Abstract
Advances in treatment options for patients with multiple myeloma have made a significant impact on the overall survival of patients and have helped achieve levels of response and duration of remission previously not achievable with standard chemotherapy-based approaches. These improvements are due, in large part, to the development of the novel agents thalidomide, bortezomib, and lenalidomide, each of which has substantial single-agent activity. In addition, a large number of second-generation agents are also in clinical development, such that the repertoire of available treatment options continues to expand. To better interpret clinical trials performed in the relapsed setting, it is important that definitions of relapse categories are used to help better pinpoint the specific benefit for a given therapy, especially in the combination therapy setting as it aids in determining if ongoing work should be continued or abandoned for a given new agent. Insights from preclinical modeling and in vitro work have identified several new combinations, new targets and second- or third-generation versions of existing targets that hold great promise in the setting of relapsed myeloma. Combinations of thalidomide, bortezomib, and lenalidomide with conventional agents or among each other have resulted in enhanced response rates and efficacy. Clinical trials of agents such as carfilzomib, pomalidomide, vorinostat, panobinostat, and elotuzomab are just a few of the many exciting new compounds that are being tested in phase 1 and phase 2 clinical trials for relapsed patients. Further clinical and translational testing are critical to better understanding how best to combine these new agents, as well as identifying patient populations that may best benefit from treatment with these developing new agents.
The spectrum of treatment options for patients with relapsed multiple myeloma has dramatically changed over the past 10 years such that many patients can now enjoy long periods of remission following relapse. These prolonged remissions are due, in large part, to the development of new classes of agents, such as thalidomide, bortezomib, and lenalidomide—all of which have substantial single-agent and combination activity. In addition, a large number of second-generation agents or new targets are also in clinical development, and the repertoire of available treatment options continues to expand.
Definition of Relapsed/Refractory Disease
In the context of relapsed and/or refractory, three groups of patients exist.1,2 The first is a group that has “relapsed” disease, which specifically includes patients whose first progression occurs in the absence of any therapy following successful initial therapy. Although the definition of relapsed disease requires a ≥ 25% increase in the serum or urine protein and ≥ 0.5 mg/dL, the presence of “biochemical” relapse alone is not indication for additional systemic therapy. Because the patient time to relapse can be quite variable (weeks to months), patients should have some form of symptomatic relapse prior to initiation of therapy, because many patients could survive for some time with biochemical progression and yet not require additional therapy beyond careful monitoring.
The next category is comprised of patients having relapsed and refractory disease, who are defined as progression on a specific therapy, or within 60 days of completion of a given therapy (International Myeloma Working Group Consensus Panel, International Myeloma Workshop, February 2009). Historically, this was limited to steroid or alkylator-based approaches; thus, “refractory” was a generic term. But, more recently, it has become associated with specific agents, such as bortezomib or lenalidomide refractory relapse. This is clearly important because patients who are refractory to bortezomib may still be responsive to lenalidomide or vice versa, and this agent-specific resistance may continue to be relevant for the sequential evaluation and integration of new agents that are in development in the relapsed setting. This group of patients may be especially challenging among the group of patients who have received multiple prior lines of therapy and outside of clinical trials have few treatment options.
The final category is primary refractory, which also represents a potentially challenging group of patients who did not achieve a response following induction therapy. As with refractory disease, this category is most useful when described in the context of specific agents or combinations, and it is particularly important to distinguish the group of patients who can have a variable course with less aggressive tempo of disease despite initial resistance.
Characteristics of Relapse
As one considers the salvage treatment options, characteristics associated with the relapsed disease are important (Table 1). First, what was the duration of the first response? It is known that, among patients with remission duration of < 6 months, their duration of response is inferior to patients with remission duration of greater than 12 months. Patients with rapid progression or aggressive relapse are also to be considered differently from those with indolent slowly progressive relapse. Additional factors for consideration include agents previously utilized for either induction or previous relapse salvage (both response to and tolerance of these agents), as well as duration of response. Each of these factors is utilized to plan for the choice of relapse therapy. For instance, a patient with indolent relapse who received a proteasome inhibitor-based treatment, followed by high-dose therapy, is a good candidate for a single-agent approach (eg, immunomodulatory agent-based approach). Conversely, a patient with short duration remission (6 months) is more likely to benefit from combination therapy, because single agents are less likely to be useful in this circumstance. Additionally, patients with a high-risk feature (del 17p, t(4:14) or t(14:16)) usually warrant combination therapy approaches, given the rapidity of relapses and its aggressive nature.
Allogeneic Transplant
The use of allogeneic transplant for the treatment of relapsed myeloma remains a strategy with limited clinical benefit. Most studies evaluating its use in this setting demonstrate long-term, disease-free survival of 10% to 20%, with a significant fraction of patients developing debilitating chronic graft-versus-host disease or relapse.3,4 Even among patients with biologically poor risk disease as defined by Injury Severity Score or cytogenetics, few patients are ultimately cured with allogeneic transplant.5 Given the significant limitations of treatment-related mortality, morbidity, and poor overall outcomes, the use of allogeneic transplant for the management of relapsed myeloma should be discouraged until more effective and less toxic approaches are established.
Chemotherapy and Second Transplant
In the setting of relapsed disease, the use of conventional or high-dose chemotherapy has been a long-standing approach as salvage therapy. Chemotherapy regimens (eg, DCEP [dexamethasone, cyclophosphamide, etoposide, and cisplatin] or DT-PACE [cisplatin, doxorubicin, cyclophosphamide, and etoposide]) have traditionally been used in the induction setting, but they have been used extensively in the relapsed setting as well.6,7 Overall response rates for salvage combination chemotherapy are between 30% and 60%, with morbidity and mortality that is related to the intensity of therapy, as well as the refractoriness of the patient at the time of chemotherapy administration. Trieu and colleagues8 presented data on the use of cyclophosphamide weekly with alternate-day prednisone as salvage therapy and demonstrated an overall response rate of 41%, with a median progression-free survival (PFS) of 18.6 months and an overall survival (OS) of 28.6 months. With regard to the role of second autologous transplant, a series from Olin et al9 reviewed 66 patients who underwent second autologous transplant as salvage therapy for relapsed myeloma and identified a median PFS of 8.5 months, with a median OS of 20.7 months. Subset analysis suggested that patients with five or more prior therapies, or a median PFS of < 1 year following the first transplant were less likely to benefit from a second transplant. Overall, patients likely to gain significant benefit from a second autologous transplant, as viewed from a PFS perspective, are those with durations of response longer than 2 years. However, autologous transplant can have value in the context of reestablishing hematopoiesis among a group of heavily pretreated patients with poor hematologic reserve, thus allowing them to receive additional cytotoxic or investigational therapy once their counts and disease status are improved.
Thalidomide
Thalidomide is one of the first novel agents to be evaluated in relapsed/refractory myeloma patients, based initially on its inhibitory effects on angiogenesis.10,11 Initial trials used doses ranging from 200 mg/day to 800 mg/day, and demonstrated activity despite a heavily, pretreated refractory patient population. A subsequent review by Glasmacher and colleagues12 demonstrated that thalidomide alone produced partial remission rates in 30% of patients, and other studies have demonstrated that the overall response rate can be significantly enhanced with the addition of concomitant dexamethasone. Toxicities associated with thalidomide include sedation, constipation, and increased risk of venous thromboembolism, as well as peripheral neuropathy that occurs more frequently if the daily dose exceeded 200 mg. In terms of a dose-response effect, investigators from Investigations and Fraud Management studied 100 versus 400 mg/day that showed no difference in 1-year OS, and patients randomized to receive 100 mg were more likely to have dexamethasone added due to suboptimal response, but clearly had a lower incidence of significant toxicity.13
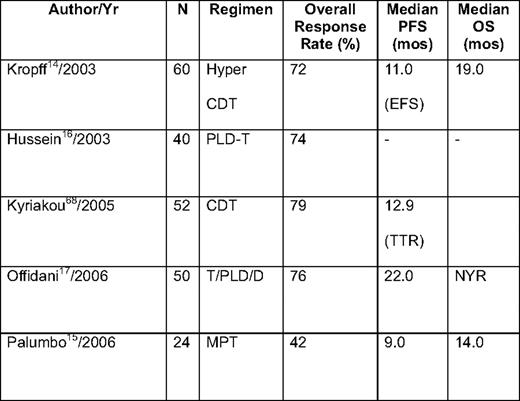
Thalidomide has also been combined with conventional cytotoxic drugs (eg, alkylating agents14,15 and anthracyclines),16,17 as well as with novel agents, such as bortezomib,18 in relapsed/refractory myeloma (Table 2). Trials combining thalidomide with conventional chemotherapy are clearly active, and result in overall response rates of 60% to 75%, with complete remission (CR) rates of approximately 20% in a number of early phase I/II studies. One case-matched study by Offidani et al19 compared thalidomide + dexamethasone + pegylated liposomal doxorubicin (ThaDD) with thalidomide + dexamethasone alone. ThaDD produced a higher overall and complete response rate than thalidomide + dexamethasone (92% and 30% vs 63% and 10%, respectively). In addition, the median PFS and OS were better with ThaDD (21 vs 11 months and 35 vs 20 months, respectively).19 Thalidomide combinations with chemotherapy, specifically anthracyclines, carry an increased risk of venous thromboembolic (VTE) complications, which often requires more intense prophylaxis than is used when patients received thalidomide with dexamethasone or bortezomib. In one series from the Arkansas group, the combination of doxorubicin with thalidomide, as part of the DT-PACE regimen, resulted in a > 25% incidence of deep venous thrombosis, necessitating more intense anticoagulation prophylaxis.20
Bortezomib
Bortezomib is a proteasome inhibitor with potent antimyeloma activity as a single agent.21 The large randomized APEX (Assessment of Proteasome Inhibition for Extending Remissions) trial demonstrated the superiority of bortezomib given intravenously on days 1, 4, 8 and 11 of a 21-day cycle over pulse dexamethasone in myeloma patients with relapsed/refractory disease who had received no more than three prior treatment regimens. The overall response rate was 38%, and median time to progression (TTP) was 6.2 months, compared with only 18% and 3.5 months with dexamethasone at the time of the first analysis.22 Further follow-up has yielded a response rate of 43%, compared with 9%, with bortezomib, and a longer median OS of 29.8 versus 23.7 months, despite the fact that over 60% of patients in the dexamethasone arm were allowed to crossover to receive bortezomib.23
The toxicity profile of bortezomib has been well-characterized, and includes nausea, diarrhea, cyclic reversible thrombocytopenia, fatigue, and peripheral neuropathy.21,22,24,25 Peripheral neuropathy occurs in about one-third of patients and may have a painful component. Dose modification or discontinuation of bortezomib is required in the latter setting; the neuropathy improves or resolves in a high proportion of affected individuals, although often over a several month period.26
Bortezomib combinations have been evaluated in a number of different settings and are being widely tested due to the minimal effect on marrow function and its ease of use in renal insufficiency27 and lack of thrombogenicity.28 From preclinical evaluations, the combination of bortezomib with immunomodulatory agents, alkylators, and other novel agents are predicted to have significant activity based on preclinical rationale (Table 3).18,29–32 These generally produce high overall response rates, in the range of 50% to 80% with CR/near CR rates of 15% to 30%, although their impact on duration of response and OS is uncertain at this time. However, one large randomized trial comparing bortezomib alone with bortezomib + pegylated liposomal doxorubicin demonstrated the superiority of the combination in terms of TTP (9.3 vs 6.5 months) and OS, despite only a modest increase in overall response rate (52% vs 44%).33 Preclinical studies have demonstrated that many new investigational antimyeloma agents demonstrate at least additive benefits when combined with bortezomib, setting the stage for a multitude of ongoing phase I/II clinical trials of such combinations.
Lenalidomide
Lenalidomide is the most recent novel agent approved for relapsed/refractory myeloma in the United States and Europe. Approval was based on the results from two parallel trials (MM-009 and MM-010), in which lenalidomide + dexamethasone was compared with dexamethasone alone in patients with progressive myeloma who had received one to three prior regimens. The dose of lenalidomide was 25 mg on days 1 to 21 of a 28-day schedule, with pulse dexamethasone given on days 1 to 4, 9 to 12, and 17 to 20 for the first four cycles and with dose reduction of dexamethasone for subsequent cycles.34,35 The results of the two trials were identical, with overall response rates of 60% and 61% for lenalidomide + dexamethasone, compared with 20% and 24% with dexamethasone as a single agent. The median TTP was approximately 11 months in both trials, whereas the median OS with the combination was not yet reached in the North American trial (MM-090)34 and was 29.6 months in the European trial (MM-010).35 The benefit of lenalidomide + dexamethasone was apparent despite extensive crossover of patients from the dexamethasone to lenalidomide + dexamethasone arm, similar to what was seen in the APEX trial.
Lenalidomide is associated with fewer and different toxicities than thalidomide, with less somnolence, constipation, and peripheral neuropathy. However, it is associated with an increased risk of VTE, similar to thalidomide, and thromboprophylaxis of some form is required.36 However, unlike thalidomide, lenalidomide is associated with myelosuppression.34,35 If significant neutropenia occurs, either the dose of lenalidomide can be reduced or granulocyte-colony stimulating factor can be given while maintaining the full lenalidomide dose. Experience with lenalidomide in patients with renal sufficiency is relatively limited, although the drug has been administered to patients with variable degrees of renal compromise without prohibitive toxicity, with close observation on myelosuppression as a main side effect.37,38
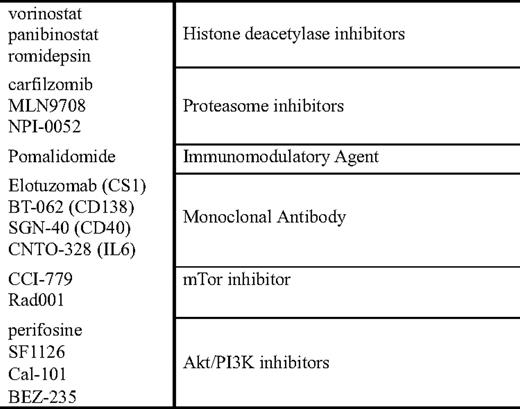
Lenalidomide-based combinations, in a similar vein to those proposed with thalidomide, are currently in progress (Table 4). These include combinations with doxorubicin or pegylated liposomal doxorubicin39,40 and cyclophosphamide.41,42 Based on preclinical models, combinations of lenalidomide with the histone deacetylase (HDAC) inhibitors vorinostat and panobinostat, as well combinations with monoclonal antibodies and proteasome inhibitors are also in progress.
Combinations in Development
Rationale for Combinations
The rationale for combination studies in relapsed or newly diagnosed disease is two-fold. First, it is clear across most of oncology that combinations are more effective than single agents at inducing responses, and, if tolerable, durable responses. Second, exposure of malignant cells to a single agent often results in preferential overactivation of a survival pathway, one that can then be targeted using a second agent. This concept of induced pathway dependence or addiction forms the basis for many rationally based combinations in myeloma, as described later. Finally, the use of combinations should also take advantage of other potential mechanisms of action for a given single agent that can be enhanced when used in conjunction with another agent.
Proteasome Inhibitor-Based Approaches
In addition to the effects of single-agent proteasome inhibitors on plasma cell apoptosis, there are a number of combination strategies that are predicted by preclinical data and appear to warrant further clinical study. The first of these involves the combination of the heat shock protein (HSP) inhibitor 17AAG with bortezomib. Early data from Mitsiades and colleagues43 suggested that exposure to bortezomib resulted in a compensatory stress response that included early and rapid upregulation of HSP 90, in addition to HSP 27 and other markers of cellular stress. In vitro and in vivo work further demonstrated that the combination of a HSP-90 inhibitor, in conjunction with bortezomib, appeared to induce significant regression of tumors in xenograft models. A phase I clinical trial conducted by Richardson and colleagues,44 combining tanespimycin with bortezomib, demonstrated not only an encouraging overall response rate (57%), but also that there were patients with bortezomib-resistant disease who responded, suggesting reversal of bortezomib resistance. Additionally, other HSP inhibitors are in development, and given the preclinical data suggesting that overexpression of HSPs are found broadly in cancer cells, the concept of combinations of agents utilizing an HSP inhibitor as part of the therapy is being tested in multiple different tumors.45
Additional data are emerging combining bortezomib with HDAC inhibitors (vorinostat, LBH 589 [panobinostat], and depsipeptide) and in preclinical models have demonstrated significant synergy. Mechanistically, this is thought to be related to the effects of HDAC inhibitors on HDAC 6, which is critical to the function of an alternative pathway of protein catabolism: the aggresome/autophagy pathway.46 When patients are exposed to proteasome inhibition, the alternative pathway is upregulated (aggresome pathway), and protein catabolism occurs via this alternative pathway. Preliminary data from two phase I studies presented at the 2007 Annual Meeting of the American Society of Hematology 2007 combining the HDACi vorinostat with bortezomib demonstrated significant responses; and, among the patients who were defined as bortezomib-resistant, the overall response rate was 30%.47,48
Preclinical data from Hideshima et al49 has also explored other potential survival responses among malignant plasma cells following treatment with bortezomib. One interesting potential pathway to target is the PI3K/AKT/mTOR pathway that appears to be activated following exposure to bortezomib. Several agents are in development both in the preclinical and clinical settings that are testing the ability to block both the proteasome and PI3K/AKT/mTOR pathways. The combination of the alkylphospholipid perifosine, an AKT inhibitor, is being tested with bortezomib in a trial from Richardson and colleagues,50 and, to date, has demonstrated encouraging preliminary activity in the relapsed myeloma setting. Additional direct PI3K inhibitors are currently in development, and their use either alone or in combination with strategies that induce cellular dependence on PI3K as part of a survival mechanism is underway as well.
Finally, the combination of the immunomodulatory lenalidomide with bortezomib was also predicted in preclinical models to have synergistic effects in combination, and this too has been tested in clinical trials both in the relapsed and newly diagnosed myeloma setting.51 Among all patients with relapsed disease, 84% of patients responded to the RVD combination (revlimid, bortezomib, and dexamethasone),52,53 and in the newly diagnosed patient population, the overall response rate was 100%, with 44% of patients achieving a CR/near CR.54
In addition to the effects of lenalidomide on directly inducing apoptosis of myeloma cells, lenalidomide, perhaps more so than thalidomide, is also an immunomodulatory agent that enhances natural killer (NK) cell function, increases T-cell secretion of interleukin (IL)-2, and increases overall immune activation.55 Given theses properties, there is strong preclinical and clinical rationales to combine lenalidomide with other “immune-based” treatment approaches, such as monoclonal antibodies and vaccines. There is clinical data suggesting that the responses with thalidomide are associated with an increase in NK cell number and function, as manifested by increased IL-2 and interferon-γ secretion. For lenalidomide, there are data supporting NK cell expansion following exposure to lenalidomide, both among normal healthy volunteers and patients with myeloma.56 For this reason, the approach of lenalidomide + monoclonal antibody holds great promise as a therapeutic strategy to enhance not only the efficacy of lenalidomide, but also to enhance the efficacy of our currently available monoclonal antibodies. In a pilot phase I/II trial combining the CS1 antibody elotuzomab with lenalidomide and low-dose dexamethasone, the overall response rate for the first 28 patients treated was 82%, and, among the cohort of patients that were lenalidomide-naive, the response rate jumped to 95%.57 Although this data is very preliminary in nature, it certainly provides clinical support for the concept of lenalidomide enhancing the efficacy of a monoclonal antibody and will be further tested with a number of different antibodies in the future as well.
Second Generation and New Versions of Active Agents
Clearly, the activity of proteasome inhibition and immunomodulatory agents is established in myeloma therapy and is being explored in other diseases as well.
Second-generation proteasome inhibitors are now in development with different functional abilities. PR-171 (carfilzomib) as an irreversible inhibitor of chymotryptic activity of the proteasome, the same site of inhibition induced by bortezomib that is currently being tested in phase I/II trials in myeloma and other diseases.58,59 Phase II trials evaluating the efficacy of carfilzomib in the relapsed and refractory populations,60 and in the bortezomib-exposed61 and bortezomib-naive62 populations thus far have demonstrated encouraging activity. Although only a small fraction of patients with bortezomib refractory disease achieve an objective response, there are a number of patients who achieve stable disease for prolonged periods; and, in the vast majority of trials to date, the incidence of peripheral neuropathy appears to be lower among patients treated with carfilzomib63 when compared with bortezomib, allowing for longer durations of therapy. Additionally, there are other proteasome inhibitors in development that have different spectrums of activity (pan-proteasome inhibition with NPI-0052), as well as oral proteasome inhibitors that are in preclinical and clinical development.
Complementary to the development of newer proteasome inhibitors, there is the development of a new immunomodulatory agent CC-4047 (pomalidomide). Data initially presented by Schey and colleagues64 showed safety and efficacy of this agent in myeloma, and demonstrated a potent immune-activating effect. Trials from the Mayo Clinic and from Richardson and colleagues66 have reevaluated the efficacy of pomalidomide in the context of sensitive and refractory relapse. Lacy and colleagues65 presented data initially on a cohort of 60 relapsed patients and demonstrated an overall response rate of 63%; and, among lenalidomide-resistant patients, the response rate was 40%. Subsequent to this, Richardson and colleagues66 performed a phase I dose escalation study increasing the dose of pomalidomide to 4 mg, and demonstrated, among patients refractory to lenalidomide and bortezomib, an overall response rate of 28%. An update of the Mayo Clinic experience among patients refractory to lenalidomide treated with pomalidomide also demonstrated a similar 32% overall response rate.67
It is clear that the availability of new classes of agents (Table 5), and new, proteasome inhibitors and immunomodulatory agents have changed the natural history of myeloma over the past 10 years. Their use both in the induction setting and the relapsed setting either alone or in combinations has resulted in significant improvements in OS for patients. The wealth of second-generation agents and new agents in development currently suggest that this improvement will continue. Ongoing enrollment to phase II/III trials (Table 6) is critical to successful drug development, and will allow physicians access to even more life-saving advances as we seek to prolong durations of remission, or eventually cure patients of multiple myeloma.
Disclosures
Conflict-of-interest disclosure: The author has been a consultant to Millennium, Celgene, Novartis, and BMS, and has received research funding from the same sources.
Off-label drug use: Use of experimental agents in the treatment of relapsed myeloma.
Correspondence
Sagar Lonial, MD, Winship Cancer Institute, Emory University, 1365 Clifton Rd., Bldg. C, Rm. 4004, Atlanta, GA 30322; Phone: (404) 727-5572; Fax: (404) 778-5530; e-mail: sloni01@emory.edu