Abstract
Myelodysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN) overlap syndromes are uniquely classified neoplasms occurring in both children and adults. This category consists of 5 neoplastic subtypes: chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia (JMML), BCR-ABL1–negative atypical chronic myeloid leukemia (aCML), MDS/MPN-ring sideroblasts and thrombocytosis (MDS/MPN-RS-T), and MDS/MPN-unclassifiable (U). Cytogenetic abnormalities and somatic copy number variations are uncommon; however, >90% patients harbor gene mutations. Although no single gene mutation is specific to a disease subtype, certain mutational signatures in the context of appropriate clinical and morphological features can be used to establish a diagnosis. In CMML, mutated coexpression of TET2 and SRSF2 results in clonal hematopoiesis skewed toward monocytosis, and the ensuing acquisition of driver mutations including ASXL1, NRAS, and CBL results in overt disease. MDS/MPN-RS-T demonstrates features of SF3B1-mutant MDS with ring sideroblasts (MDS-RS), with the development of thrombocytosis secondary to the acquisition of signaling mutations, most commonly JAK2V617F. JMML, the only pediatric entity, is a bona fide RASopathy, with germline and somatic mutations occurring in the oncogenic RAS pathway giving rise to disease. BCR-ABL1–negative aCML is characterized by dysplastic neutrophilia and is enriched in SETBP1 and ETNK1 mutations, whereas MDS/MPN-U is the least defined and lacks a characteristic mutational signature. Molecular profiling also provides prognostic information, with truncating ASXL1 mutations being universally detrimental and germline CBL mutations in JMML showing spontaneous regression. Sequencing information in certain cases can help identify potential targeted therapies (IDH1, IDH2, and splicing mutations) and should be a mainstay in the diagnosis and management of these neoplasms.
Learning Objectives
Define the landscape of cytogenetic and molecular abnormalities in patients with MDS/MPN overlap neoplasms including chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia (JMML), BCR-ABL1–negative atypical chronic myeloid leukemia (aCML), MDS/MPN-ring sideroblasts and thrombocytosis (MDS/MPN-RS-T), and MDS/MPN-unclassifiable (U)
Characterize molecular signatures that can be used in the context of appropriate clinical and morphological features to help diagnose CMML, JMML, MDS/MPN-RS-T and BCR-ABL1–negative aCML
Underscore the importance of molecular profiling in MDS/MPN overlap syndromes with regard to diagnosis, prognosis, and clinical therapeutics
Case
A 71-year-old man presents with a 6-month history of effort intolerance, weakness, intermittent drenching night sweats, and low-grade fevers. His last complete blood count 2 years ago had demonstrated mild thrombocytopenia. On examination his vital signs are stable. He his spleen is palpable 10 cm below the left costal margin. He has no hepatomegaly or lymphadenopathy. His past medical history is significant for hypertension controlled with lisinopril. His blood counts reveal hemoglobin of 9.6 g/dL, white blood cell count 15 × 109/L, absolute monocyte count 2.3 × 109/L, and platelet count 110 × 109/L. His blood smear did not have elevated blasts or promonocytes, but there were circulating metamyelocytes and myelocytes. A bone marrow biopsy was 90% cellular with megakaryocytic atypia and hyperplasia. Bone marrow blasts were estimated at 7%. The karyotype was normal, and next-generation sequencing identified mutations involving ASXL1: c.1934dup; p.Gly646Trpfs*12 (20%), TET2 c.1648C>T; p.Arg550* (41%), SRSF2 c.284C>T; p.Pro95Leu (43%); and NRASc.38G>A; p.Gly13Asp (46%) (variant allele frequency for each mutation added in parentheses).
What is the diagnosis, and how would you risk stratify this patient?
Introduction
Myelodysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN) overlap syndromes are well-defined myeloid neoplasms characterized by overlapping features of MDS and MPN.1 This uniquely classified entity consists of 4 adult-onset subtypes: chronic myelomonocytic leukemia (CMML), BCR-ABL1–negative atypical chronic myeloid leukemia (aCML), MDS/MPN-ring sideroblasts and thrombocytosis (MDS/MPN-RS-T), and MDS/MPN-unclassifiable (MDS/MPN-U). There is also one pediatric subtype: juvenile myelomonocytic leukemia (JMML) (Table 1).1 Although the classification of these neoplasms relies largely on clinical features and peripheral blood and bone marrow (BM) morphology, the incorporation of next-generation sequencing (NGS) techniques has helped in defining the molecular landscape and ability to diagnose, risk stratify, and plan appropriate treatment strategies. Among the subtypes, CMML is the most common, demonstrating marked clinical heterogeneity and an inherent tendency to transform to acute myeloid leukemia (AML).2
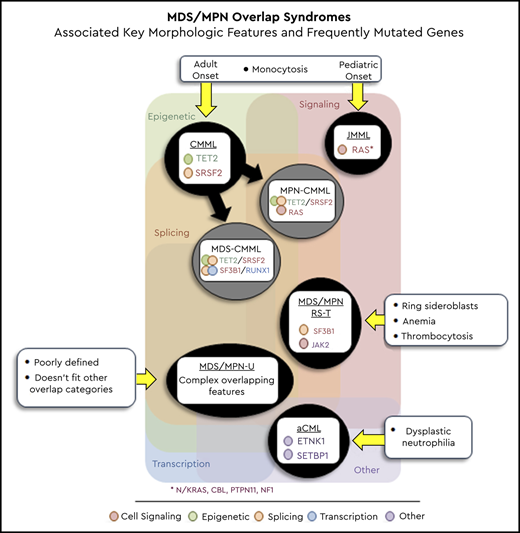
Whereas CMML and JMML are defined by the presence of clonal monocytosis, aCML presents with dysplastic neutrophilia, MDS/MPN-RS-T with anemia and thrombocytosis, and MDS/MPN-U with poorly defined overlapping features. Table 1 outlines the 2016 World Health Organization (WHO) criteria for the diagnosis of MDS/MPN overlap syndromes, including key associated genes and epidemiologic features (incidence, median age, and median overall survival [OS]).1 Although cytogenetic abnormalities are seen in a small fraction of patients with overlap neoplasms, molecular aberrations occur in most (>90%). In this review, we highlight salient features with regard to the genetic landscape of MDS/MPN overlap neoplasms.
Molecular aberrations in MDS/MPN overlap neoplasms
To establish a diagnosis of an MDS/MPN overlap syndrome, molecularly defined neoplasms that present with similar or overlapping features must be ruled out.1 These include BCR-ABL1–driven CML (especially the p190 variant), PDGFRA, PDGFRB, FGFR1, and PCM1-JAK2 rearranged myeloid neoplasms.1,3 In patients with overlap who present with monocytosis and eosinophilia, aberrations in PDGFRA, PDGFRB, FGFR1, and the PCM1-JAK2 fusion should be assessed by fluorescence in situ hybridization or quantitative polymerase chain reaction studies. Of note, whereas the most common PDGFRA abnormality, the FIP1L1-PDGFRA fusion secondary to CHIC2 deletion, is karyotypically occult, the ETV6-PDGFRB fusion and FGFR1 rearrangements are regularly detected by conventional karyotyping.4
Chronic myelomonocytic leukemia
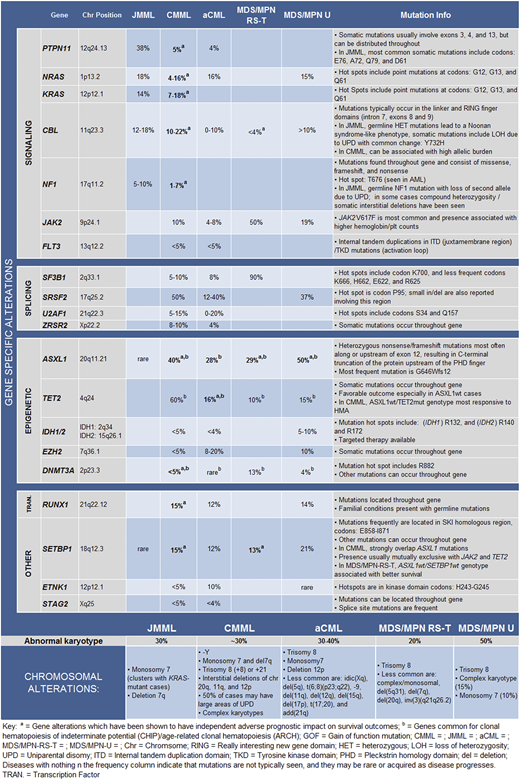
Gene mutations are seen in >90% of patients with CMML and most commonly involve TET2 (60%), SRSF2 (50%), ASXL1 (40%), and the oncogenic RAS pathway (30%).5-7 Additional genes mutated at lower frequencies include SETBP1 (15%), RUNX1 (15%), and JAK2V617F (10%), with DNMT3A, IDH1, IDH2, STAG2, PHF6, CEBPA, ETNK1, and EZH2 occurring at < 5%.2,8,9 Unlike in MPN and chronic neutrophilic leukemia (CNL), driver mutations in MPL, CALR, and CSF3R (CNL) are very infrequent, and if found they suggest an alternative diagnosis.10 Similarly, leukemia-associated driver mutations including NPM1 and FLT3 are very uncommon and if present suggest AML in evolution.11
CMML is a disease of aging, resulting from the acquisition of clonal hematopoiesis–related mutations (TET2, ASXL1, and SRSF2), resulting in monocyte-biased hematopoiesis and disease progression secondary to acquisition of additional driver mutations along with cell-intrinsic and -extrinsic factors.12 Among mutations seen in CMML, truncating (frameshift and nonsense) ASXL1 mutations are universally deleterious, adversely affecting both OS and leukemia-free survival (LFS),2,13,14 whereas TET2 mutations are associated with favorable outcomes, especially in the absence of ASXL1 mutations, with the ASXL1wt/TET2mt genotype predicting best survival rates.10,15 In fact, this genotype is also most predictive of responses to hypomethylating agent therapy (HMA) in CMML.10,15 Heterozygous splicing mutations (SRSF2, SF3B1, U2AF1, and ZRSR2) are common, with SRSF2 (P95 hot spot) being most frequent, with no clear impact on survival.16 Acquisition of oncogenic RAS pathway mutations (NRAS, CBL, KRAS, PTPN11, and NF1) drives a proliferative phenotype (MPN-CMML), with marked leukocytosis/monocytosis, pronounced constitutional symptoms, splenomegaly, and lower survival.13 RUNX1 and SETBP1 mutations are seen in 15% of patients, with RUNX1 mutations associated with severe thrombocytopenia, and both mutations negatively affect outcomes.2,17 Of note, TP53 mutations are uncommon in CMML (<1%) and if seen are usually present in the context of therapy-related CMML.18
Juvenile myelomonocytic leukemia
JMML is the only pediatric-onset neoplasm in this category and is considered a bona fide RASopathy, with germline and somatic mutation in the RAS/RAF/MEK/ERK pathway giving rise to disease.19 Germline mutations associated with JMML include NF1, RAS mutations in the context of Noonan syndrome (PTPN11, KRAS, NRAS, RIT1), and CBL, with CBL mutant JMML often demonstrating spontaneous regression.20,21 Somatic mutations that give rise to JMML include PTPN11 (38%), NRAS (18%), KRAS (14%), RRAS and RRAS2 (<10%). Unlike in CMML, mutations in epigenetic regulators including ASXL1 and SETBP1 and in signaling genes such as JAK3 are late events and are often responsible for disease progression to AML.19,22
MDS/MPN-ring sideroblasts and thrombocytosis
This is a unique overlap neoplasm, most recently formally assigned to this category in the 2016 WHO classification, characterized largely by features of MDS with ring sideroblasts (MDS-RS) and concomitant thrombocytosis.23 Unlike for other neoplasms in this category, the median OS is favorable at 5 to 7 years, with AML transformation rates of <5%.23,24 The disease is defined by the specific presence of SF3B1 (90%) and JAK2V617F (50%) mutations, and apart from BM RS it also demonstrates atypical megakaryocytes in the BM with peripheral blood thrombocytosis.23,24 It is believed that SF3B1-mutant MDS-RS clonally evolves into MDS/MPN-RS-T, because of the acquisition of signaling mutations such as JAK2V617F. Of note, although CALR mutations have been documented in a small fraction of patients with JAK2/MPL wildtype MDS/MPN-RS-T, they tend to be infrequent (<5%).24,25 Additional mutations seen in MDS/MPN-RS-T include ASXL1 (29%), DNMT3A (13%), SETBP1 (13%), and TET2 (10%),23,24 with the ASXL1wt/SETBP1wt genotype associated with better outcomes.23,24
BCR-ABL1–negative atypical CML
This is a rare overlap neoplasm characterized by dysplastic neutrophilia in the absence of monocytosis and basophilia.1 Gene mutations encountered include ASXL1 (28%), TET2 (16%), EZH2 (15%), NRAS (15%), SETBP1 (12%), and RUNX1 (12%), with ETNK1 mutations seen in 10%.26,27 Initial data ascribed CSF3R mutations to 30% of patients with aCML, but in our experience these mutations are uncommon in WHO-defined aCML and are more reflective of CNL.28 aCML and CMML share overlapping mutational profiles largely differentiated by the frequencies of NRAS, CBL, TET2, SRSF2, and ETNK1 mutations.15,29
MDS/MPN-U
This subtype consists of a conglomerate of poorly defined MDS/MPN overlap syndromes, not meeting criteria for other well-defined entities in this group1 . Frequencies of gene mutations encountered include ASXL1 (30% to 50%), SRSF2 (23% to 37%), SETBP1 (11% to 21%), JAK2 (19% to 25%), NRAS (10% to 15%), and TET2 (15% to 27%).30,31 Less frequent occurrences of TP53 and CBL mutations have also been documented, with a negative impact on survival.30 Although MDS/MPN-U does not have a specific prognostic scoring system, 2 studies have shown that MDS-centered prognostic models such as the international prognostic scoring system can be used to risk stratify affected patients.30,31
Functional categories of mutated genes encountered in MDS/MPN overlap neoplasms
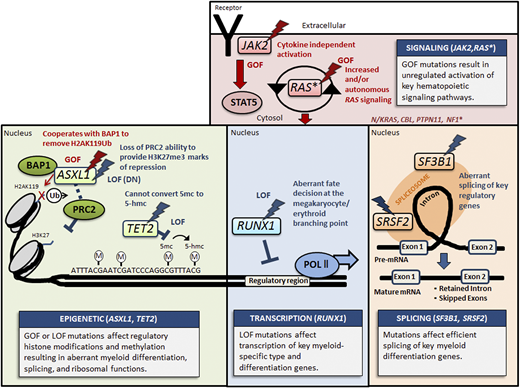
Mechanisms of key mutations in gene categories represented in MDS/MPN overlap syndromes. This figure illustrates the top represented mutated genes in each of four categories: signaling (pink), epigenetic (green), transcription (blue), and splicing (orange). Key mutated genes in each panel are highlighted by lightning, and the color red corresponds to gain-of-function (GOF) mutations, whereas the color blue denotes a loss-of-function (LOF) or dominant negative (DN) mutant effect. Other RAS pathway genes* include NRAS, KRAS, CBL, PTPN11, and NF1.
Mechanisms of key mutations in gene categories represented in MDS/MPN overlap syndromes. This figure illustrates the top represented mutated genes in each of four categories: signaling (pink), epigenetic (green), transcription (blue), and splicing (orange). Key mutated genes in each panel are highlighted by lightning, and the color red corresponds to gain-of-function (GOF) mutations, whereas the color blue denotes a loss-of-function (LOF) or dominant negative (DN) mutant effect. Other RAS pathway genes* include NRAS, KRAS, CBL, PTPN11, and NF1.
Epigenetic regulator genes
Key altered epigenetic regulator genes include TET2, ASXL1, EZH2, DNMT3A, IDH1, and IDH2. TET2 is a critical dioxygenase that helps convert 5-methylcytosine to 5-hydroxymethylcytosine and other oxidative metabolites, which regulate the state of DNA accessibility (methylation).10 TET2 is mutated in 60% of patients with CMML, and in the absence of ASXL1 mutations it has a favorable prognostic impact. ASXL1 regulates chromatin dynamics through its interaction with the polycomb group repressive complex proteins (PRC1 and PRC2).32 It is believed that ASXL1 mutations result in loss of PRC2-mediated H3K27 (histone 3 lysine 27) tri-methylation.33 In addition, recent data suggest that ASXL1 truncations confer enhanced activity on the ASXL1-BAP1 (BRCA associated protein 1) complex. Both of these pathways result in a global erasure of H2AK119Ub and depletion of H327Kme3, promoting dysregulated transcription.34 EZH2 is a key catalytic component of PRC2, and loss-of-function mutations result in dysregulated chromatin dynamics. EZH2 mutations are seen in aCML (15%) but are uncommon in CMML (<5%), where they often co-occur with ASXL1 mutations and are associated with poor outcomes.9,35 DNMT3A encodes for the DNA methyltransferase responsible for the conversion of cytosine to 5-methylcytosine. DNMT3A mutations are seen in <5% of patients with CMML and are associated with poor outcomes.8 IDH1 and IDH2 are key components of oxidative phosphorylation, with mutant IDH1/2 generating the oncometabolite 2-hydroxyglutarate (2-HG). 2-HG in turn suppresses TET2 activity, mimicking a TET2 mutant effect on methylation, with IDH1/2 mutations being infrequent (<5%).5 It is believed that because of the convergence of pathways (2-HG–mediated suppression of TET activity), TET2 and IDH1/2 mutations are largely mutually exclusive.
Splicing mutations
Spliceosome components are critical regulators of pre-mRNA splicing, with gene mutations involving SRSF2, SF3B1, U2AF1, and ZRSR2 implicated in myeloid oncogenesis.16 In CMML, SRSF2 mutations are seen in 50% of patients, with no clear impact on survival, whereas SF3B1 mutations are less common and phenotypically associated with BM RS.16,36 SF3B1 mutations are seen in 90% of patients with MDS/MPN-RS-T, often co-occurring with JAK2V617F.24
Signaling mutations
Aberrant signaling in overlap neoplasms involves mainly the oncogenic RAS pathway and is secondary to mutations involving NRAS, KRAS, CBL, PTPN11, and NF1.37 In MPN-CMML, these mutations can be early clonal/dominant events and are associated with poor outcomes,5 whereas they occur later and impose transformation risk in MDS-CMML. JAK2 is the other common signaling mutation seen, with JAK2V617F identified in 50% of patients with MDS/MPN-RS-T and in 10% of patients with CMML.38 CSF3R, MPL, CALR, and FLT3 mutation are uncommon (<5%). Signaling mutations in MDS/MPN overlap neoplasms are associated with cytokine deregulation and inflammation. Mutations involving the JAK/STAT pathway (JAK2/CALR/MPL) and the oncogenic RAS pathway (NRAS, KRAS, CBL, PTPN11, and NF1) result in complex ligand-independent deregulation in cytokine production and secretion. Cytokines significantly elevated in patients with CMML and signaling mutations include IL-10, CCL2/MCP-1, CD44, IL-1RA, and CXCL7, whereas lower IL-6 levels have been seen in TET2-mutant CMML.39 Although granulocyte–macrophage colony-stimulating factor (GM-CSF) levels were not statistically different between patients with CMML and controls, GM-CSF hypersensitivity has been well documented in both JMML and RAS pathway mutant CMML patient samples.40
Transcription factors
RUNX1, a critical transcription factor gene, can be mutated in CMML (15%), MDS/MPN-U (14%), and aCML (12%).17,30 RUNX1 mutations are associated with lower platelet counts and a shortened LFS.17 These mutations should be curated manually to ensure that they are not germline, given that RUNXI-FPD (familial platelet disorder) is associated with an inherent risk for myeloid neoplasms.17 This is particularly relevant when RUNX1 mutation variant allele frequencies are in the heterozygous range (40% to 60%). Based on a family history of thrombocytopenia and myeloid neoplasms, personal history of antecedent thrombocytopenia, and the clinical scenario (eg, choosing matched related donors), germline tissue (skin biopsy–derived fibroblast or hair follicle–derived DNA) assessments should be considered. In addition, RUNX1-FPD can result from gene deletions, which are often missed by amplicon based-NGS assays.17 In these circumstances, copy number analysis can be carried out with array comparative genome hybridization assays.
Others
SETBP1 mutations are found in 15% of patients with CMML and aCML and are associated with inferior outcomes.2,41 Various oncogenic mechanisms have been proposed, including binding to the SET region and interfering with methylation of lysine residues on histone tails. TP53 is a critical tumor suppressor gene, and mutations are infrequent in MDS/MPN overlap syndromes. STAG2 and RAD21 are components of the cohesion complex, with mutations seen in <10% of patients, with no clear impact on outcomes.
Cytogenetic abnormalities in MDS/MPN overlap neoplasms
Clonal cytogenetic abnormalities are seen in ∼30% of patients with CMML, with common alterations including trisomy 8 (+8), –Y, abnormalities of chromosome 7 (monosomy 7 and del7q), trisomy 21, and complex karyotypes.42-44 The CMML-specific cytogenetic risk stratification (CPSS) system categorizes patients in three groups: high risk (+ 8, chromosome 7 abnormalities, or complex karyotype), intermediate risk (all except for those in the high- and low-risk categories), and low risk (normal karyotype or –Y), with 5-year OS of 4%, 26%, or 35%, respectively.43 The Mayo–French cytogenetic risk stratification system was developed to refine this prognostication and has three distinct risk categories: high (complex and monosomal karyotypes), intermediate (all abnormalities not in the high- or low-risk groups), and low (normal, sole –Y, and sole der(3q)), with median survivals of 3 (hazard ratio, 8.1; 95% confidence interval, 4.6-14.2), 21 (hazard ratio, 1.7; 95% confidence interval, 1.2-2.3) and 41 months, respectively.45
Cytogenetic abnormalities in JMML are uncommon; monosomy 7 is the most common, with this abnormality clustering with KRAS-mutant JMML.21 Although cytogenetic abnormalities are uncommon in MDS/MPN-RS-T (80% with normal karyotype), approximately 50% of patients with MDS/MPN-U have cytogenetic aberrations (+8 and complex karyotypes, 15% each, and monosomy 7, 10%).23,30 Cytogenetic changes are seen in approximately 30% to 40% of patients with aCML, with +8 being most common.26
Integration of molecular and cytogenetic abnormalities for diagnosis, prognostication, and therapeutics of MDS/MPN overlap syndromes
Diagnosis
Although none of the aforementioned gene mutations or cytogenetic abnormalities are specific to a single MDS/MPN subtype, molecular signatures can be used in combination with clinical and morphological features to help establish a diagnosis (Figure 2). Data from clonal hematopoiesis and clonal architectural studies in CMML have shown that coexpression of TET2 and SRSF2 mutations result in clonal monocytosis, with the acquisition of subsequent driver mutations defining dysplastic (RUNX1, SETBP1, DNTM3A, ASXL1) or proliferative (NRAS, KRAS, CBL, PTPN11, JAK2) CMML subtypes.46,47 Based on their frequency and co-occurrence, the presence of ASXL1, TET2, and SRSF2 mutations in the presence of adult-onset sustained monocytosis (>3 months) can be used to establish a diagnosis of CMML.1 As mentioned before, MPL and CALR mutations are uncommon in CMML, and their presence points toward a differential diagnosis of MPN with monocytosis.38 In MDS/MPN-RS-T, there is acquisition of driver signaling mutations, most commonly JAK2V617F in the context of antecedent SF3B1 mutant MDS-RS, giving rise to anemia and thrombocytosis.48 SF3B1 mutations correlate strongly with the presence of BM ring sideroblasts, and the presence of JAK2/SF3B1 mutations with BM RS and thrombocytosis can be used to establish a diagnosis of MDS/MPN-RS-T.36 The presence of germline or somatic RAS pathway mutations, in the context of early-onset monocytosis (infants and children), can be used to establish a diagnosis of JMML, whereas subsequent clonal hematopoiesis (SETBP1, ASXL1, and JAK3) is usually a marker of disease progression. Although aCML and MDS/MPN-U do not have classic molecular features, the relative enrichment of SETBP1 and ETNK1 mutations in aCML can be helpful in the presence of dysplastic neutrophilia.
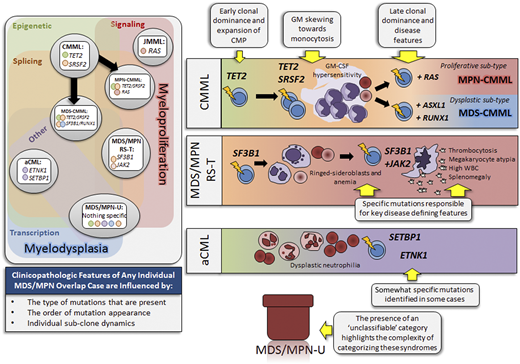
Clonal architecture and molecular signatures of MDS/MPN overlap syndromes. The panel on the left illustrates all 5 MDS/MPN overlap syndrome entities with corresponding specific mutational signatures. CMML has additional subcategories based on the relative enrichment of mutation types in proliferative (MPN-CMML) or dysplastic (MDS-CMML) CMML. Each entity is spatially placed according to mutation type in relation to myeloproliferative (on the right) and myelodysplastic (on bottom) features. The five mutated gene categories are represented in the left panel: epigenetic (green), signaling (pink), splicing (orange), other (purple), and transcription (blue). The panels on the right depict the influence of mutations on each MDS/MPN overlap subtype. aCML, atypical chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; CMP, common myeloid progenitor; GM, granulocytic-monocytic; JMML, juvenile myelomonocytic leukemia; MDS/MPN-RS-T, MDS/MPN-ring sideroblasts and thrombocytosis; MDS/MPN-U, MDS/MPN-unclassifiable.
Clonal architecture and molecular signatures of MDS/MPN overlap syndromes. The panel on the left illustrates all 5 MDS/MPN overlap syndrome entities with corresponding specific mutational signatures. CMML has additional subcategories based on the relative enrichment of mutation types in proliferative (MPN-CMML) or dysplastic (MDS-CMML) CMML. Each entity is spatially placed according to mutation type in relation to myeloproliferative (on the right) and myelodysplastic (on bottom) features. The five mutated gene categories are represented in the left panel: epigenetic (green), signaling (pink), splicing (orange), other (purple), and transcription (blue). The panels on the right depict the influence of mutations on each MDS/MPN overlap subtype. aCML, atypical chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; CMP, common myeloid progenitor; GM, granulocytic-monocytic; JMML, juvenile myelomonocytic leukemia; MDS/MPN-RS-T, MDS/MPN-ring sideroblasts and thrombocytosis; MDS/MPN-U, MDS/MPN-unclassifiable.
Prognosis
Gene mutations have prognostic value in MDS/MPN overlap neoplasms. ASXL1 mutations are universally detrimental across myeloid neoplasms and have a particularly poor outlook in CMML.2,13,14 In CMML, these mutations have been incorporated into three molecularly integrated prognostic models: Mayo Molecular Model, CPSS-molecular, and the Groupe Francophone des Myelodysplasies model.2,13,14 All three models effectively integrate clinical and molecular features and help risk stratify patients with regard to OS and LFS (Table 3). In addition to ASXL1 mutations, the CPSS-molecular model includes NRAS, RUNX1, and SETBP1 mutations and also incorporates clonal cytogenetic abnormalities (genetic score).13 In JMML, the presence of germline mutations in CBL and PTPN11 can be associated with spontaneous regressions, and the secondary acquisition of SETBP1 and JAK3 mutations is associated with disease progression and inferior OS.19,22 In fact, in JMML, knowledge on specific nucleotide changes is informative, with somatic NRAS and KRASG12S mutations being associated with better outcomes than the typical G12D mutations.49 In MDS/MPN-U we recently demonstrated the negative prognostic impact of TP53 and CBL mutations, and the ASXL1mt/SETBP1mt genotype is associated with adverse outcomes in aCML.26,30 Gene mutations are also predictive of allogenic hematopoietic cell transplantation (HCT) outcomes. In a molecularly annotated cohort of 52 CMML patients who underwent HCT, NRAS mutations were associated with higher relapse rates, whereas ATRX and WT1 mutations were associated with relapse and an inferior OS.50 This study also showed that higher mutational burdens (≥10) and mutations involving ≥4 epigenetic regulator genes were associated with poor outcomes.50
Clinical therapeutics
Currently, allogenic HCT remains the only curative option for higher-risk MDS/MPN overlap neoplasms, with HMA being used for HCT-ineligible patients. Although HMA epigenetically restores hematopoiesis in a subset of patients with CMML (30% to 40%), serial monitoring of somatic mutations has shown that they do not affect mutational allele burdens, with disease progression occurring in most.51 Gene mutations that serve as therapeutic targets in myeloid neoplasms are uncommon in MDS/MPN overlap neoplasms. Effective targets such as mutations involving IDH1, IDH2, and FLT3 are seen in <10% of patients,8 and emerging targets such as TP53 are even more uncommon (<5%). Given the ubiquitous nature of splicing mutations in these diseases, spliceosome component inhibitors in clinical trials are being eagerly watched. MEK inhibition in RAS mutant subtypes has not proven to be an effective strategy.52 In CMML, the presence of the ASXL1wt/TET2mt genotype is best associated with responses to HMA,10,53 whereas clonal RAS pathway mutations (MPN-CMML) are associated with resistance. Gene mutations affecting prognosis (ASXL1, NRAS, RUNX1, and SETBP1) in CMML also help with important decisions with regard to timing and the need for allogenic HCT.
Our patient is a 71-year-old man who presented with constitutional symptoms, splenomegaly, anemia, leukocytosis, monocytosis, and thrombocytopenia (Figure 3). His BM has features of dysplasia, and NGS testing has identified mutations involving ASXL1, TET2, SRSF2, and NRAS. These features suggest a diagnosis of CMML-1. According to the CPSS-molecular model, he fits into the intermediate-2 risk category, with an estimated median OS of 18 months and a 48% cumulative incidence of AML at 48 months.13 According to the Mayo Molecular Model, he fits into the high-risk category, with a median OS of 16 months.2 This patient will benefit from an allogenic transplant consult and will probably need pretransplant cytoreductive therapy with HMA.
MDS/MPN overlap case study. Shown is the current clinical vignette with symptoms, laboratory results, diagnosis, and resulting prognostication. AML, acute myeloid leukemia; HMA, hypomethylating agent; OS, overall survival.
MDS/MPN overlap case study. Shown is the current clinical vignette with symptoms, laboratory results, diagnosis, and resulting prognostication. AML, acute myeloid leukemia; HMA, hypomethylating agent; OS, overall survival.
Conclusions
MDS/MPN overlap neoplasms are a well-defined group of myeloid neoplasms with unique molecular signatures. Mutations in ASXL1, TET2, and SRSF2 are common in CMML, whereas the SF3B1/JAK2V617F genotype often defines the pathobiology of MDS/MPN-RS-T. JMML is a RAS-driven disease, with germline and somatic mutations in the RAS pathway accounting for most cases. aCML is enriched in SETBP1 and ETNK1 mutations, and MDS/MPN-U is the least defined in this group. Understanding the molecular landscape in overlap neoplasms is important, because it helps with establishing a diagnosis, helps with disease prognostication, and in certain cases allows selection of appropriate treatment strategies.
Acknowledgment
This publication is supported by a grant from the Henry J. Predolin Foundation for Research in Leukemia, Mayo Clinic, Rochester, Minnesota.
Correspondence
Mrinal M. Patnaik, Division of Hematology, Mayo Clinic, Rochester, MN 55905; e-mail: patnaik.mrinal@mayo.edu.
References
Competing Interests
Conflict-of-interest disclosure: M.M.P. has served on the advisory boards for Kura Oncology and Stemline Therapeutics. T.L.L. has no competing interests to declare.
Author notes
Off-label drug use: None disclosed.