Abstract
Hodgkin lymphoma (HL) has become a curable malignancy for most patients during the last decades. However, many controversies still exist on the optimal strategy of how to cure our patients. The key question is how to balance the risks and toxicities of chemotherapy and radiotherapy against the need for a definite treatment for early or advanced-stage HL patients. However, although many studies have been conducted and reported during the past decade, interpretation of their results and treatment recommendations might vary significantly in different countries. For example, early-stage HL might be divided into two different subgroups: early favorable and early unfavorable or not. Treatment of early-stage HL might include radiotherapy (“combined modality”) or not. Depending on the extent of radiotherapy, the schedule and number of chemotherapy cycles are also questioned. For advanced-stage HL, the situation is not much different. Compared with ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine), the more aggressive escalated BEACOPP regimen (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone) is highly effective, but also raises concern due to excessive toxicity. Thus, there is a controversy about the standard of care for advanced HL patients. Because no mature results comparing these approaches with each other are currently available, it remains our duty to share the preliminary information with our patients and to figure out the most appropriate individual treatment strategy. Of course, the discussion of these issues is influenced by experiences and preferences. In contrast, in this article, we will try to focus on the available scientific evidence regarding the first-line treatment of HL. Of course, focusing on the last decade necessarily exclude the most recent results from ongoing studies. Thus, even though this article comprises treatment recommendations for HL patients, the best treatment certainly still is within properly designed prospective clinical trials.
Early-Stage HL
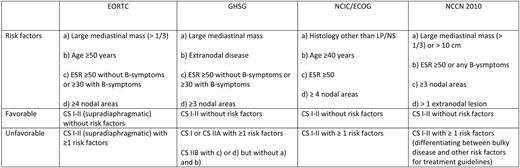
In the past, patients with limited-stage I/II disease were treated with extended- field radiotherapy (EFRT). The increasing awareness of serious long-term toxicity after EFRT promoted the development of combined modality treatment approaches. Combined modality has the evident advantage of combining two efficacious treatment modalities so that the extent of both radiotherapy (RT), as well as chemotherapy (CT), can be reduced in the combined treatment design. However, even in stage I/II, the extent of disease and the individual prognosis vary substantially. For example, mediastinal bulky disease is known to be an unfavorable prognostic factor. Other poor prognostic clinical factors include higher age, increased number of involved nodes, and elevated erythrocyte sedimentation rate, accompanied by B symptoms. Clinical stage (CS) I/II HL patients are generally divided into an early favorable and an early unfavorable (intermediate) subgroup (see Table 1). In the past, patients in North America presenting with bulky disease had been are treated like stage III/IV disease, which of course had a large impact for the patient. However, the updated National Comprehensive Cancer Network guidelines classify stage I/II patients with bulky disease as early unfavorable, and future studies might be directly comparable with European trials.
Should One Distinguish Between Early Favorable and Early Unfavorable HL?
These risk factors and the prognostic groups were originally defined in the context of treatment with EFRT, and modern combined treatment might reduce their significance. However, over the last decades, there have been > 10 early-stage randomized clinical trials (with > 9000 HL patients) using these disease subgroups. In addition, there are different outcomes seen in these trials with slightly worse outcome in unfavorable early-stage HL. Additionally, there is one large recent randomized trial that included a joint experimental treatment arm for both, favorable and unfavorable subgroups, thus possibly reflecting the current impact of predictive factors. In this trial (ie, EORTC [European Organization for Research and Treatment of Cancer] H7), the unfavorable subset of patients was randomized between six cycles of EBVP (epirubicin, bleomycin, vinblastine, and prednisone), a combination presumed to be less toxic and equally effective to ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine), and six cycles of MOPP/ABV (mechlorethamine, vincristine, procarbazine, prednisone, adriamycin, bleomycin, vinblastine, and dacarbazine), both followed by 30 to 36 Gy involved-field RT (IFRT).1 After a median follow-up of 9 years, patients treated with EBVP had a significantly higher rate of tumor progression and relapse than those treated with MOPP/ABV, thus resulting in a significantly inferior 10-year event-free survival (EFS) of 68% versus 88% (P < .001). The favorable subset of patients was randomized between six cycles of EBVP followed by IFRT and subtotal nodal irradiation (STNI), considered standard treatment at the time of initiation of the trial. Those treated with EBVP had a superior 10-year EFS, compared with patients treated with STNI alone: 88% versus 78% (P = .01). Although the less toxic EBVP regimen produced superior results in the favorable subset of patients, the poor results in the unfavorable patients reflect the necessity for a more potent and intense treatment for this subgroup. Thus, the clinical relevance of the prognostic factors appeared to be maintained. Indirect evidence supporting the presence of two different prognostic subgroups also comes from other studies.2,3 To summarize, dividing the early stages into two distinct subgroups is strongly supported by these data.
Treatment of Early Favorable HL
Treatment of early favorable HL has been investigated in large clinical trials, mainly by the EORTC/GELA (Groupe d'Etude des Lymphomes de l'Adulte) and the German Hodgkin Study Group (GHSG). The GHSG HD7 trial had shown that the addition of two cycles of ABVD to EFRT dramatically improved the freedom from treatment failure from 75% to 91% at 5 years.4 The EORTC/GELA H8-F trial showed the superiority of combined modality treatment using three cycles of MOPP/ABVD followed by IFRT over STNI.5 However, neither EFRT nor MOPP/ABVD are generally accepted as standard therapies for this patient cohort with a favorable risk profile. Therefore, the lately published HD10 study adds important information. This study compared two cycles with four cycles of ABVD; both followed either 30 or 20 Gy RT, respectively. Importantly, the weakest combination was not inferior to any other combination. Thus, two cycles of ABVD, followed by 20 Gy IFRT, are the new treatment standard for early favorable HL patients.6
Treatment of Early Unfavorable HL
The open questions for the treatment of early unfavorable HL include the number of CT cycles (four or six?), the CT regimen (is ABVD enough or even too much?), and the extent or even indication of RT.
Regarding the number of cycles, the EORTC/GELA H8-U study compared four cycles to six cycles MOPP/ABV hybrid (followed by IFRT).5 The EFS at 7 years did not differ significantly (86% and 84%, respectively). In the more recent EORTC/GELA H9-U trial, 533 patients were randomized between four cycles and six cycles of ABVD, followed by IFRT, again with no significant difference (87% and 91%, respectively, at 4 years).7 In conclusion, four cycles of ABVD in a combined modality setting is currently considered standard treatment.
In terms of CT intensity, the reduction from ABVD to presumably less toxic regimens has failed in several trials due to impaired tumor control.1,8 But, also, more aggressive regimens than ABVD with a presumably superior tumor control have been tested. However, both the EORTC H9-U and the GHSG HD11 studies failed to show a significant progression-free survival (PFS) advantage using the BEACOPP baseline regimen (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone).7,9 Since the effective dose (ED), according to the Hasenclever-score of BEACOPP baseline, is only very moderately increased compared with ABVD (15 to 15.2), the GHSG follow-up trial for early unfavorable patients, HD14, compared four cycles of ABVD with two cycles of escalated BEACOPP, followed by two cycles of ABVD (“2 + 2,” ED 17.3), followed by IFRT.3,10 The third preplanned interim analysis of this trial demonstrated a significantly better PFS for the more intensive “2 + 2” arm at 3 years (“2 + 2”: 97%; ABVD: 91%, P < .0017).11 Upfront intensification with only two cycles of escalated BEACOPP improves outcome in this group of patients; however, one can argue about clinical relevance of an absolute improvement in PFS of 6% in the face of the putative increased toxicity (eg, gonadal damage and secondary malignancies). Similar considerations should also be applied to appreciating whether the 12-week intense CT regimen, Stanford V, can improve treatment outcome—compared with ABVD—as investigated currently in the ongoing US Intergroup Study.
To summarize, four cycles of ABVD followed are still the standard CT for early unfavorable HL patients.
Concerning the role of RT, available studies still have their limitations. In the GATLA (Grupo Argentino de Tratamiento de la Leucemia Aguda) study, a nonstandard CT was used; other studies included pediatric patients or all stages of disease that used divergent definitions of unfavorable prognostic features or had not enough statistical power to detect clinically significant differences in outcome parameters between RT and non-RT arms (as summarized Table 2).12 The National Cancer Institute of Canada/Eastern Cooperative Oncology Group (ECOG) study on the early stages is probably the most relevant at present, although bulky disease as an unfavorable prognostic factor was an exclusion criterion for entry into the study: this study failed to show a survival benefit for adding RT to ABVD in the early unfavorable cohort of patients, notwithstanding a significant advantage in PFS for those who received the outdated large-field RT.13 Thus, the question on the role of RT in early unfavorable stages disease cannot yet be answered unequivocally.
Randomized clinical trials in unfavorable CS I/II disease on combined modality treatment vs. chemotherapy alone
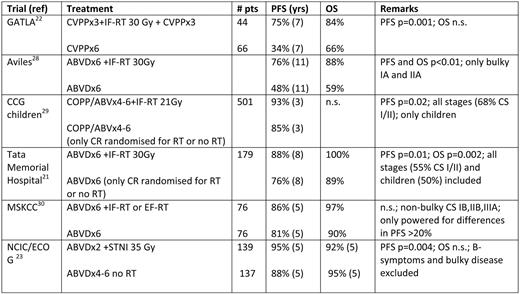
Pts indicates patients; CCG, Children's Cancer study Group; MSKCC, Memorial Sloan Kettering Cancer Center; NCIC, National Cancer Institute of Canada; n.s., statistically not significant.
In summary, there is no generally accepted evidence that RT can really be omitted in unfavorable stage I/II without jeopardizing the long-term outcome. Thus, the combined modality treatment with four cycles of ABVD, followed by IFRT, remains the preferred treatment approach for patients with unfavorable CS I/II disease.
Advanced-Stage HL
Before the introduction of combination CT, more than 95% of patients with advanced HL succumbed to their disease within 5 years. Thus, remission rates in excess of 50% achieved with MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) were a major breakthrough in oncology.14,15 MOPP was successfully introduced almost 40 years ago and used for many years for advanced-stage disease, resulting in long-term remission of nearly 50%.14,16 It was then replaced by ABVD, after a series of large multicenter trials had investigated ABVD versus alternating MOPP/ABVD or MOPP alone.16–18 Bonnadonna et al16 were the first to report on the relevance of ABVD and anthracyclines for the treatment of advanced-stage HL. This study showed an impressive benefit of ABVD in terms of efficacy when added to MOPP with an increase in OS (83.9% vs 63.9%; P < .06). Also, ABVD alone, when compared with MOPP, gave superior results favoring ABVD. Santoro et al18 investigated 3 × MOPP + RT + 3 × MOPP versus 3 × ABVD + RT + 3 × ABVD. In this trial, the 7-year results indicated that ABVD was superior to MOPP, even in terms of OS (77.4% vs 67.9%; P = .03). A US trial also tested six to eight cycles of ABVD against six to eight cycles of MOPP or MOPP alternating with ABVD for 12 cycles.19 In this trial, ABVD was equally effective and less toxic than MOPP/ABVD; thus, the use of ABVD alone as first-line therapy for advanced-stage HL was supported. Another US intergroup trial tested ABVD versus the MOPP/ABV hybrid.17 Clinically significant acute pulmonary and hematologic toxicity were more common with MOPP/ABV (P =.06 and .001, respectively). More therapy-associated fatal outcomes and more secondary malignancies were reported for the hybrid regimen (ABVD = 9, MOPP/ABV = 15, P =.057). Of 13 patients developing myelodysplastic syndrome or acute leukemia, 11 were initially treated with MOPP/ABV, and only two were treated with ABVD. Both subsequently received MOPP-containing regimens and RT before developing leukemia (P = .011).17 Therefore, it was concluded that ABVD and the MOPP/ABV hybrid are equally effective; but, due to significantly less toxicity, ABVD should be considered the standard regimen for treatment of advanced-stage HL.
Taken together, ABVD has become widely accepted as standard regimen for advanced-stage HL. A major advantage of this regimen is its tolerability. ABVD is a safe outpatient treatment without the need for close white blood cell monitoring and can be also administered in developing countries.20 Several studies have reported the feasibility of 100% dose density with the ABVD schedule regardless of the neutrophil counts. It is speculated that a higher dose density might increase the efficacy of ABVD. However, so far, only retrospective analyses or phase II studies are available, and the evidence is certainly not sufficient to draw firm conclusions regarding this issue. One has to keep in mind, though, that a long-term follow-up report of 123 patients treated with ABVD for advanced HL revealed a failure-free survival of only 47% and an OS of 59% after 14.1 years.21
Challenging ABVD
Hybrid and Alternating Regimens
To improve the outcome parameters of ABVD, it has been tested versus multiple alternating or hybrid multidrug polychemotherapy regimens, including the Stanford V regimen (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone) and the MOPP/EBV/CAD (mechlorethamine, vincristine, procarbazine, prednisone, epidoxorubicin, bleomycin, vinblastine, lomustine, doxorubicin, and vindesine) program in an Italian cooperative study, or ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisolone)/PABlOE (prednisolone, doxorubicin, bleomycin, vincristine, and etoposide) and ChlVPP/EVA (etoposide, vincristine, and doxorubicin) in a UK study (as summarized in Table 3).
“Forth generation” trials for advanced stage HL
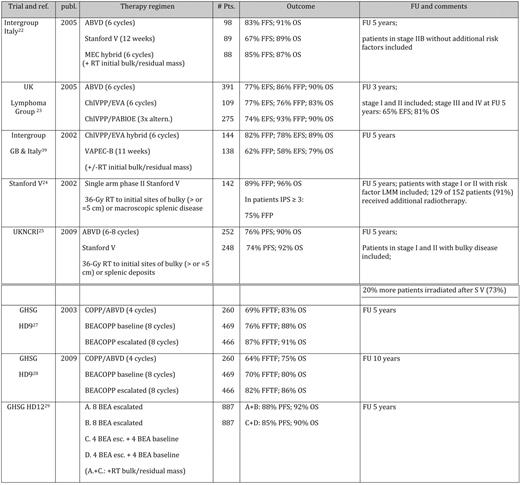
Publ. indicates publication date; pts., patients; FU, follow-up; UK, United Kingdom; UKNCRI, United Kingdom National Cancer Research Institute; MEC, MOPPEBVCAD (mechlorethamine, vincristine, procarbazine, prednisone, epidoxorubicin, bleomycin, vinblastine, lomustine, doxorubicin, and vindesine); VAPEC, doxorubicin, cyclophosphamide, etoposide, vincristine, bleomycin, and prednisolone; BEA, bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone; esc., escalated; FFS, failure-free survival; LMM, large mediastinal mass.
The Italian cooperative study tested two CT regimens (Stanford V and MOPP/EBV/CAD) against ABVD.22 The complete remission (CR) rates for ABVD, Stanford V, and MOPP/EBV/CAD were 89%, 76%, and 94%, respectively; the 5-year failure-free survival was 78%, 54%, and 81%, respectively (P < .01 for comparison of Stanford V with the other two regimens). Corresponding 5-year OS rates were, respectively, 90%, 82%, and 89%. The authors concluded that ABVD is still the treatment of choice when combined with optional, limited irradiation. The reported failure free survival for ABVD is high compared with other studies that might be explained by the inclusion of stage IIB patients without additional risk factors, resulting in a relatively high percentage of good prognosis patients according to the International Prognostic Score (IPS; 35%).
The UK study compared ABVD with two multidrug regimens: alternating ChlVPP with PABIOE or hybrid ChlVPP/EVA.23 At 52 months median follow-up, the primary objective EFS at 3 years was 75% (95% CI, 71%–79%) for ABVD and 75% (95% CI, 70%–79%) for multidrug regimens. However, patients receiving multidrug regimen experienced more grade 3/4 side effects, including infection, mucositis, and neuropathy. It should be mentioned that this study reported a better EFS and OS for ABVD than other trials. Again, patients with stage I/II disease who had systemic symptoms, multiple sites of involvement, or bulky disease were included. Looking at stage III/IV patients only, the 5-year EFS was 65% only. Taken together, in both trials, hybrid regimens did not show any superiority over ABVD.
Stanford V
Stanford V was developed as a short-duration, reduced-toxicity program and was applied weekly over 12 weeks. Consolidating RT to sites of initial disease was employed.24 Data were generated in a single-center setting with a very limited number of patients (142 patients). With a median follow-up of 5.4 years, the 5-year freedom from progression (FFP) was 89%, and the OS was 96%. However, FFP was significantly inferior among patients with an IPS of 3 and higher (94% vs 75%; P = .0001). One hundred twenty-nine of 142 patients (91%) received additional RT. The regimen was then used in the previously described Italian study and was inferior in terms of response rate and PFS in a multicenter setting.22 These conflicting results might be partially explained by the use of less RT in the randomized setting and the better treatment quality in single-center setting. Furthermore, in a large intergroup trial, including all US cooperative study groups, Stanford V was compared with ABVD ± RT.25 In this multicenter, prospective, randomized controlled trial, weekly alternating Stanford V was compared with the standard twice-weekly ABVD regimen. Patients had stage IIB/III, or stage IV disease, or stage I/IIA disease with bulky disease or other adverse features. RT was administered in both arms to sites of previous bulk (> 5 cm) and to splenic deposits, although this was omitted in the latter part of the trial for patients achieving CR in the ABVD arm. Five hundred patients received protocol treatment, and RT was administered to 73% in the Stanford V arm and to 53% in the ABVD arm. The overall response rates after completion of all treatment were 91% for Stanford V and 92% for ABVD. During a median follow-up of 4.3 years, there was no difference in the projected 5-year PFS and OS rates (76% and 90%, respectively, for ABVD; 74% and 92%, respectively, for Stanford V). Thus, in this large randomized trial, Stanford V was not superior to standard ABVD when given in combination with RT. However, 20% more patients had to be irradiated in the Stanford V arm, and the 5-year PFS was about 15% lower than reported in the single-center setting. This inferiority in terms of PFS is in the magnitude seen in the Intergruppo Italiano Linfomi.22 Finally, a large US intergroup (E2496) study comparing Stanford V to ABVD has been fully recruited and results will be available soon. To summarize, the compelling single-center phase II data could not be confirmed in multicenter randomized trials so far.
Escalated BEACOPP
The BEACOPP regimen has been introduced by the GHSG, with a substantial increase of dose density and dose intensity, compared with ABVD and hybrid regimens. Although some indications for a role of dose intensity were available in the early 1990s, no prospective randomized trial had been undertaken. To obtain an impression of the shape of the essential dose-response characteristic, Hasenclever and coworkers10 developed a novel statistical model to analyze a set of data in which dose variations had been used. The model took tumor growth and CT effects into account and was applied to correlate tumor control in relation to treatment intensity. On the basis of simulations using the model, it was predicted that shortening cycle intervals from 4 to 3 weeks should lead to small benefits (about 3% in 5-year tumor control rates), but a moderate average dose escalation by 30% of a standard CT would lead to a potential benefit in the range of 10% to 15% in tumor control at 5 years. Thus, the BEACOPP regimen was designed and evalutated.26 The subsequent HD9 trial compared COPP/ABVD (cyclophosphamide, vincristine, procarbazine, prednisone, adriamycin, bleomycin, vinblastine, and dacarbazine), BEACOPP baseline, and escalated BEACOPP. Results from 1195 randomized patients showed a clear superiority of escalated BEACOPP over BEACOPP baseline and COPP/ABVD at 5 years and also at 10 years.27 The follow-up data at 10 years confirmed these results (freedom from treatment failure [FFTF] and OS: 64% and 75% for COPP/ABVD, 70% and 80% for BEACOPP baseline, and 82% and 86% for escalated BEACOPP).28 The 10-year update of the HD9 study showed that this advantage is particularly evident in the subset of intermediate prognosis patients, as defined by the IPS 2 to 3, which is the largest subset of patients.28
However, toxicity of escalated BEACOPP remains a concern. The subsequent GHSG HD12 trial was thus aimed at deescalating CT and RT by comparing four courses of escalated BEACOPP with four courses of escalated BEACOPP and four courses of baseline BEACOPP (“4 + 4”).29 The role of RT was tested by a second randomization between consolidating radiation to initial bulky and residual diseases and no RT. Overall, at 5 years, OS was 91%, FFTF was 85.5%, and PFS was 86.2%. Statistically significanty, more patients with progressive disease were documented with the 4 + 4 arm. There was a trend toward a loss of tumor control in the no-RT arm for patients with residual disease. Thus, outside clinical trials, the GHSG still considers eight cycles of escalated BEACOPP, followed by RT to residual disease as standard for advanced-stage HL patients.
What Is the Standard Treatment Today: ABVD or Escalated BEACOPP?
In the advanced-stage HL trials reported thus far, 5-year PFS for ABVD is between 60% and 75%. It can be estimated that about half of the relapsed patients can be rescued.17–19,22,23,25 ABVD is certainly a good regimen, but can′t we do better with BEACOPP?
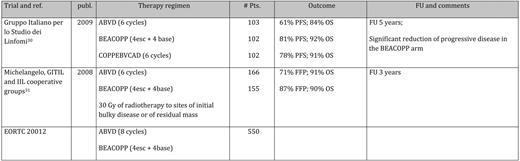
Three studies have been initiated comparing these two approaches in a prospective randomized setting (summarized in Table 4). So far, only one trial has undergone final analysis. Immature data have been reported for the second, and no data are available for the third. The final results of the HD2000 trial showed a significant superiority of BEACOPP over ABVD in terms of FFP, but not for OS.30 At 5 years, the FFP rate was 68% for ABVD and 81% for BEACOPP (4 escalated + 2 baseline, “4 + 2”); the OS was 84% for ABVD and 92% for BEACOPP, respectively. However, the lack of significance is likely due to the low power of this study that enrolled 307 patients in three different treatment arms. In the IIL-GITIL-Michelangelo study, ABVD (6–8 courses) or BEACOPP given in 4 + 4 fashion plus preplanned high-dose salvage produced a comparable 3-year outcome.31 BEACOPP upfront showed a superior 3-year FFP (87 vs 71%, P < .04). Finally, a large intergroup trial organized by the EORTC is currently ongoing (#20012). In this trial, ABVD is compared with BEACOPP 4 + 4; results are pending. To summarize, the difference in FFTF at 3 to 5 years is around 15%, which is likely to result in an estimated OS difference of about 7% once a sufficient observation time (5–10 years) is reached. Thus, there is also indirect evidence of a clinically meaningful superiority of BEACOPP escalated over ABVD in terms of OS. Data also highlight the need for large patient numbers if OS is selected as primary objective.
Do We Need RT?
The role of consolidating RT for advanced HL depends on the efficacy of the prior CT. A randomized EORTC study demonstrated that consolidation with IFRT did not improve the outcome in CR patients after 6 to 8 courses of alternating MOPP and ABV, but potentially improved the outcome of PR (partial remission) patients.32 A randomized GELA trial showed that consolidation with IFRT after doxorubicin-induced CR was not superior to two additional cycles of CT.33 The GHSG HD12 study randomized consolidating RT to residual disease versus observation only and showed a noninferiority of the observation arm.29 Unfortunately, the study was biased by a central review. Almost 10% of patients were irradiated, which had originally been randomized to the observation arm. Thus far, unpublished data from the HD12 trial indicate a benefit of RT to residual disease > 1.5 cm in terms of PFS. To conclude, in patients achieving a CR with CT, consolidating RT does not seem to improve the overall results.
What Do We Know About Positron Emission Tomography?
It has been demonstrated for HL patients that response to CT has an impact on the final treatment outcome.34,35 However, response as measured by computed tomography scan might occur with some delay in advanced HL. This is likely due to the fibrotic tissue infiltrating lymph nodes in this disease, which often results in residual masses remaining several months after therapy. In the GHSG HD15 trial, 311 of 817 patients (38%) showed residual disease > 2.5 cm, as determined by computed tomography after the completion of CT.36 However, 79% (n = 245) of these patients at the same time had a negative fluorodeoxyglucose-positron emission tomography (FDG-PET) scan. These patients did not receive any additional RT and, with a rather short median observation time of 18 months, their outcome was not inferior, compared with patients reaching a CR after CT. These data indicate, at least, that the biologic response determined by FDG-PET is superior to the morphologic response in terms of its negative predictive value. Gallamini et al 37 and Hutchings et al38 must be mentioned in this context. They were able to show that the early PET response (after two cycles of ABVD) overshadows the prognostic value of the IPS and thus is an important tool for planning risk-adapted treatment in advanced HL. Therefore, current concepts include early PET-determined response evaluation into treatment strategies and will hopefully define a new standard of care in which each patient receives as much therapy as needed. There are many ongoing prospective clinical trials for newly diagnosed HL (early stage and advanced stage) throughout the world investigating the predictive value of PET and using it for treatment guidance. Unless no final results of these trials are available, the use of PET is not advised outside clinical trials.
Conclusions
Advanced-stage HL has become a curable disease for the majority of patients. First-line treatment with six to eight cycles of ABVD is the widely accepted standard. However, the more aggressive escalated BEACOPP regimen induces a clinically relevant superior PFS, which is expected to translate into an improved OS in indirect comparisons. Ongoing, well-designed prospectively randomized studies are currently evaluating these two approaches, and mature results will be available in the near future. It might well be, though, that in the meantime, the early PET response-adapted design of the latest study generation will render this question obsolete. Scientific interest is currently focused on the questions of whether (i) two cycles of the less toxic regimen ABVD should be escalated into the more aggressive BEACOPP schedule in the case of PET-2 positivity; or (ii) if, after an aggressive induction therapy with two cycles of BEACOPP, further treatment can be deescalated. Both approaches promise to find the best balance between toxicity and efficacy for the benefit of each individual patient. Unfortunately, these different approaches are not tested against each other within a single randomized trial and, therefore, the current debate on the standard treatment of advanced-stage HL will continue to keep us excited.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Off-label drug use: None disclosed.
Correspondence
Peter Borchmann, MD, Klinik I für Innere Medizin, Universitätsklinikum Köln, Kerpener Str. 62, 50937 Köln, Germany; Phone: +49 221 478 7216; Fax: +49 221 478 7991; e-mail: peter.borchmann@uni-koeln.de