACOPP and four cycles of standard BEACOPP. The lack of a survival difference in these trials is likely due to the better prognosis following second-line therapy for patients initially treated with ABVD, compared with escBEACOPP. In the trial by Gianni et al, 48% of the patients who failed ABVD remained in continuous CR after salvage therapy, compared with only 18% of the BEACOPP failures. Results of EORTC 20012 comparing escBEACOPP to ABVD are still pending.
Failure to embrace the escBEACOPP regimen outside of Germany is a result of physicians choosing to expose fewer numbers of patients to sterilizing and potentially leukemogenic therapy, with the tradeoff being that a higher percentage of patients will need salvage therapy, including an autologous stem cell transplant. Interestingly, autologous transplant is now associated with < 2% acute mortality in most centers and in many ways may be a more tolerable treatment than eight cycles of escBEACOPP. In addition to PET-directed therapy approaches as described below, a recent report of “dose dense, dose intense” ABVD for advanced-stage cHL resulted in a 5-year EFS of 91%, compared with 73% for historical controls treated with standard ABVD.38 ABVD was administered on days 1 and 11 of a 21-day cycle, and doxorubicin was increased from 25 to 35 mg/m2. Perhaps tweaking ABVD in this manner will improve outcomes without increasing the risks of infertility or leukemia. The increased dose intensity and density of anthracyclines with this approach raise the concern of cardiovascular toxicity and would need to be studied in a randomized trial.
The Stanford V (SV) regimen, a 12-week chemotherapy course followed by planned RT to sites of initial bulk, has been compared with ABVD in three large randomized trials. An Italian study showed inferior results with SV, but RT was not administered as initially prescribed.39 A phase III study conducted by the UK National Cancer Research Institute demonstrated no difference in the projected 5-year PFS and OS rates for SV and ABVD.40 Results of a large US intergroup trial, comparing SV and ABVD, has been reported at the 2010 annual meeting of the American Society of Hematology. Although SV uses lower cumulative doses of doxorubicin and bleomycin, RT must be used in the majority of patients, again raising concerns of long-term toxicity. Because ABVD offers similar results without the use of RT, it remains the standard of care.
PET-directed Therapy
Optimal therapy for advanced-stage patients may lie somewhere in between ABVD and escBEACOPP; and, as in early-stage disease, the interim PET/CT scan may allow us to tailor therapy more accurately than the initial IPS. As described previously, in the joint report from an Italian-Danish study evaluating the prognostic significance of early interim PET scans (PET-2), only PET-2 was significant in a multivariate analysis that included all IPS factors.25 Still under investigation is whether altering therapy on the basis of the interim PET will result in more favorable outcomes. Dann et al41 described 69 patients treated with two cycles of standard-dose BEACOPP, followed by a 67Ga or PET/CT scan. Eleven patients had a positive interim 67Ga or PET/CT scan, and therapy was intensified to escBEACOPP in 10 of 11 patients. Only 2 of 11 patients relapsed. In a retrospective review of 164 newly diagnosed, advanced-stage cHL patients treated in 2006 and 2007 at one of nine Italian or US centers, with two cycles of ABVD followed by escBEACOPP × 4 plus standard BEACOPP × 4 if an interim PET/CT scan was positive, and an additional four cycles of ABVD if PET/CT was negative, the 2-year FFS and OS were 61% and 96%, respectively, for PET+ patients, and 92% and 98% for PET– patients.42 This result is in contrast with the 2-year PFS of 12% reported by Gallamini et al25 for patients with a positive interim PET/CT who continue treatment with ABVD.
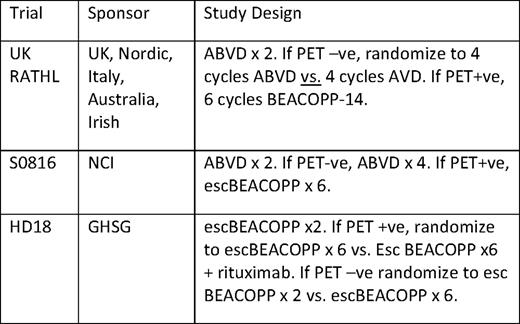
Several ongoing clinical trials in advanced-stage cHL are designed to confirm prospectively with the high negative predictive value of an interim negative scan. With approximately 20% of patients expected to have positive interim PET scans, the outcomes in this subset of patients treated with more intensive therapy will be primarily observational. A randomized trial of standard chemotherapy versus intensified therapy for PET+ patients will never be done in advanced cHL. Table 5 summarizes ongoing trials that utilize a postcycle 2 PET to make treatment decisions.
Optimal Approach to Therapy in Advanced-Stage Disease
Standard of care outside the setting of a clinical trial is six cycles of ABVD. Retrospective studies suggest that growth factors are often unnecessary despite neutropenia. Current recommendations include administering full-dose ABVD therapy on schedule regardless of neutrophil count and restricting growth factors to patients with complications associated with neutropenia. There are no data to support a better outcome with eight cycles of ABVD, and the higher cumulative dose of doxorubicin increases the long-term risk of cardiomyopathy. Hodgson et al43 recently showed a significant increase in cardiac-related hospitalizations in patients treated with ABVD alone, compared with the general population. Eight cycles of escBEACOPP is an acceptable alternative in higher risk patients (IPS 3–7). It is unclear whether fewer cycles of escBEACOPP are associated with similar results. As results from the ongoing clinical trials described in Table 5 become available, the hope and expectation are that we will be able to tailor therapy much more effectively by escalating to more toxic therapy only in the small subset of patients with an early positive interim PET and perhaps by eliminating cycles or agents such as bleomycin in those patients with a negative PET.
Conclusions
Based on statistics from the SEER (Surveillance Epidemiology and End Results) database, the prognosis for patients with cHL treated from 2000 to 2004 was significantly better than that for patients treated from 1980 to 1984.44 Importantly, during this interval, splenectomy was abandoned, RT fields and doses decreased significantly, MOPP (mustargen, oncovin, procarbazine, and prednisone)-like drugs were eliminated from first-line therapy, and results of therapy for relapsed disease—specifically stem cell transplant—improved. Although indirect evidence, these population-based findings provide credence to the idea that reducing the intensity of first-line therapy has not resulted in worse overall outcomes for patients with cHL and supports increasing efforts to minimize therapy further.
More than 90% of patients with early-stage cHL and 80% of advanced-stage patients will be cured of their cHL. With the use of PET/CT scans, it appears both chemotherapy and RT use will be able to be further reduced without compromising the long-term outcome of patients. It is no longer appropriate to continue CMT in all early-stage patients while awaiting long-term survival results. Historically, overtreatment with both chemotherapy and RT has been the rule in most patients with cHL.45 Our treatment choices must reflect our obligation to ensure patients have a future without therapy-induced sequelae.44 Hopefully, confirmation of the utility of interim PET/CT scans, the development of better markers that reliably predict outcome, and the discovery of more effective and less toxic targeted therapies will result in more personalized and optimal therapy for all patients with cHL.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests.
Off-label drug use: None disclosed.
Correspondence
Nancy L. Bartlett, MD, Professor of Medicine, 660 S. Euclid, Box 8056, St. Louis, MO 63110; Phone: (314) 362-5654; Fax: (314) 747-5123; e-mail: nbartlet@dom.wustl.edu