Abstract
Approximately 300,000 patients in the world are diagnosed annually with acute myeloid leukemia (AML). The median age at presentation has been steadily increasing over the past few decades and now is approaching 70 years. Although considerable progress has been made over the past 3 decades in the therapy of AML, two thirds of young adults still die of their disease. The therapy of AML, unlike acute lymphoblastic leukemia (ALL), is based on maximally tolerated induction and post-remission therapy, all given within a few months from diagnosis. While complete remission can be achieved in the majority of young patients, ultimate cure of the disease depends on disease eradication through the administration of post-remission therapy. This is most often done with intensive chemotherapy. Harnessing the immunologic effect of graft-versus-leukemia, as in allogeneic transplantation, has further improved the outcome for many patients. Treatment of older adults, representing the majority of patients with AML, remains quite unsatisfactory. While 40% to 50% can achieve a complete remission, less than 10% are long-term survivors, and the cure rate of older patients has only minimally improved over the past three decades. Significant progress in the treatment of this age group is urgently required. New and targeted agents have much promise, but a definitive clinical role for these has yet to be conclusively established.
Curative treatment in acute myeloid leukemia (AML) depends on successful induction therapy to achieve a complete remission (CR) and subsequent post-remission therapy to prevent relapse. While over two thirds of young adults and approximately half of fit older adults can achieve a CR, only a proportion of these patients will be cured with current post-remission strategies. Thus, the greatest challenge in AML (as in other forms of acute leukemia, such as ALL) is to maintain the remission. Among patients younger than 55 to 60 years old, about one third of all AML patients can be cured, although there is enormous heterogeneity such that about half of patients with the best prognostic features at diagnosis can be cured and only about 10% in the most unfavorable groups are long-term survivors. Published data from cooperative group clinical trials, while crucially important, are often not representative of the outcome in the real world community; in the US only about 5% to 10% of adults with AML enroll on a clinical trial, and this in itself is a select population. Acute promyelocytic leukemia (APL), comprising about 10% of adults with AML, is currently treated with a therapy based on a targeted approach to a specific genetic lesion. This subtype of AML, not considered in detail in this chapter, can be cured in over 75% of cases and represents the paradigm and therapeutic aspiration for all other types of AML. With a median age of 70 years for AML, older adults represent the largest group of such patients. Much progress has been made in successfully achieving a good response to induction therapy; however, fewer than 10% of older patients with AML can be cured. The challenge for improved therapy for AML remains enormous.
Induction Therapy
Modern induction therapy in AML began in the late 1960s, when both cytarabine and anthracyclines were shown to have significant activity as single agents. It was but a short leap to studying the combination of cytarabine and anthracyclines, where CR was achievable in more than 50% of young adults.
A series of classic experiments in the USA by the Cancer and Leukemia Group B (CALGB) some 25 years ago established what really became standard of care for at least two decades. Data from carefully controlled randomized studies established that continuous infusion cytarabine was most effective; 3 + 7 was more effective than 2 + 5; daunorubicin (DNR) was less toxic than adriamycin; and anything less than 45 mg/m2 was significantly less effective. Furthermore, there was no advantage in giving 200 mg/m2 rather than 100 mg/m2 of cytarabine and 6-thioguanine did not improve the overall results of induction. The early 1990s ushered in a new era, during which newer anthracyclines were studied.
Based on these studies, standard induction consisted of DNR 45 mg/m2 intravenously for 3 days and cytarabine 100 mg/m2 by continuous infusion for 7 days. This has been the standard against which most new regimens have been tested. With this regimen 60% to 80% of young adults and 40% to 60% of older adults can achieve a CR. Data from published studies need to be cautiously interpreted as they represent data that are perhaps not representative of real world outcome. Patients referred to cooperative group trials often are selected for having adequate cardiac, renal and hepatic functions and in many instances those with prior malignancies or antecedent hematologic disorders, such as myelodysplastic or myeloproliferative syndromes, are excluded from AML trials. Nevertheless, it is clear that CR can be achieved in a high proportion of adults and this is a crucial and necessary goal in an attempt to achieve a long-term survival.
The major issues to be addressed in induction are the following:
Is there a preferred anthracycline or anthraquinone?
What is the optimal dose of daunorubicin?
Should alternative regimens be offered to patients with secondary leukemia or those with unfavorable cytogenetics?
Should induction be attenuated for older patients?
What is the role of new agents?
In the early 1990s, a series of randomized studies compared 45 or 50 mg/m2 of DNR with a variety of newer agents, including idarubicin, mitoxantrone, aclarubicin or amsacrine. Of note, in every one of these studies the anthracycline or anthraquinone was superior to DNR 45 mg/m2 either in CR rate, disease-free survival (DFS), overall survival (OS) or in the number of courses needed to get into CR1. Furthermore, sequential studies of induction by the same groups of investigators showed a significant reduction in CR rate when the dose of DNR was reduced from 70 or 60 mg/m2 to 45 mg/m2.1 Therefore, while there are no convincing data that any anthracycline or anthraquinone is preferred for induction, it is surprising that despite all these randomized studies DNR at 45 mg/m2 remained as the standard of care for so many years and has been widely used both in major cooperative groups and in the community at large.
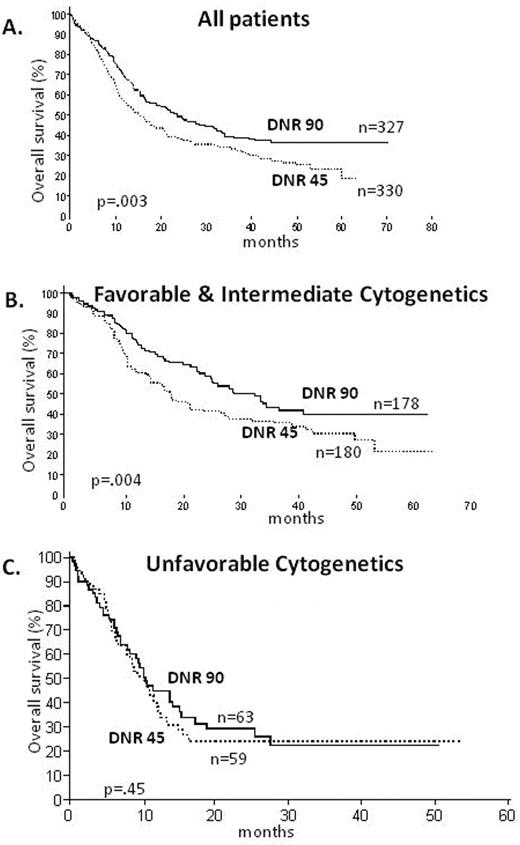
Several major studies, particularly CALGB 9621 and the French ALFA 9000 study, have demonstrated that higher doses of DNR up to 80 or 90 mg/m2 can be administered safely. Recently two major cooperative groups have prospectively compared 45 mg/m2 of DNR to 90 mg/m2. The Eastern Cooperative Oncology Group (ECOG) studied younger patients up to age 60 and very recently reported a significantly higher CR rate for patients receiving 90 mg/ m2;2 72% versus 57% (P = .004). More importantly, the OS was also significantly prolonged among patients receiving the higher dose of DNR, particularly among patients with favorable or intermediate cytogenetics (Figure 1 ). The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON)/Swiss Group for Clinical Cancer research (SAKK) recently completed a study in older adults administering 45 mg/m2 or 90 mg/m2.3 The CR rate for patients receiving the 90 mg/m2 dose was superior to that of patients receiving 45 mg/m2, 64% versus 54%, respectively (P = .002), with a greater percentage of patients achieving remission after only one course of treatment, 52% versus 35%, respectively (P ≤ .001). There were no significant safety issues in the high dose, finally putting to rest the myth that older patients, even if fit, should be treated in induction with attenuated doses. Thus, based on historic trials and the most recent data, it appears probably fair to say that 45 mg/m2 should no longer be considered as the standard of care. For induction therapy, also for older patients, the dose should be clearly higher—somewhere between 60 and 90 mg/m2 for 3 days—and the optimal dose has not been established.
In the Medical Research Council (MRC) AML15 study of younger adults, 1115 patients were randomized in induction to receive, or not, gemtuzumab ozogamicin (GO) in addition to the induction regimen. There was also a randomization between 3 different induction regimens, but all patients underwent the GO randomization. The post-remission therapy was identical in both arms of the study. The data were initially presented in 2006, reporting a similar CR in both arms, but a significantly improved DFS among patients receiving GO—51% versus 40% at 3 years (P = .008).4 Further follow-up is anxiously awaited. Disappointingly, these improvements in induction therapy, increasing the DNR dose or the addition of GO, do not seem to benefit patients with unfavorable cytogenetics—patients who need better treatment most.
Several other important issues in induction need to be addressed. First, there is no evidence that any form of induction therapy is better than the standard combination of anthracyclines and cytarabine for secondary leukemia or any cytogenetic or molecular subtype. Almost an inbuilt reflex, given the worse prognosis for these patients, it is tempting to offer such patients alternative regimens in induction. To date, there is no evidence that would justify such an approach. If indeed a regimen were to lead to an improved CR rate for the unfavorable prognostic groups, it should be just as beneficial for the better prognostic groups in whom an improvement in induction results would still be welcome. Secondly, for fit older patients attenuation of induction therapy is definitely not recommended. Once the decision is made to treat older patients with the intent of achieving CR, standard doses should be given—anything less will not reduce the toxicity, but will only result in lesser efficacy with probably equal myelosuppression-related toxicity. A recent comprehensive population-based study from the Swedish Acute Leukemia Registry5 reported the outcome for older patients with AML. The survival was improved for patients up to 80 years of age in the geographical regions where most were given standard induction therapy, and the early death rate was also lower with intensive therapy than with only palliative therapy. It is possible that in many instances an overly cautious approach to older patients with AML may not be in the patient’s best interest.
Currently, multiple new agents are being evaluated in an attempt to further improve the overall outcome of induction therapy or at least reduce the toxicity, particularly among older patients. Several new agents have demonstrated activity in AML. Clofarabine, laromustine and rapamycin have demonstrated activity in patients with more advanced AML and are currently being investigated as agents in induction in an attempt to further improve the overall results and, especially, ameliorate the toxicity. Whether such agents will replace standard induction with an antracycline and cytarabine, wholly or partially, is unknown, but preliminary data indicate that any benefit from such new drugs as initial agents to be used in induction is likely to be marginal. If they have a role, it may be in older patients with impaired performance status, in whom a reduction in toxicity may be a major consideration.
Post-remission Therapy
Despite initial high CR rates, the OS of adults with AML is unsatisfactory, even in the most favorable cytogenetic groups, such as the core binding factor translocations.6 The need for any post-remission therapy was established in the landmark study conducted by the ECOG in 1983 which prospectively included an observation arm as part of the post-remission strategy.7 Clearly, this was a study that could not be done today, but was ethically appropriate in 1983. Importantly, the study was stopped early by the National Cancer Institute (NCI) in the USA when virtually every patient who was in the observation arm relapsed by 18 months.
While the prescribed regimen for induction is generally identical for all patients (except those with APL), the options for post-remission treatment are broad and the ultimate choice of therapy is determined by the prognostic factors at diagnosis and beyond. Clearly, patient and physician biases also impact the therapy that is offered. A variety of strategies to prevent relapse have been explored. Intensive consolidation chemotherapy remains the standard against which other strategies are compared. These include allogeneic hematopoietic stem cell transplantation (allo HSCT), auto HSCT or low-dose maintenance therapy. Multiple studies of post-remission therapies have been conducted over the past two decades, and significant controversy and uncertainty remains. The major trials report their results, appropriately, based on intention-to-treat analyses in most cases either from diagnosis or from randomization. However, with the evolving delineation of differing prognostic groups, the published data are often based on small cohorts of patients. Furthermore, such data are often not helpful in the clinical setting when advising the patients who have successfully undergone induction therapy and perhaps one or two cycles of consolidation therapy and would like to know the risks and benefits of various strategies at specific time points. Such information, important for the clinician and the patient, is hard to come by from published data and may present significant gaps in the ability of clinicians to properly advise patients at different time points.
Chemotherapy
Non-transplant post-remission consolidation consists of intensive chemotherapy. A variety of studies have suggested that increasing the intensity of post-remission therapy prolongs remission duration and increases the likelihood of cure in adults up to age 55 or 60. Once a patient is assigned to consolidation chemotherapy as the post-remission strategy, the choice of drugs and mode of administration are, as a rule, not dependent on the prognostic factors.
A classic trial by the CALGB studied post-remission therapy in young adults up to age 60 and compared 3 different doses of cytarabine given for 4 cycles followed by maintenance therapy. The data from this prospective study demonstrated a significant improvement in the OS for patients less than 60 years of age when a high dose of cytarabine, at 3 g/m2 for 6 doses, was given.8 The problem is that these data may sometimes be misunderstood. These data demonstrate only that 3 g/m2 is better than 400 mg/m2 or 100 mg/m2 when given for 4 cycles and followed by maintenance therapy; they do not indicate how many cycles should be given and they also provide no evidence that the same results could not be achieved with other agents. Of note, in the CALGB study the impact of cytarabine dose on long-term survival was most marked among patients with favorable cytogenetics.9
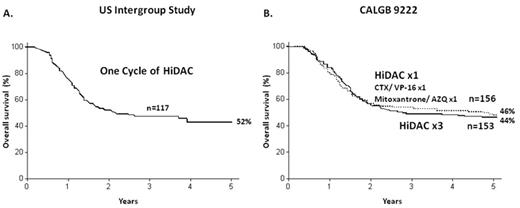
Many other questions remain unanswered regarding post-remission therapy in young adults. Just as the optimal number of cycles of any form of consolidation therapy is not known, so is the optimal drug. Regimens that have not used high-dose cytarabine, such as in studies conducted by the MRC in Britain, report results that are comparable to the data using high-dose cytarabine. In addition, data using only one cycle of high-dose cytarabine, as published by the US Intergroup in 1998,10 are very similar to the published results when 3 to 4 cycles of high-dose cytarabine were used11 (Figure 2 ).
An important study by the Finnish Leukemia Group attempted to prospectively determine how much consolidation needs to be given. Patients were given 2 courses of consolidation and were then randomized between receiving additional 4 cycles of consolidation versus observation. No difference was seen in survival from randomization.12 Thus, for younger adults it is fair to say that the knowledge at the present time suggests that in a non-transplant setting some form of post-remission chemotherapy is critical, and reasonable data show that such therapy should include at least 1 course of very intensive consolidation. Although most clinicians administer more than 1 cycle, in part based on some retrospective comparisons of small cohorts of patients, it is important to note that there are no prospective data that clearly establish this approach.
Regarding older adults, the issues concerning post-remission therapy are even more fundamental. Unlike induction therapy, dose attenuation is critical when administering post-remission therapy to older patients. The gastrointestinal and central nervous system toxicity from high-dose cytarabine is prohibitive at the standard doses given to young adults. Furthermore, as opposed to younger adults, the benefit of any post-remission therapy has never been unequivocally established for older patients. It has never been shown that any form of post-remission therapy makes a difference; although in common practice, fit older adults almost always receive consolidation therapy. What is also not known is the number of cycles that should be given. The problem of treating older adults relates most importantly to the inability to maintain a remission. While CR can be achieved in 40% to 60% of patients on clinical trials, depending on age and prognostic factors, the DFS is only 6 to 9 months and with an OS of 7 to 10 months (Table 1 ). The reason for the inability of post-remission therapy to effect a cure in more than a very small proportion of older patients is likely to be multifactorial: a cohort of patients with more adverse prognostic factors who are unable to tolerate the dose-intense post-remission therapies given to younger adults.
The largest study of older patients with AML was the MRC AML 11, which was published 8 years ago.13 Essentially this trial compared older patients who went into CR with induction therapy, got one course of consolidation with daunorubicin, cytarabine and 6-thioguanine (DAT) and were then randomized to 3 further cycles versus observation only. The data demonstrated clearly that within the range of doses given in this study there was no particular value for further intensification after a single course of consolidation therapy. Studies from other groups, such as the German AML Cooperative Group (AMLCG) or CALGB also suggested that there is no benefit to giving more than one cycle of intensive chemotherapy. A possible exception may be for the relatively uncommon older patients with favorable cytogenetics, among whom 20% to 30% may be cured with maximally tolerated post-remission therapy.14
The role of cytogenetics, crucial for assigning post-remission therapy in young adults, is also an important one for older patients. Data from several studies show that post-remission therapy, as currently given, provides little benefit for older patients with unfavorable cytogenetics.14,15 It is therefore even more difficult to make a case for giving any post-remission therapy to such patients outside of a clinical trial.
Thus, the issue of non-transplant consolidation therapy is even more confounding than the uncertainties in induction therapy. Ideally, future studies should shed more light on these fundamental issues, although at the present time there appears to be little enthusiasm among the major cooperative groups to embark on such clinical trials.
Maintenance Therapy
Unlike ALL, the role of maintenance therapy in AML, with the exception of APL, remains controversial. AML is clearly curable without maintenance therapy and such an intervention is, in most centers, not used routinely, especially in younger adults. The AMLCG has studied prolonged myelosuppressive maintenance chemotherapy for all patients with AML and reported that such a strategy is efficacious in maintaining long-term remission.16 However, the regimen intensity of maintenance therapy given in these reports, based on historic studies by the CALGB, is exceptional compared with most published regimens, which involve a far less toxic regimen that is also given for a shorter period of time. For younger patients, such therapy, although somewhat efficacious,7 cannot replace any of the more intensive post-remission strategies. Whether low-dose maintenance can have a role following intensive consolidation with chemotherapy or any form of transplant has not been adequately investigated.
The issue of maintenance therapy is particularly pertinent for older patients. An important study by the European Organization for Research and Treatment of Cancer (EORTC) and the HOVON group demonstrated an improved DFS after maintenance with low-dose cytarabine, although the OS was not significantly improved.17
Several strategies have been proposed as maintenance therapy apart from low-dose chemotherapy. An immuno-therapeutic approach is attractive conceptually. However, to date there have been five major randomized studies of interleukin-2 (IL-2) as maintenance therapy in AML; disappointingly, none of them demonstrated a clear advantage for IL-2. Of interest is the multi-center study of IL-2 and histamine, given as maintenance post consolidation. In 320 randomized patients a significant benefit in leukemia-free survival was demonstrated for patients receiving IL-2 and histamine compared to no therapy (P = .008).18
Whether targeted agents such as tipifarnib, tyrosine kinase inhibitors or hypomethylating agents will prove to be useful as maintenance agents is currently the subject of clinical investigations. Because many studies using maintenance regimens consistently show at least a modest benefit in DFS, although usually not in the OS, this form of therapy needs to be evaluated, especially for older patients who typically have a short DFS of approximately 6 to 9 months (Table 1 ).
Allogeneic Hematopoietic Stem Cell Transplantation
Allogeneic hematopoietic stem cell transplantation (allo HSCT) from an HLA-matched related donor provides the most potent anti-leukemic effect of any post-remission therapy in AML, as demonstrated by the lowest rates of relapse. The clinical impact of the graft-versus-leukemia effect has by now been clearly established. Thus, such a transplant often provides the best chance of long-term survival. The most important limiting factor for allo HSCT remains the high transplant-related mortality. The long-term mortality from established grade IV graft-versus-host disease has not changed significantly over the past three decades. The current recommendations for the performance of allo HSCT for patients in CR1 are limited to those whose risk of relapse significantly exceeds the incremental mortality from allo HSCT over standard chemotherapy.
The most important issues regarding allo HSCT are the following:
Which patients should be offered this in CR1?
How much, if any, additional post-remission therapy should be administered prior to allo HSCT?
Should patients who do not have an HLA-matched sibling be offered a transplant from a matched unrelated, a genetically haploidentical donor or an unrelated umbilical cord?
Given the high procedural mortality, should such a procedure be preferably reserved for patients in second remission or at relapse?
The decision to undergo an allo HSCT will depend on the prognostic factors at diagnosis and, no less importantly, at the point of achieving CR1. Thus, allo HSCT in CR1 should be considered in patients with unfavorable cytogenetics and those with intermediate-risk cytogenetics, except possibly those with the NPM1+/Flt3-ITD− subgroup (Table 2 ). Patients with favorable cytogenetics should not be transplanted in CR1 unless they have particularly unfavorable factors, such as mutations in c-kit. Post-induction consolidation needs also be taken into account, such that a patient who had not responded to one or two cycles of induction therapy and required a salvage regimen to achieve CR1 is also at high risk, even if the cytogenetics at presentation were of a favorable subtype.
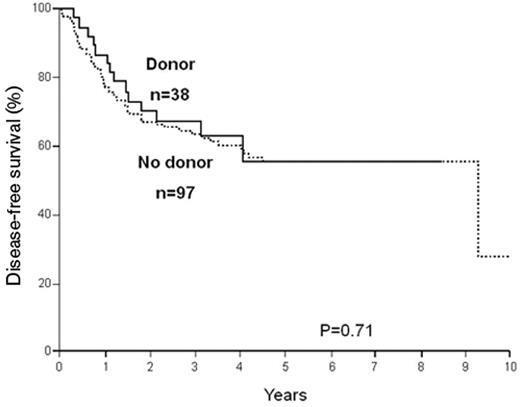
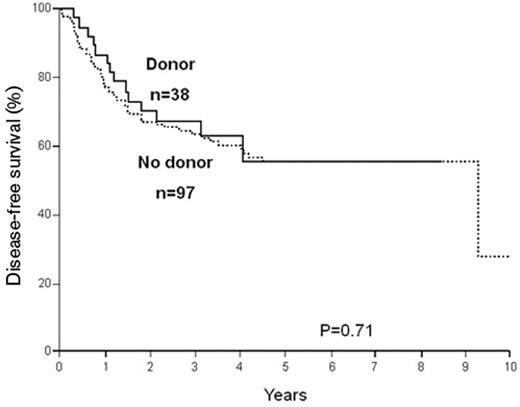
It must be understood that as further mutations are being described and their prognostic significance evaluated, this simple algorithm may change to either include more high-risk patients among the so-called favorable leukemias (for example, CD56 expression or trisomy 4) or assign a lesser risk to patients with intermediate-risk cytogenetics (for example, the CEBP-alpha mutations). A degree of caution is also appropriate in interpreting published data. While it is becoming increasingly accepted that patients with intermediate risk who have the NPM1+/Flt3-ITD− subgroup belong more appropriately in the favorable risk group and may not benefit from allo HSCT (Figure 3 ), these are based on only 38 patients in the donor group.19 Clearly, these data need to be confirmed in studies with larger number of patients.
There are no prospective studies that indicate whether any form of post-remission consolidation will further reduce the risk of post-transplant relapse. However, two retrospective analyses have been performed from international registries, and the data from these studies strongly suggest that there is no benefit to adding any therapy prior to embarking on allo HSCT.20,21
Alternative Donor Strategies
Since only about 25% of individuals have an HLA-matched sibling, interest has turned to the rapidly growing experience of using either a matched unrelated donor transplant or haploidentical transplants. The advent of high-resolution allele typing by molecular methods has had a major impact on the more successful performance of matched unrelated HSCT. The German AML01/99 is to date the only study that attempted to prospectively address the value of unrelated HSCT for AML in CR1.22 In this study patients with unfavorable cytogenetics, or those with residual leukemia on the day 15 post-induction marrow, were assigned to receive an allo HSCT from a matched sibling, if such a donor were available. Otherwise, patients were to receive a matched unrelated donor transplant, if a suitable donor could be found, or an auto HSCT. The 4-year OS was 68% with sibling allo HSCT and 56% with an unrelated allo HSCT; both significantly better than an auto HSCT or chemotherapy. Based on these data, and on the exceedingly poor prognosis of such patients without a transplant, there is an evolving consensus to refer such patients to a transplant from an unrelated donor in CR1. There is lesser consensus whether such a strategy should be routinely adopted for patients with intermediate cytogenetics who do not have a matched sibling.
While an increasing number of reports describe an equivalent survival for sibling and matched unrelated HSCT for AML in CR1,23 it is not clear that such reports are free of inherent selection biases when performing a matched unrelated donor HSCT. A recent report, based on patients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) described the characteristics of patients who underwent a myeloablative fully matched unrelated donor transplant or an HLA-identical sibling transplant between 1995 and 2004.24 One thousand seventy-one patients with AML who underwent an HLA-identical sibling transplant were compared with 304 patients who received a matched unrelated donor transplant. A large proportion of these patients were in CR1. Despite a prevailing suggestion that the immunologic graft-versus-leukemia effect may be more potent using matched unrelated donors due to a higher likelihood of mismatching at minor histocompatibility antigens,25 this report described an increased relapse rate in the unrelated donor transplant group for patients with AML in CR1 (P = .02). The leukemia-free survival was also significantly improved for patients receiving a sibling allograft, although this was only true for patients with no graft-versus-host disease.24 Thus, the indications for the performance of unrelated donor transplants for patients with intermediate-risk cytogenetics are less well defined due to the lack of prospective studies and inherent selection biases among patients chosen for such procedure; nevertheless, the emerging data indicate that this procedure provides a curative option for many patients with AML and is a viable alternative for those patients who do not have a sibling donor. The data in appropriately selected patients are close to those that are typically reported for sibling allografts.
Genetically haploidentical transplants present another alternative for patients with a poor prognosis for whom no sibling donor is available. The advantage of this procedure is the immediate availability of a donor for almost all patients. Although there are less cumulative data on using this procedure than on using well-matched unrelated donor transplants, reports over the past few years, based on the pioneering experience of the group in Perugia, Italy, indicate that the results for high-risk AML in CR1 are similar to what has been reported for matched unrelated donor transplants.26
Another evolving option for patients who do not have a sibling donor is the use of unrelated umbilical cord blood transplantation. Although the reported data for AML in CR1 are scarce, there are increasing data on its use for adult patients with leukemia with results that suggest that this is also a viable option when a sibling donor or a matched unrelated donor are not available. This approach has had limited applicability in adults due to the cell dose requirements. Recent investigations using 2 partially matched umbilical cord units have raised hopes that this procedure may be more widely applied also to adults.27
The decision to undergo an allo HSCT for AML in CR1 must be based on a careful consideration of the evidence. If the evidence suggests a clear benefit for this in CR1 (as in Table 1 ), such transplants should preferably be performed at that time and not “reserved” for CR2. Although there are several reports of successful allo HSCT in CR2, with a curative potential of 25% to 35%, such data are highly selective and should not influence the appropriate decision to transplant patients with AML in CR1. The survival of all relapsed patients is exceedingly poor,28 and reports of successful strategies in CR2 relate only to the select patients who survived a relapse and were fit enough to get into CR2.
Autologous Transplantation
Auto HSCT has also been extensively studied for patients with AML in CR1. After allo HSCT, auto HSCT is the most potent form of post-remission therapy relying in this instance on the antileukemic effect provided by the myeloablative regimen. Further evidence for the anti-leukemic potency of this procedure comes from the curative potential of this also for AML patients in CR2.29 The use of auto HSCT for AML in CR1 has generated much controversy, partly because of the difficulty in interpreting the historic outcome data and applying them to current practice. Data from even the largest studies are difficult to interpret due to the small number of patients per subgroup. The data are further confounded by the degree and intensity of prior therapy and patient selection. Lastly, a significant proportion of patients, 50% or more, randomized to auto HSCT never go on to receive their assigned therapy.10,30 Intention-to-treat analyses are difficult to interpret when half of the patients do not receive their intended therapy. Most of the major prospective studies published over the past decade have described a lower relapse rate for patients undergoing an auto HSCT in comparison with chemotherapy and a meta-analysis of six trials, including 4410 patients, also indicated that auto HSCT is associated with a modest improvement in DFS.31 Importantly, the lower relapse rate following an auto HSCT has also been demonstrated in prospective studies in patients with favorable cytogenetics.14,32 In most prospective studies of auto HSCT the OS was not significantly improved, mostly because of the very high mortality reported in patients undergoing such transplant, where bone marrow was used as the source of hematopoietic stem cells; 14% in the US Intergroup study, 18% in the MRC trial.10,30 Currently, an auto HSCT performed in experienced centers, using hematopoietic stem cells collected from the peripheral blood, has a very low mortality rate ranging between 0% and 2%, which is not greater than for patients undergoing intensive consolidation with high-dose cytarabine.33
The approach to post-remission therapy must be based on regimens with the most potent anti-leukemic activity, provided this effect is not abrogated by unacceptably high mortality. Clearly, auto HSCT is a potent strategy for post-remission therapy in AML.34 Thus, for adult patients who are not candidates for allo HSCT, auto HSCT using peripheral blood should be a cornerstone of consolidation therapy, also for patients with favorable cytogenetics, preferably after one or more cycles of chemotherapy.
There remains a significant controversy at which point in the course of post-remission therapy auto HSCT is most effective. Should this replace chemotherapy entirely or should this be offered only after intensive chemotherapy and, if so, after how many cycles? No prospective studies have evaluated this and, in fact, much like the data on intensive chemotherapy, the published reports of auto HSCT performed as the sole strategy for post-remission therapy are similar to the survival curves when this was performed following several cycles of chemotherapy.35 Nonetheless, current practice, based on retrospective registry data and sound rationale, is to offer one or more cycles of consolidation prior to auto HSCT.
Conclusion
While there has been impressive progress in the treatment of AML, the majority of patients still die from this disease. Clearly, the major curative potential is while patients are in CR1; once in relapse, the options are very limited. Multiple studies have attempted to define the optimal strategies for post-remission therapy. Nevertheless, much uncertainty and controversy persists, especially among patients with the intermediate cytogenetics. This group comprises a heterogeneous population that only recently is becoming more clearly defined based on molecular prognostic groups. The major areas of uncertainty relate to the type of post-remission therapy that should be offered. Who should get an allo HSCT, and who should be offered an alternative donor when a matched sibling donor is not available? Who should be offered an auto HSCT and when?
A multitude of new agents have activity in AML and several targeted agents have biological potential that is intriguing. Unfortunately, to date, any role for a new agent is likely to be of marginal significance for AML in CR1. Targeted agents potentially could serve as important adjuncts to standard therapy, if they have efficacy with minimal toxicity. Despite such hope, to date, in AML none of the new targeted agents has therapeutic activity that matches all-trans retinoic acid (ATRA) or arsenic in APL or imatinib in chronic myeloid leukemia (CML). The therapy of AML remains a formidable challenge.
Overall survival in AML ≤ 60 years, based on the ECOG randomized study (E1900) of anthracycline dose in induction. A. All patients on study B. Favorable and intermediate cytogenetics C. Unfavorable cytogenetics Reproduced with permission from Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259.
Overall survival in AML ≤ 60 years, based on the ECOG randomized study (E1900) of anthracycline dose in induction. A. All patients on study B. Favorable and intermediate cytogenetics C. Unfavorable cytogenetics Reproduced with permission from Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259.
Overall survival in young adults with AML using different number of cycles of post-remission therapy. A. US Intergroup Study (ECOG E3489) for patients < 55 years; survival for patients who only received one cycle of post-remission therapy. Reproduced with permission from Cassileth PA, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339:1649–1656.10 B. CALGB 9222 for patients < 60 years; survival for patients who received 3 cycles of post-remission therapy. Reproduced with permission from Moore JO, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as postremission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B Study 9222. Blood. 2005;105:3420–3427.11
Overall survival in young adults with AML using different number of cycles of post-remission therapy. A. US Intergroup Study (ECOG E3489) for patients < 55 years; survival for patients who only received one cycle of post-remission therapy. Reproduced with permission from Cassileth PA, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339:1649–1656.10 B. CALGB 9222 for patients < 60 years; survival for patients who received 3 cycles of post-remission therapy. Reproduced with permission from Moore JO, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as postremission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B Study 9222. Blood. 2005;105:3420–3427.11
Disease-free survival, comparing patients with or without a donor, among patients with a normal karyotype and NPM1+/Flt3-ITD−. Reproduced with permission from Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918.19
Disease-free survival, comparing patients with or without a donor, among patients with a normal karyotype and NPM1+/Flt3-ITD−. Reproduced with permission from Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918.19
Disclosures Conflict-of-interest disclosure: The author is a consultant for Teva Pharmaceuticals and EpiCept Corporation. Off-label drug use: None disclosed.
References
Author notes
Department of Hematology and Bone Marrow Transplantation, Rambam Medical Center and Technion, Israel Institute of Technology, Haifa, Israel