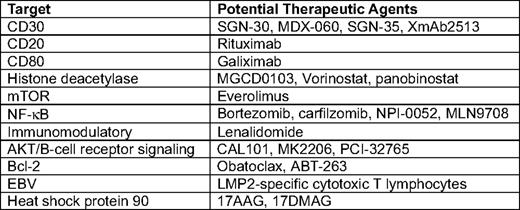
Abstract
With improvements in therapy, increasing dose intensity, early recognition of toxicity, and enhanced supportive care, current outcomes in patients with classical Hodgkin's lymphoma (HL) may be superior to disease-free and overall survival (OS) predicted by existing prognostic models, including the International Prognostic Score (IPS). The addition of biologic markers to recognized clinical prognostic factors, including those of the IPS, may to improve patient risk stratification and guide therapy in the future. However, the identification of these markers has been problematic due to the lack of large, confirmatory prospective trials, reproducibility and feasibility of the assays, and failure to improve upon already recognized clinical risk factors. One biomarker in particular, CD68, present on tumor infiltrating macrophages and detectable by immunohistochemical staining, is significantly associated with both shortened progression-free and disease-specific survivals in patients with HL at diagnosis and at relapse. In addition, less than 5% CD68+ cells correlates with a 100% disease-specific survival in patients with early-stage HL. CD68 represents just one of the many prognostic markers that could eventually be used to risk-stratify therapy. In addition, biologic markers may not only serve as prognostic markers, but also as therapeutic targets in HL. This review examines current data using the IPS to determine patient outcome, discuss several potential biologic prognostic markers, and summarize new therapies that are currently in clinical development in HL.
Introduction
Historically, outcomes in patients with classical Hodgkin's lymphoma (HL) have been predicted using recognized clinical variables such as bulk of disease, patient age, number of nodal sites, and erythrocyte sedimentation rate or the International Prognostic Score (IPS) in early- and advanced-stage HL, respectively.1,2 The IPS consists of seven clinical variables, including serum albumin less than 4 g/dL, hemoglobin less than 10.5 g/dL, male gender, age 45 years or greater, stage IV disease, white blood cell count at least 15,000/mm3, and absolute lymphocyte count less than 600/mm3 (or less than 8% of the total white blood cell count).2 Utilizing these risk factors in 5141 patients treated at 25 centers from 1983 to 1992, the predicted 5-year freedom from progression (FFP) rates were 84%, 77%, 67%, 60%, 51%, and 42% for patients with 0, 1, 2, 3, 4, or ≥ 5 risk factors (Table 1), respectively.2 In a re-examination of the IPS in 579 Canadian patients treated between 1990 and 2008, IPS scores of 0, 1, 2, 3, 4, and ≥ 5 correlated with 5-year FFP rates of 83%, 86%, 80%, 74%, 67%, and 66%, respectively (Table 1).3 OS also improved across all risk groups.3 The improvements in OS may be secondary to advances in transplantation, increased salvage therapy options, reductions in secondary malignancies, improved supportive care, or earlier recognition of toxicity. The changes in FFP after initial therapy may be related to more accurate staging or increases in treatment intensity with fewer delays or dose reductions. In this series, the IPS did not adequately risk-stratify subgroups of patients with excellent, intermediate, and poor outcomes, because patients with 0 to 3 risk factors demonstrated similar 5-year FFP rates ranging from 80% to 86%, and patients with 4 or more risk factors had similar outcomes.3 Therefore, a re-evaluation of the IPS in a larger population of patients treated at multiple centers is necessary to confirm these findings and to determine if the IPS in the current era segregates patients into clearly defined risk groups suitable for risk-adapted therapeutic strategies designed to minimize treatment for patients with the most favorable outcomes and intensify or utilize novel therapy for those patients most likely to relapse.
Improvements in patients' outcomes over time according to International Prognostic Index
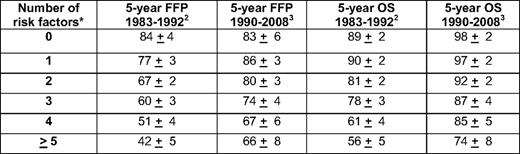
* Risk factors include age at least 45 years, male gender, white blood cell count at least 15,000/mm3, hemoglobin less than 10.5 g/dL, absolute lymphocyte count less than 600/mm3 or less than 8% of the white blood cell count, albumin less than 4.0 g/dL, and stage IV disease.
Hopefully, in the future, a greater understanding of the biology of HL will determine and individualize patient therapy. Biologic factors that influence not only the pathogenesis but also the progression of the disease may serve as prognostic markers and therapeutic targets. This article reviews some of the recently described clinically relevant biologic prognostic factors and novel targets for therapy. Several excellent recent review articles,4–7 including Dr. Kupper's review of the biology of HL from the 2009 American Society of Hematology meeting,7 address the current understanding of the pathogenesis of HL in greater detail than can be provided in the current article. This article addresses the biology of HL within a clinical and therapeutic context and is limited to biologic markers with forthcoming prognostic and therapeutic applications and to novel therapies currently in clinical investigation in classical HL.
Biologic Prognostic Factors in HL
Numerous biologic markers have been identified as prognostic factors in HL, and include surface receptors, intracellular proteins, cytokines, and genetic abnormalities comprised of amplifications, deletions, epigenetic silencing, or alterations in microRNA in Hodgkin's Reed-Sternberg (HRS) cells or surrounding inflammatory cells. However, many of the techniques used to detect these markers are not easily reproducible in local reference laboratories or are not validated prospectively. Also, the effects on patient outcomes are often not large enough to guide initial therapy, and the association of these factors with patient outcomes at relapse are not known. However, recently, the association between infiltrating tumor-associated macrophages with prognosis both at diagnosis and at relapse has been recognized as an important prognostic factor in patients with HL, and CD68 expression by immunohistochemistry has been shown to be a reliable marker to detect these cells.8
CD68
Using gene-expression profiling in 130 diagnostic fresh-tissue samples from patients with HL, Steidl et al. identified two distinct gene clusters associated with treatment success and treatment failure (p = 0.02).8 In the treatment-failure group, there was an overexpression of gene signatures associated with tumor-infiltrating macrophages (p = 0.02), tumor-infiltrating monocytes (p = 0.01), angiogenic cells (p = 0.04), adipocytes (p = 0.01), and HRS cells (p = 0.047). The germinal center B-cell signature was underexpressed in the treatment-failure group (p = 0.01). This correlation of tumor-infiltrating macrophages with poor prognosis has also previously been reported by other investigators.9,10 Therefore, Steidl et al. used a tissue microarray constructed from 166 independent classical HL paraffin-embedded diagnostic lymph node specimens to examine if CD68, a macrophage marker, could be assessed by immunohistochemistry and expression levels correlated with patient outcomes.8 CD68 staining was scored from 1 to 3, with a scores of 1, 2, and 3 representing < 5%, 5% to 25%, and > 25% CD68+ cells relative to overall cellularity, respectively. In univariate analysis, the presence of a high number of infiltrating CD68+ cells (immunohistochemical score of 2–3) was correlated with reduced progression-free (p = 0.03) and disease-free (p = 0.003) survivals. Median progression-free survival was 2.7 years in patients with more than 25% CD68+ infiltrating macrophages, 6.2 years in patients with 5% to 25% CD68+ cells, and not reached after 16.4 years of follow-up in patients with less than 5% CD68+ cells. The 10-year disease-specific survival rate was 88.6%, 67.4%, and 59.6% in patients with CD68 immunohistochemical scores of 1, 2, and 3, respectively. In a multivariate analysis, CD68 expression was superior to the IPS in predicting disease-free survival (p = 0.003 vs. p = 0.03). Similar findings correlating the presence of tumor-infiltrating macrophages with adverse patient outcomes have also been described in follicular non-Hodgkin's lymphoma,11,12 suggesting that interactions between the malignant lymphoma cell and the microenvironment are critical to the pathogenesis and progression of these diseases. Perhaps macrophage-produced cytokines provide HRS cell survival and proliferation signals or facilitate immunologic escape of the HRS cell; however, the interactions of tumor-infiltrating macrophages with HRS and the mechanisms of how these interactions affect patient outcome remain unclear.
Interestingly, CD68 could also identify early-stage HL patients with excellent long-term outcomes. In an analysis of stage I and II patients (N = 41), less than 5% CD68+ cells correlated with a disease-specific survival rate of 100%. In addition, there was a significant association with CD68 expression and relapse after second-line therapy or autologous stem-cell transplantation (p = 0.009 and p = 0.008, respectively). Second-line therapy failed in 12.5%, 51.7%, and 62.5% patients with < 5%, 5% to 25%, and > 25% CD68+ cells. Therefore, pretreatment CD68 staining may be used not only to identify those patients at a high risk for relapse with induction and salvage therapy in whom novel treatments should be explored, but also to identify early-stage patients with excellent outcomes in whom perhaps chemotherapy and/or radiotherapy could be reduced while maintaining excellent outcome.
CD20
CD20 expression by immunohistochemistry in the inflammatory infiltrate and on HRS cells has also been evaluated with respect to prognosis. In the same trial by Steidl et al.,8 CD20 expression was scored from 1 to 4, with scores stratified for high (3 or 4) or low (1 or 2) CD20 staining. CD20 expression in both infiltrating small B cells and on HRS cells were analyzed independently. In univariate analysis, an increased number of tumor-infiltrating CD20+ small B cells (score > 2) was associated with both prolonged progression-free (p = 0.02) and disease-free (p = 0.02) survivals. Similar findings, in which a high number of CD20+ cells in the inflammatory infiltrate correlated with improved event-free and OS, have also been previously reported by Chetaille et al.13
Steidl found no correlation with the number of CD20+ HRS cells and patient outcome.8 In another study of 598 patients, the presence of CD20+ HRS cells (N = 132, 22%) failed to correlate with failure-free survival in patients treated with ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine), MOPP (mustargen, oncovin, procarbazine, and prednisone), or radiotherapy, and no differences in patient outcomes were observed utilizing cutoffs of 5, 10, 20, 30, and 50% CD20+ HRS cells.14 However, in a study of 248 patients, 11% had CD20+ HRS cells, and both time to treatment failure and OS were significantly lower in patients treated with ABVD with CD20+ HRS cells (defined as any CD20 staining on CD30+ HRS cells).15 Interestingly, in a third study, greater than 10% CD20+ HRS cells was associated with inferior patient outcomes in patients treated from 1974 to 1980, but failed to significantly impact failure-free survival in patients treated from 1981 to 1999.16 Therefore, the prognostic relevance of CD20 in HL may be dependent on its location (on the HRS cell or on infiltrating CD20+ small B cells), the therapeutic modality, and the criteria defined for CD20 positivity. Recent data suggest that an increased number of CD20+-infiltrating B cells coupled with low CD68 expression on tumor infiltrating macrophages in the reactive infiltrate in HL samples may identify patients with very favorable HL, who would be most suitable for minimization of therapy.8 Prospective studies are needed to determine if these findings are reproducible among pathologists and if therapy can be risk-stratified on the basis of these immunohistochemical markers.
MMP11
MMP11, a marker of matrix metallopeptidases expressed by tumor-associated macrophages, is associated with treatment failure by both gene-expression profiling and immunohistochemistry.8 MMP11 was one of the 30 genes with superior discriminative power with respect to patient outcome, and more than 1% MMP11 staining by immunohistochemistry predicted a reduced progression-free survival in both univariate (p = 0.008) and multivariate (p = 0.009) analyses. Unfortunately, because MMP11 stains many cell types in classical HL specimens, including HRS, endothelial cells, and macrophages, it is difficult to determine the biologic mechanism and contributing cell type responsible for this protein's association with poor patient outcomes.
Other Markers
A variety of other prognostic markers have been described in HL, although none with the predictive power both at diagnosis and relapse reported for the biologic marker CD68.8 These markers include soluble CD30 (sCD30), interleukin (IL)-10, tumor necrosis factor-alpha (TNFα), B-cell-activating factor (BAFF), thymus and activation regulated chemokine (TARC), vascular endothelial growth factor (VEGF), bcl-2, p53, and the ratio of cytotoxic and regulatory T cells within the inflammatory infiltrate.6,9,17–19
Increased serum levels of sCD30, IL10, BAFF, TNFα, TARC, and VEGF have all been correlated with adverse patient outcomes,6,20–27 including an increased risk for relapse or shortened progression-free or OS. For sCD30, serum levels greater than 100 to 200 U/mL are generally associated with worse outcomes.25–27 In several studies, combining sCD30 level with the IPS improved the predictive power of the IPS.24–27
In 707 patients with HL, bcl-2 positivity in HRS cells was observed by immunohistochemistry in 65% of nodular sclerosis (n = 551), in 47% of mixed cellularity (n = 147), and in all five lymphocyte-depleted cases.17 For all classical HL subtypes, the 5-year failure-free survival was 74% for patients with bcl-2 expression versus 84% for patients without bcl-2 expression (p = 0.0016). Multivariate analysis confirmed that bcl-2 expression was independently associated with reduced failure-free survival in addition to age over 45, stage IV disease, low albumin, and elevated lactate dehydrogenase. Likewise, in a retrospective study of 327 patients with classical HL, bcl-2 expression was correlated with low overall response rates to initial therapy and shortened survival.28 In a third series of 107 patients with classical HL, evaluation of bcl-2, p53, and p21 expression by immunohistochemistry using tissue microarrays demonstrated that bcl-2 was expressed in 26% of patients and was significantly associated with reduced failure-free survival in both univariate and multivariate analyses, along with age over 45 and advanced-stage disease.29 However, the relationship between bcl-2 expression and patient outcome in HL remains controversial because other studies have not demonstrated the same correlation between bcl-2 expression and failure-free survival.30 Similarly, the association of p53 with patient outcomes in HL remains controversial,28–30 although more studies suggest a prognostic role for bcl-2 than for p53.
The presence of T cells in the inflammatory infiltrate also seems to influence patient outcomes in HL. Recently, several studies have examined the ratio of regulatory T cells, defined as CD4+CD25+ T cells with FOXP3 expression, and cytotoxic T cells, defined by either TIA-1 or granzyme B expression, in HL.9,13,19,31 In one study from Alvaro et. al., patients with a high number of FOXP3+ cells by immunohistochemistry and low numbers of TIA-1+ cytotoxic T-cells had 5-year predicted overall and disease-free survivals of 100% and 93%, compared with 73% and 67%, respectively, in patients with few FOXP3+ cells and high TIA-1+ staining.19 Similarly, in a second study, patients with fewer than 25 FOXP3+ cells per high-powered field were found to have a 5-year failure-free survival of 64%, compared with 85% for 25 or more FOXP3+ cells per high-powered field.31 The ratio of regulatory FOXP3+ T cells to cytotoxic T/NK (natural killer) cells with granzyme B expression also independently predicted patient survival, with a ratio of FOXP3+/granzyme B+ cells of 1 or less indicative of a poor failure-free survival.31 Therefore, not only are biologic markers on the HRS cell prognostic in HL, but also the composition of the surrounding cellular matrix and microenvironment.
Despite this plethora of available prognostic markers in HL, there is no current consensus on how best to incorporate these biologic markers with accepted clinical prognostic risk factors or how to use this information to alter treatment. Further prospective studies with incorporation into front-line therapeutic trials are needed to assess the value of CD68 and other markers in predicting patient outcomes prior to the widespread use of these markers in determining patient therapy.
Biologic Targets and Novel Therapies in HL
Due to the multiple pathways that are dysregulated or altered in HL, including nuclear factor (NF)-κB, phosphatidylinositol (PI3)-kinase/AKT, mammalian target of rapamycin (mTOR), surface receptor signaling through CD30, CD40, and RANK, and immunologic defects through alterations in the tumor microenvironment, the future of Hodgkin's therapeutics may involve targeting multiple pathways simultaneously. Table 2 and the text below describe some of the agents in clinical development selective for these pathways that may one day become accepted therapeutic options for patients with relapsed or poor-prognosis HL.
CD30
Although the degree of HRS surface CD30 expression is not prognostic,8 surface CD30 represents an excellent target for monoclonal antibody therapy because it is restricted to the HRS cell and is otherwise absent on normal tissue (with the exception of activated B and T cells).32–34 The chimeric anti-CD30 monoclonal antibody SGN-30 has activity in murine xenograft models of HL, with treatment resulting in a dose-dependent reduction in tumor mass and prolonged survival.34 However, in phase I and II testing of single-agent SGN-30, the activity in 62 patients with relapsed HL was modest, with a partial response in 1 patient and stable disease in 15 patients for 6 to 16 months.35,36 Combining SGN-30 with GVD (gemcitabine, vinorelbine, and liposomal doxorubicin) chemotherapy also did not seem to improve response rates (overall response rate of 65% with the combination, compared with 57% with GVD alone), and the combination of SGN-30 and GVD led to unexpected severe pulmonary toxicity.37 In a phase I/II study with the anti-CD30 antibody MDX-060, results were similarly disappointing, with responses only observed in 6 of 72 patients with CD30+ malignancies.38 The minimal efficacy of these agents is poorly understood, but may be related to binding with sCD30, poor effector cell binding, and limited antibody-dependent cellular cytotoxicity or limited direct cellular cytoxicity due to continued signaling and downstream anti-apoptotic effects through other tumor necrosis factor surface receptors, including CD40.
Several strategies have been employed to improve the clinical efficacy of anti-CD30 antibodies, including humanizing the antibodies, altering the heavy chain to optimize effector cell binding, using drug-antibody conjugates, creating bi-specific antibodies that bind both effector and HRS cells to ensure proximity and trigger antibody-dependent cellular cytotoxicity, and radiolabeling antibodies. Two agents that are farthest along in clinical development using these approaches include XmAb2513, a humanized anti-CD30 antibody with an Fc region engineered to enhance Fcγ receptor effector cell-binding affinity, and SGN-35, an anti-CD30 antibody conjugated with the tubulin inhibitor monomethyl auristatin E. A phase I trial with XmAb2513 was recently completed exploring six dose levels ranging from 0.3 to 12 mg/kg every other week for a maximum of eight doses. In the phase I study with this agent, toxicity was limited and a partial response was observed in one patient receiving 9 mg/kg of the drug.39
The antibody-drug conjugate SGN-35 has been the most promising of the CD30-directed therapies. In two clinical trials, SGN-35 was administered either every 3 weeks or weekly. With the every-3-week schedule, 45 patients with CD30+ lymphoma received doses of 0.1 to 3.6 mg/kg SGN-35. Responses were observed in 37% of patients, and tumor reductions were observed in up to 88% of patients.40 The maximum tolerated dose was 1.8 mg/kg, and dose-limiting toxicity consisted of febrile neutropenia and hyperglycemia. With weekly dosing, the maximum tolerated dose of SGN-35 was 1.4 mg/kg, and dose-limiting toxicities included diarrhea, vomiting, and hyperglycemia.41 The overall response rate with weekly dosing was 46%, with 29% complete responses. Common grade 1 or 2 toxicities reported with SGN-35 include peripheral neuropathy, nausea, fatigue, dizziness, diarrhea, and neutropenia. Pivotal phase II multi-center studies of single-agent SGN-35 have recently been completed, and the agent is under Food and Drug Administration (FDA) review. Based on preclinical models demonstrating improved anti-tumor activity with ABVD42 and encouraging single-agent activity, clinical trials are also now exploring combinations of SGN-35 with ABVD in patients with high-risk, previously untreated HL and as maintenance therapy following autologous stem-cell transplantation.
mTOR and AKT
The PI3-K pathway and the downstream tyrosine kinase AKT are critical for B-cell survival and proliferation. Immunohistochemistry showed that 64.3% (n = 27 of 42) of patient-derived primary HL specimens expressed phosphorylated AKT in the HRS cells, but not in the surrounding reactive cells.43 In HL cell lines, AKT is constitutively activated, resulting in phosphorylation of glycogen synthase kinase 3 and the mTOR substrates 4E-BP1 and p70 S6 kinase, all downstream effectors of AKT.44 Furthermore, AKT phosphorylation is induced by CD30, CD40, and RANK binding.43 Inhibition of PI3-kinase and inhibition of mTOR individually both modestly affect HL cell survival,43–45 suggesting that AKT and its downstream targets are up-regulated in HL through multiple mechanisms, and that targeting multiple pathways may be necessary for therapeutic efficacy. In fact, combined inhibition of the mTOR and AKT pathways with the mTOR inhibitor temsirolimus and the PI3-kinase inhibitor LY294002 significantly increased autophagy and reduced HRS cell viability compared with mTOR inhibition alone.45 In addition, combined therapy with the mTOR inhibitor rapamycin and doxorubicin also synergistically reduced HL cell survival.44
In a clinical trial of the oral mTOR inhibitor everolimus (RAD001), 17 patients with relapsed HL received 10 mg/d for up to 12 cycles.46 Partial responses were observed in 7 of 15 evaluable patients, for an overall response rate of 47%. Toxicities consisted primarily of thrombocytopenia, anemia, and neutropenia. A multi-center phase II trial is currently recruiting patients, and several trials are also exploring combinations of lenalidomide, panobinostat, and sorafenib with everolimus. In addition, several AKT inhibitors, including MK2206, CAL-101, and PCI-32765, are in development in non-Hodgkin's lymphoma and should be considered for further study alone and in combination with other agents, including everolimus, in patients with relapsed HL.
Histone Deacetylase
Transcriptional silencing through hypermethylation and histone deacetylation (HDAC) contributes to genomic silencing and consequent down-regulation of B-cell-specific genes, including CD19, CD79b, Syk, BOB.1/OBF, and the immunoglobulin heavy chain in HRS cells, and this may be reversible with DNA methyltransferase or HDAC inhibitors.47–49 One of the available DNA methyltransferase inhibitors, decitabine, has been difficult to administer to patients with relapsed lymphoma due to myelosuppression, even at low doses ranging from 10 to 15 mg/m2/d.50,51 However, activity has been observed with a variety of HDAC inhibitors in patients with HL.52–55
In vitro, the nonselective HDAC inhibitor vorinostat induces cellular apoptosis and may affect chemotaxis of cytotoxic and regulatory T cells to the surrounding cellular infiltrate through down-modulation of TARC.49 In a multicenter study with vorinostat, one of 27 patients achieved a partial response; however, prolonged stable disease was observed in five patients who received vorinostat for 9 months or more.53 After a surprising number of partial responses (overall response rate of 58%) were observed in 12 patients with HL enrolled on a phase I trial of the nonselective HDAC inhibitor panobinostat,54 a multi-center phase II trial with this agent was conducted.55 In this phase II trial, the overall response rate was lower at 21%, with 11 responders among 53 evaluable patients; however, similar to vorinostat, 31 patients have had prolonged stable disease, with a disease control rate (complete response + partial response + stable disease) reported as high as 79%. In this trial, the median duration of treatment was 89 d (range 5–300+ d).55 In a phase II study of the class 1-specific HDAC inhibitor, MGCD0103, Younes et al. reported an overall response rate of 40%, with two of 20 evaluable patients with a complete response and six patients with a partial response.52 In addition, 75% of patients had reductions in tumor size. Toxicities consisted primarily of fatigue and gastrointestinal symptoms (vomiting, diarrhea, and anorexia); however, two patients developed significant pericardial effusions that have precluded further clinical development of this agent.
Due to preliminary efficacy in patients with relapsed HL, HDAC inhibitors may be ideal agents to combine with chemotherapy or to use as maintenance therapy in high-risk patients. In vitro studies suggest that combinations of vorinostat with bortezomib, doxorubicin, and gemcitabine are synergistic.56 In contrast, in these same studies, no added benefit was observed when vorinostat was combined with temsirolimus. Therefore, preclinical data should be utilized to assist in the development of rational combination approaches with the HDAC inhibitors.
Immunomodulatory Approaches to Hodgkin's Therapy
It is well recognized from the variety of publications identifying the number of tumor-infiltrating macrophages, cytokine profiles, and the ratio of FOXP3+-regulatory T cells to granzyme B+-cytotoxic T cells in the inflammatory infiltrate as prognostic factors in HL,6–8,31 that the interplay between the HRS cell and the tumor microenvironment is critical in the pathogenesis and progression of HL. The thalidomide derivative lenalidomide is an oral agent with multiple postulated immunomodulatory mechanisms of action, including cytokine alterations, T-cell and NK-cell modulation, inhibition of angiogenesis, and direct effects on tumor cells. At least three different studies have examined the single-agent activity of lenalidomide in HL. In 14 patients treated with lenalidomide at 25 mg/d on d 1 to 21 of a 28-d cycle, the overall response rate was 14%, with two partial responses and seven patients with stable disease.57 Similarly, in a multi-center trial of 35 patients with HL using the 21-d lenalidomide schedule, Fehniger et al. reported an overall response rate of 17% and a disease-control rate (complete response + partial response + stable disease) of 34%, including one complete response, five partial responses, and six additional patients with prolonged stable disease for more than 6 months.58 Cytopenias were the most commonly encountered toxicities, and accrual to the study is continuing in order to examine continuous 28-d dosing of lenalidomide. A third study of lenalidomide utilizing the 25 mg/d d 1 to 21 schedule every 28 d reported an overall response rate of 50%, with one complete response, five partial responses, and stable disease in six patients.59 Therefore, with response rates of 14% to 50% and the encouraging numbers of patients with prolonged stable disease observed in three different trials of single-agent lenalidomide, further evaluation of lenalidomide as a single agent, in a maintenance setting following combination chemotherapy or stem-cell transplantation, and in combination with other novel agents is warranted. Combinations with lenalidomide and HDAC inhibitors (panobinostat), mTOR inhibitors (everolimus), SGN-35, and chemotherapy are in development.
One other potential approach to targeting the tumor microenvironment in HL includes the development of therapy that targets directly the inflammatory infiltrate. The anti-CD20 antibody rituximab may lead to depletion of reactive small B cells that surround the malignant HRS cell, and this B-cell killing may disrupt cytokine and ligand signaling utilized for apoptotic escape of the HRS cell. Secondly, rituximab may directly target previously described circulating clonal HL stem cells that are CD19, CD20, CD27, and aldehyde dehydrogenase positive.60 Younes et al. conducted a pilot study of single-agent rituximab in patients with relapsed or refractory classical HL and observed a response rate of 22%, with a median response duration of 7.8 months.61 Responses were observed regardless of CD20 expression on the HRS cell. As a result, this group then added rituximab to ABVD as a front-line therapy for patients with advanced-stage HL.62 Compared with historical controls, the 5-year event-free survival was 87% with rituximab plus ABVD (R-ABVD), a significant improvement over the 66% observed with ABVD alone (p = 0.0036). In addition, improvement in event-free survival was observed with R-ABVD both in patients with IPS scores of 0 to 2 (event-free survival 89% with R-ABVD compared with 71% with ABVD; p = 0.0248) and in those with IPS scores above 2 (EFS 80% with R-ABVD compared with 55% with ABVD alone; p = 0.0532). As a result of these encouraging results, randomized multi-center trials of R-ABVD compared with ABVD are ongoing in patients with early- and advanced-stage HL. These clinical results are encouraging despite the pathologic data that CD20+-infiltrating B cells favorably affect prognosis in HL,8,13 suggesting that the mechanism of rituximab's activity in HL is unknown.
Chemotherapy
Numerous chemotherapy agents, including gemcitabine, vinorelbine, liposomal doxorubicin, oxaliplatin, cyclophosphamide, etoposide, ifosfamide, cisplatin, and carboplatin, have activity alone or in combination in patients with relapsed HL. Recently, clinical efficacy has also been observed with single-agent bendamustine.63 Bendamustine was administered at 120 mg/m2 on d 1 and 2 of a 28-d cycle with pefilgrastim support for a maximum of 6 cycles. In 24 evaluable patients, responses were observed in 15 patients, including eight complete responses and seven partial responses, for an overall response rate of 63%, rivaling the results of many combination salvage regimens. However, in this heavily pretreated patient population (median four prior therapies including four patients with prior allogeneic transplant), the median response duration was only 2.6 months. Toxicity consisted primarily of myelosuppression leading to treatment delays or reductions, fungal pneumonia in one patient, and pyelonephritis. Therefore, bendamustine is a promising cytotoxic agent for patients with relapsed or refractory HL who have failed or are not candidates for autologous stem-cell transplantation. Additional study is needed using bendamustine prior to stem-cell transplantation to determine its effect on stem-cell mobilization. Future combinations of bendamustine with other chemotherapeutic agents and with the targeted agents discussed previously are also warranted.
NF-kB
NF-κB is constitutively active in HRS cells through receptor-mediated (CD40, CD30, Notch1, RANK, TACI, and BCMA) signaling, genomic amplification of the REL gene, and inactivation of the NF-κB inhibitors IκBα and A20.7 The proteasome bortezomib inhibits proteasomal degradation of IκB, consequently sequestering NF-κB in the cytoplasm and preventing its activity as a transcription factor. In preclinical studies, bortezomib has activity in HL cell lines.64 However, despite this preclinical activity, two separate studies demonstrated limited single-agent activity of bortezomib in patients with relapsed HL.65,66 Perhaps simultaneously targeting the multiple pathways of NF-kB overexpression with several different therapeutic agents or combining NF-κB inhibitors with chemotherapy will be more efficacious than inhibition of proteasomal degradation with bortezomib alone. In one trial, combined bortezomib and gemcitabine resulted in an overall response rate of 22% and was associated with severe liver toxicity67 ; however, in a phase I combination study with ICE (ifosfamide, carboplatin, and etoposide), response rates were high, with an overall response rate of 75%, and toxicities consisted primarily of cytopenias.41 More potent or irreversible novel proteasome inhibitors (carfilzomib, MLN9708, NPI-0052)68–72 may be more effective in heavily pretreated patients with HL than bortezomib. However, phase I and II trials with these agents are ongoing in hematologic malignancies and their efficacy in HL has not yet been evaluated.
Conclusions
A greater understanding of the biology of HL and recognition of prognostic biomarkers has led to the development of targeted therapy for patients with HL. While several of these agents have significant single-agent activity in patients with relapsed disease, the multiple alterations in the malignant HRS cells responsible for apoptotic and immunologic escape will likely make combination therapy necessary for superior efficacy. Preclinical studies to determine which agents are synergistic with selected chemotherapeutics or other targeted agents are ongoing, and will be required in conjunction with randomized clinical trials to rationally develop and determine the clinical benefit of these combinations. These novel therapies should also eventually incorporated into front-line regimens or utilized as maintenance therapy with the aim of reducing toxicity in patients with very favorable disease and to improve outcomes in patients at high-risk for relapse. Several agents with activity in the relapsed setting are currently being examined in the front-line setting in combination with ABVD or as maintenance therapy after autologous stem-cell transplantation in high-risk patients, and include rituximab, SGN-35, lenalidomide, and panobinostat. Confirmation of the predictive value of several new biomarkers, including CD68 and CD20, will hopefully help to guide future therapeutics with these new agents and will permit the development of individualized therapy in HL.
Acknowledgments
KAB is supported by National Cancer Institute Career Development Award number K23 CA109004–01A1.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Novartis, Seattle Genetics, and Celgene. Off-label drug use: SGN-35, panobinostat, everolimus, bendamustine, and lenalidomide are not FDA-approved for the treatment of Hodgkin's lymphoma.
Correspondence
Kristie A. Blum, MD, Associate Professor of Medicine, The Ohio State University, Division of Hematology, Starling Loving Hall, Room B315, Columbus, OH 43210; Phone: (614) 293-8858; Fax: (614) 293-7484; e-mail: kristie.blum@osumc.edu