Introduction
A 64-year-old man with a history of multiple myeloma presents with new back pain. He has a history of International Staging System stage 1, IgG kappa multiple myeloma with normal cytogenetics that was diagnosed 4 years ago when he presented with a pathological fracture of the left humerus. He was initially managed with mechanical stabilization and four cycles of bortezomib-dexamethasone, as well as 2 years of bisphosphanates. Following induction therapy, he achieved a very good partial response. He subsequently received high-dose melphalan and autologous stem-cell transplantation (auto-SCT) and achieved a complete response post-transplant. He did not receive maintenance therapy and had been lost-to follow-up for about a year. He now presents 5 years after initial diagnosis with back pain and is noted to have a new lytic lesion with a compression fracture at T8. A serum protein electrophoresis demonstrates reappearance of his original monoclonal protein. After appropriate stabilization, he comes to you to discuss additional treatment options.
Treatment of relapsed multiple myeloma depends upon a number of different patient- and disease-related factors. These include duration of first response, exposure to treatment options, age, performance status, and toxicity associated with previous treatments. More recently, the emergence of novel agent-based therapies has significantly changed the clinical outcome for patients with myeloma at all phases of the disease. It is estimated that the use of agents such as thalidomide, lenalidomide, and bortezomib have improved the overall median overall survival by 50%.1 While these agents and auto-SCT have clearly improved response rates, their use in the induction therapy setting has created a new set of challenges: how to best manage patients who relapse after having already been exposed to two or three of these agents during their initial therapy. In this review, we discuss an evidence-based strategy for treatment of patients with relapsed or refractory myeloma who have already been treated with novel agents and auto-SCT.
We performed three separate literature searches for this review. In studying auto-HCT as a salvage therapy, we queried the PubMed database for all combinations of the terms “transplant” (or “transplantation”), “myeloma,” “second,” “salvage,” and “relapsed,” limited to studies published in English. This search yielded eight results, and two additional studies were found in the reference sections of the aforementioned eight articles. Of these, four studies were excluded because they were performed before the era of novel therapeutics. For Table 1 we searched the PubMed database using the terms “myeloma” and “relapsed,” with the limitation of “clinical trial,” “human” and “English.” This yielded 127 hits, of which 34 studies did not included adequate number of patients with previous exposure to novel agents, 15 studies had equivocal results, 36 studies were not relevant for our clinical question, 10 studies were not relevant to our patient population, and four studies were updates. This yielded 43 results. In studying early-phase novel therapeutics (Table 2), we performed a search of the most recent oral presentations at the annual American Society of Hematology and American Society of Oncology meetings. We then cross-referenced the cited agents in a search with “myeloma” and “relapsed” in PubMed. This yielded 11 results.
Overview of combination regimens in relapsed/refractory myelooma patients with previous exposure to novel agents with or without auto-SCT
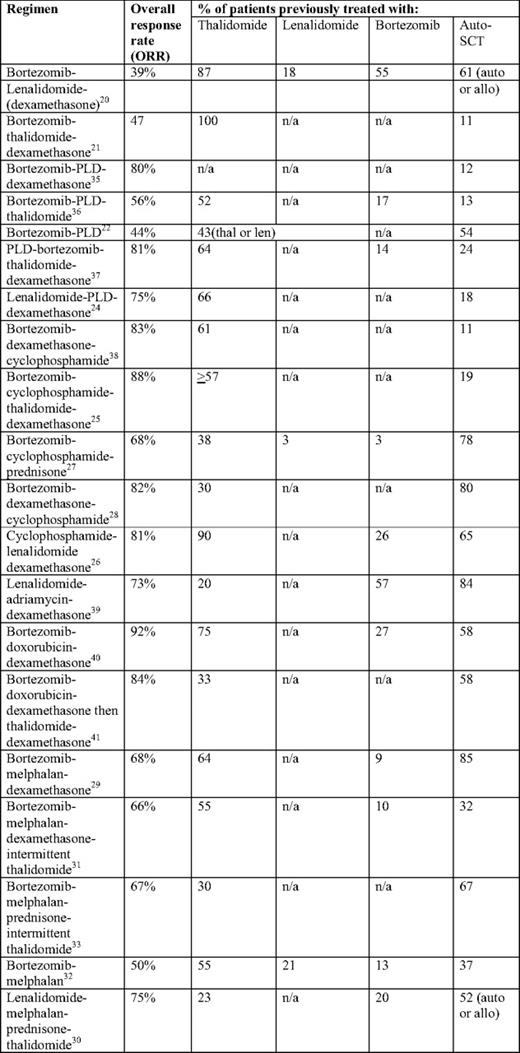
*Overall response rate = complete response + very good partial response + partial response unless otherwise noted.
Early trial results of novel agents in relapsed/ refractory myeloma
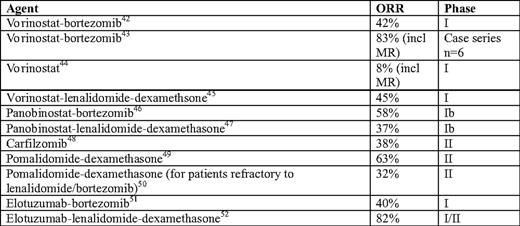
*Overall response rate = complete response + very good partial response + partial response; MR, minimal response.
In the patient who achieved a complete response after auto-SCT and maintained a disease-free interval of approximately 3 years, one could consider another auto-SCT. Available data on second autologous transplants for relapsed patients suggests that these procedures are relatively well-tolerated, with a 100-d mortality of 2% to 8%.2–5 The more recent studies of second, salvage transplants include a sizeable proportion of patients who have received thalidomide, lenalidomide, or bortezomib in the induction setting. The overall response rates in studies done in the past 5 years range from 55% to 69%.2,3,5,6 Because of the limited number of patients in each of these studies, it has been difficult to determine the most important factors in selecting ideal candidates for a salvage auto-SCT. However, one small study suggests that a relapse-free survival of more than 18 months after the first auto-SCT is the most reliable predictor of clinical outcome after a second auto-SCT.7 Although there are no official guidelines, the general consensus is that a salvage transplant with the intent of inducing long-term remission should be offered only to those patients who had a durable response for at least 18 to 24 months after their first auto-SCT.
The role of allogeneic SCT (allo-SCT) in relapsed disease is thus far limited. Patients undergoing allo-SCT as a salvage treatment demonstrate a long-term disease-free survival of 10% to 20%, with a significant portion of patients experiencing treatment-related morbidities, including chronic graft-versus-host disease.8,9 In addition, patients with high-risk disease do not appear to benefit from this treatment, even in the up-front setting.10 Finally, the prospective data for salvage allo-SCT in the era of novel therapeutics is scant. Therefore, the use of allo-SCT for patients with relapsed myeloma is not recommended as a standard salvage approach.
When deciding which agents to use in the relapsed/refractory setting, exposure to previous therapy is an important consideration. Among patients who received bortezomib-based induction, the use of immunomodulatory-based therapy in early relapse makes logical sense; the reverse is true for a patient who received an immunomodulatory-based induction.11–16 In addition, updated analyses from the MM-009 and MM-010 studies (lenalidomide-dexamethasone vs. dexamethasone) suggest that the benefit of lenalidomide was maintained despite prior exposure to auto-SCT or thalidomide.17,18 Similarly, a subgroup analysis from the APEX trial (bortezomib vs. dexamethasone) confirmed that bortezomib was better than dexamethasone regardless of prior treatment with thalidomide or auto-SCT.19
In addition to the activity of novel agents delivered either alone or in combination with steroids, there is emerging data suggesting that combinations of novel agents—either with each other or with cytotoxic chemotherapy—may offer the best chance of response in relapsed/refractory patients. Examples of successful combinations include bortezomib-lenalidomide,20 bortezomib-thalidomide,21 bortezomib or lenalidomide with pegylated liposomal doxorubicin,22–24 bortezomib or lenalidomide with cyclophosphamide,25–28 and bortezomib or lenalidomide with melphalan.29–33 In particular, dedicated subgroup analyses performed in the DOXIL-MMY-3001 study (bortezomib-pegylated liposomal doxorubicin vs. bortezomib) showed a benefit in the combination arm regardless of prior auto-SCT34 or prior immunomodulatory exposure.23 As detailed in Table 1, each of the above combinations has yielded promising results in the relapsed/refractory patient population. These studies are notable because they include patients who, like our patient, had been previously treated with both a novel agent (thalidomide, lenalidomide, or bortezomib) and auto-SCT.
In addition to combinations of established anti-myeloma agents, a host of novel agents have recently been studied in the relapsed/refractory setting. Among the most promising are histone deacetylase inhibitors, carfilzomib, pomalidomide, and elotuzumab (Table 2). Although the data for most of these drugs are from phase I or II clinical trials, many of the included patients are similar to our patient, with a prior exposure to thalidomide, lenalidomide, or bortezomib and/or auto-SCT. The importance of these agents (and their necessary clinical trials) cannot be underscored enough; many of us are utilizing our current “novel” agents much earlier and in combination with each other. As patients live longer, we will have greater opportunity and need for the incorporation of these newer agents in the post-transplant setting.
Based on available phase III data, our recommendation for the patient described above would be salvage treatment with lenalidomide-dexamethasone (Grade 1B). Both the MM-009 and MM-010 trials included patients who had previously been treated with bortezomib.12,13 Additionally, given the long duration of response following bortezomib and auto-SCT, it would be reasonable to use bortezomib with or without dexamethasone as salvage therapy (Grade 2B).53 If the patient could achieve a partial response and had a good performance status, he would be considered for a second, salvage auto-SCT. He would most likely receive lenalidomide maintenance therapy post-transplant, although this would depend on his clinical course and his post-transplant response. If at all possible, a clinical trial should be considered at each of these steps (salvage treatment, salvage transplant conditioning regimen, and post-transplant maintenance), because phase III data are still lacking for most of the combinations detailed above.
Disclosures
Conflict-of-interest disclosure: SL has been a consultant to Millennium, Celgene, and Novartis, and has received research funding from the same sources.
Off-label drug use: Use of experimental agents in the treatment of relapsed myeloma.
Correspondence
Nina Shah, MD, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 0423, Houston, TX 77030; Phone: (713) 794-5745; Fax: (713) 794-4902; e-mail: nshah@mdanderson.org
