Abstract
Acute myelogenous leukemia (AML), either de novo or arising out of antecedent myelodysplasia, increases with age and is rarely curable by standard treatments used for younger patients. Recent clinical trials using reduced-intensity allogeneic transplantation regimens suggest that a proportion of patients with this disease can be cured, with results comparable to those achieved in younger patients undergoing fully ablative transplant. Although those patients who undergo transplant in a first remission often do well, the vast majority of older patients have not benefited because of the low successful remission achieved with standard therapy, the delay in initiating a donor search, and the lack of significant benefit from transplantation in patients who are not in remission. New approaches to induction, improvements in reduced-intensity regimens, and earlier donor identification will help expand the potential clinical benefit to a larger number of older patients with the disease.
Myeloablative conditioning regimens for the treatment of acute myelogenous leukemia (AML) have been very effective in improving the cure rate for this disease, especially in younger patients. Refinements in the classification of AML based on molecular genetic characteristics have helped refine the indications for transplantation. These include patients with poor-risk cytogenetics, the requirement for more than one cycle of induction chemotherapy to achieve a remission, and those patients within the good-risk and intermediate-risk categories when there is the presence of a c-kit mutation associated with an 8;21 or inversion 16 chromosome abnormality or an FLIT-3 mutation in patients with otherwise normal cytogenetics. Currently, the decision to offer transplantation to a patient is determined by the assessment of the risk of leukemia relapse with standard consolidation therapy, the availability of a donor and the condition and age of the patient.
AML in older patients, over the age of 55–60 years, is extremely difficult to treat for several reasons, including the disease-related biology of AML and factors related to the patient’s general health. Remission induction is often unsuccessful as the disease often demonstrates intrinsic drug resistance related to poor-risk cytogenetics and the patient is often unable to tolerate conventional chemotherapy. Although the cure rate with chemotherapy may approach 40% in some groups of adult patients and 60% in those patients undergoing transplantation, the standard chemotherapy approach is very rarely successful in patients over the age of 55 to 60, with fewer than 1 in 5 patients expected to live 3 years after diagnosis. Many trials performed over a number of years have confirmed that the cure rate for this group of patients has not improved, even with improvements in supportive care. In addition to issues related to the leukemia itself, the disease becomes more common as people age. Thus, although transplantation has been used effectively to cure patients with AML who are younger, treatment, including allogeneic transplantation, has generally not been used for the vast majority of such patients who develop the disease.
AML in Older Adults
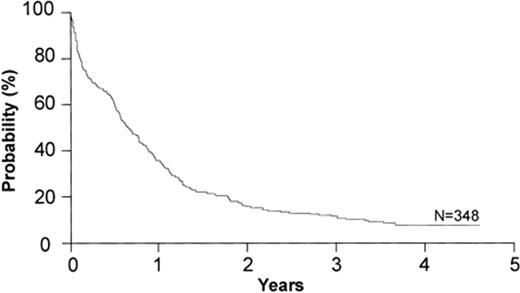
As noted above, the biological nature of AML changes as the age of the patient increases. Most notably, compared with patients who are younger, in patients older than 60 AML is more often associated with an antecedent hematologic disorder, is less proliferative, presents with lower white blood cell count and lower percentage of marrow blasts, and is more associated with the expression of p-glycoprotein in AML blasts. Most importantly, the disease is more likely to have an unfavorable cytogenetic profile and a reduced chance of having good-risk cytogenetics.1–5 These factors conspire to make the leukemia much less likely to respond to treatment with standard chemotherapy, and for those patients who are fortunate enough to achieve a remission, it is of short duration. When combined with a diminished performance status and the increased number of co-morbidities, the likelihood of a favorable response to chemotherapy is small.6 The data suggest that the complete remission rate is approximately 40% with a median duration of 6 to 8 months, and survival at 3 years is less than 20% in virtually all studies.7–9 A recent study from the Eastern Oncology Cooperative Group, a patient group that was younger than 75 years old with good performance status, showed that 2- to 4-year survival was quite low and basically unchanged over time (Figure 1 ).7
Graft Versus Leukemia
It has now been approximately 20 years since the first studies were published that showed that patients who developed graft-versus-host disease following allogeneic transplantation had a lower risk of leukemic relapse than those who did not, suggesting that immunologic alloreactivity contributed to the overall cure of the disease.10,11 The increased rate of relapse following a twin transplant, the remission that is sometimes achieved by infusion of donor leukocytes, or withdrawal of immunosuppression at the time of relapse all support the idea that the graft contributes greatly to the cure. When patients are undergoing full allogeneic transplantation, it is not possible to determine how much of the benefit is attributed to the high-dose preparative regimen and how much is related to the graft-versus-tumor regimen. However, the development of more specifically immunosuppressive regimens with minimal cytoreduction allowed the exploration of allogeneic transplant (alloimmunotherapy) in those patients who were considered either too old or infirm for full-dose transplantation, as well as showing whether such an approach could achieve engraftment or cure the disease. Over the last 15 years, several groups of investigators have developed reduced-intensity or non-myeloablative conditioning transplant regimens less toxic than fully myeloablative transplants in order to extend the treatment to older patients who might also have co-morbidities that preclude full-intensity transplants. These regimens vary in their intensity from those that are truly non-ablative (fludarabine/single-dose TBI) to those that incorporate additional chemotherapy drugs such as fludarabine/ busulfan or fludarabine/melphalan, which do result in cytopenias requiring blood and platelet support. These regimens are the three most commonly used in the treatment of older patients undergoing transplantation for most hematologic malignancies, including AML. They all have the capacity to provide, in combination with post-transplant immunosuppressive drugs, adequate host immunosuppression to facilitate engraftment of related, unrelated or cord blood stem cells. It is not known whether one of these regimens is more effective than the other, and all of them can be used in the treatment of patients in remission. Among the observations is that the reduced-intensity approach has produced major anti-tumor effects, relying on both pre- and post-transplant immunosuppression to overcome host-versus-graft disease, ie, graft rejection, and to facilitate alloimmune anti-tumor responses.
Reduced-intensity Transplant: Influence of Remission Status on Outcome
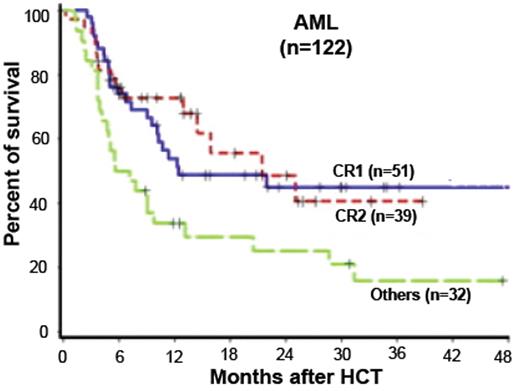
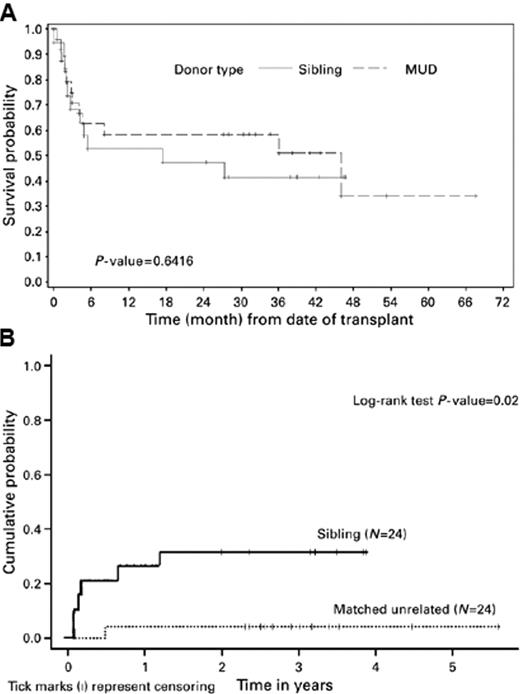
The initial reports summarized the outcome of a group of 122 patients treated with fludarabine 90 mg/m2, combined with low-dose total body irradiation (TBI 2 Gy), followed by cyclosporine and mycophenolate mofetil.12 Survival at 3 years was 46% for 51 patients transplanted in first remission and 42% for 39 patients transplanted in second remission, while those patients who had more active disease had a less optimistic result (Figure 2 ). Of note in this and other trials was that somewhat better results (reduced chance of relapse) were seen in recipients of unrelated donor transplants compared with recipients of matched related donor transplants, raising the issue of whether alloreactivity was stronger when there was some degree of mismatching (Figure 3 ).13 Another report from Shimoni using a slightly more intensive regimen of fludarabine and busulfan, followed by cyclosporine and methotrexate with ATG given to those patients with unrelated donors, showed that among the 27 patients in first remission undergoing transplant, 74% reported disease-free survival at 3 years, while those patients who were not in remission did not survive disease free.14 A retrospective study from the Cooperative German Transplant Study reported an event-free survival of 52% at 24 months among 25 patients transplanted in first remission, most of whom received a fludarabine/busulfan regimen.15 For those patients in second remission, the event-free survival was 40%. For those patients with advanced disease, less than 20% were alive at 2 years. Consistent with the concept that reduced-intensity transplant is a form of allo-immunotherapy, the development of chronic GVHD after transplant improves survival by reducing relapse16 (Figure 4 ). Despite the apparent benefit on survival, this complication is more challenging to treat in patients who are older. Others have published similar results and support the observation that 1) allogeneic transplantation using a reduced-intensity preparation is feasible in older patients, 2) in selected patients, particularly those who undergo transplantation in first remission, the outcomes were encouraging, and 3) those with more advanced disease or not in remission did not benefit.17,18 Although some patients achieve long-term remission if transplanted in early relapse or with less than a complete first remission, the results are inferior to those achieved if the patient is in remission. As many investigators have pointed out, all of these reports are in older patients who were referred specifically for transplantation after achieving a remission, most of whom had a donor, and thus overestimate the impact of the approach in this group of patients with high-risk AML.
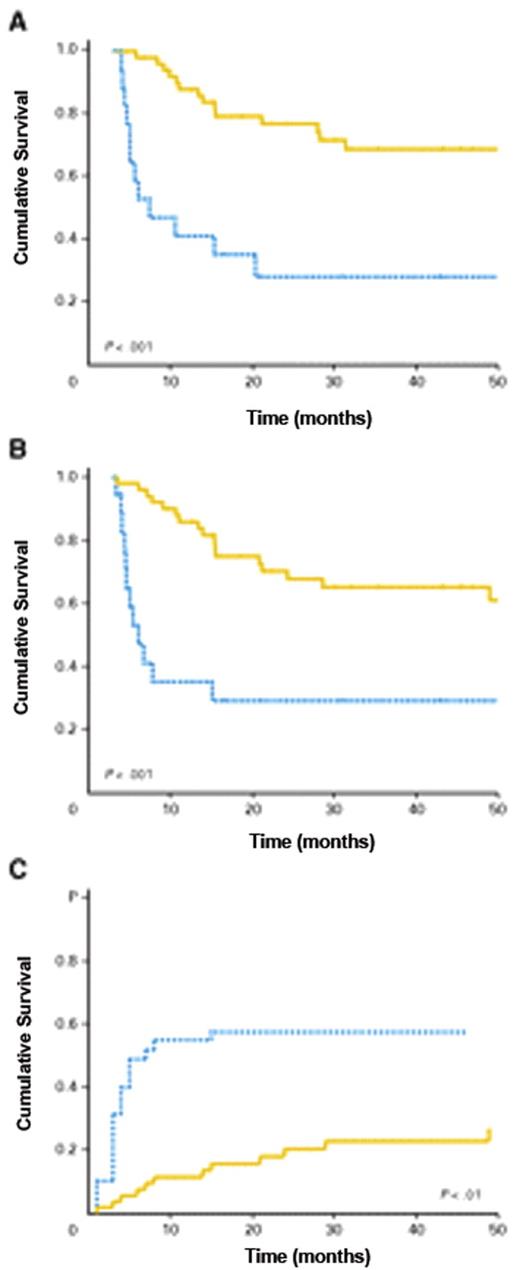
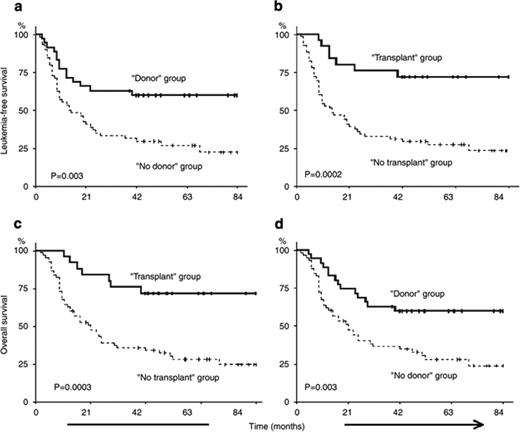
Some investigators have tried to better understand the impact of transplantation in older patients by identifying patients at the time of diagnosis to determine the percentage of patients who would actually proceed to transplant following a diagnosis of AML and the eventual overall outcome.19 In one study, 95 patients with AML in first remission were studied who had known siblings, 35 of whom had an HLA-matched sibling and were considered the donor group, while in 60 patients there was not a match. Twenty-five of the 35 in the donor group actually proceeded to transplant using a reduced-intensity transplant regimen of fludarabine, busulfan and ATG, and the intent-to-treat analysis showed leukemia-free survival at 4 years was superior in the donor group. This study has recently been updated and leukemia-free survival remains better in the donor group (72% vs 24% at 7 years, P = .002). The cumulative incidence of transplant-related mortality was 12% (Figure 5 ).20 Similar to other studies that begin the evaluation after biological allocation to one group of patients (donor – no donor), this study begins to come close to assessing the possible beneficial impact of the treatment in patients who are older with AML in remission, but also is retrospective and only dealt with those patients who had an available sibling.
Estey and his colleagues, rather than studying patients in first remission, which selects patients for their chemosensitivity and good performance status, attempted to evaluate patients at the time of diagnosis by seeing all patients admitted to the University of Texas M.D. Anderson at the time of initial evaluation.21 If transplantation was felt to be plausible, a search for the appropriate donor was to be conducted. A total of 259 patients entered the study. Complete remission was achieved in 99 patients (38%), typical of what has been seen in the cooperative group studies. Of these 99, 53 were then referred to transplant and 46 were not. In this latter group, the reasons for not proceeding were likely related to age, underlying health, patient choice and the attitude of the physician. Of the 53 who were actually referred for transplantation, a matched sibling was identified in 21 and a matched unrelated donor was found in an additional 5 patients. This suggests that a donor was found for nearly 50% of the patients who actually were referred for transplantation, similar to what is achieved in younger patients. Fourteen patients actually underwent transplantation, 13 from a matched sibling and 1 from an unrelated donor. Consistent with the results of other studies of reduced-intensity transplant, 57% of the patients who underwent transplantation were disease-free at 4 years. This result seemed very good when one accounts for age, cytogenetics, performance status and lead time bias, than patients who did not undergo transplant. However, in considering the impact of transplantation on the whole population of patients newly diagnosed with AML, these patients represented only 26% of those who were actually seen by the transplant service. Only 14% of those achieved a complete remission and only 5% of the original 259 patients entered onto the study. Thus, even with an optimistic result for transplant, the vast majority of patients who are older have yet to benefit from such an approach. The data to date suggest that for older patients who are able to achieve a first remission and have either a related or unrelated donor, allogeneic transplantation during first remission offers a better chance of improved disease control. The results for patients actually undergoing transplant are comparable to those achieved in patients who are younger with less high-risk features and represents a reasonable therapeutic option for older patients in remission for whom a donor can be identified.
Limitations and Potential Solutions to Improve Outcome of Transplantation for Older Patients
The apparent improved survival seen with allogeneic reduced-intensity transplant in older patients and the recognition that only a small minority of patients are able to benefit highlight the limitations imposed by both the disease and the patient. These include 1) the need to improve the chances of achieving a remission in older patients with AML, 2) how to improve the transplant regimen to help reduce the chances of relapse or complications without compromising the patient’s wellbeing during the procedure, and 3) identification of a donor in a timely way.
Improving Initial Response to Treatment
As noted above, an initial factor limiting the application of transplantation is the low complete response rate for older patients with AML undergoing induction therapy. Most induction studies have been based on an anthracycline and cytarabine combination; however, no approach to date, including attempts to reverse the effects of p-gp, have resulted in improvement. However, in recent studies, investigators have explored alternative initial approaches, including the use of hypomethylating agents in combination with other agents, such as gemtuzumab and HDAC inhibitors. One report from Nand on using azacytidine and gemtuzumab observed a nearly 70% complete response rate in a group of patients whose average age was about 70.22 This trial is now being expanded to determine the impact of this approach in newly diagnosed patients with AML who are older. In addition, Kirschbaum reported on a group of older patients who had either MDS-related AML or de novo AML who were older and were treated with a completely epigenetic-based therapy utilizing decitabine and SAHA.23 Approximately 40% of the patients who were previously untreated achieved a remission or adequate clinical benefit. Thus, these and other novel approaches may provide an opportunity to improve both the CR rate and facilitate patients being able to go to transplant in remission.
Requirement for a Complete Remission
Among the common observations in most studies in the use of reduced-intensity transplantation for hematologic malignancy is that patients with active disease at the time of transplant do not benefit. These patients likely require a full intensity regimen in order to have a significant chance of disease-free survival, but this approach obviously cannot be used in older patients. However, some studies have now begun to explore the augmentation of reduced-intensity regimens using the regimen as a platform for different approaches. Some investigators have shown that it is possible to use monoclonal antibodies to specifically target radioisotopes to the marrow and other sites of leukemia. Initial studies utilizing Iodine-131–labeled anti-CD45 antibody have shown that this regimen is capable of delivering between 2- to 3-fold more radiation to the marrow and spleen than to the liver, and approximately 10-fold more radiation to the marrow than to the whole body.24 In an initial phase I/II trial, adding this radiolabeled antibody to the least myeloablative reduced-intensity regimen of fludarabine and TBI showed that a small number of patients treated with the maximum tolerated dose of radioimmunotherapy had a survival of 54% at 2 years. Thus, patients who are not in a complete remission, or possibly even those who are but who are at high risk for relapse, may benefit from such approaches, hopefully without augmented toxicity, but a better anti-leukemic treatment. In addition, other investigators have begun to evaluate different drugs in the pre- and post-transplant setting. Several studies are ongoing using clofarabine, a drug with more anti-leukemic activity than fludarabine, as a partner with alkylating agents such as melphalan or busulfan.25 The early results show significant activity and tolerability, and phase I/II trials are ongoing to determine the appropriate dose and schedule for such patients as a prelude to larger phase II trials of this approach. Other investigators have begun, as in other diseases, the use of post-transplant interventions, including the use of hypomethylating agents to delay or prevent recurrence of leukemia in combination with the allogeneic effect of the donor graft, which may be augmented by the use of these drugs.26
Alternatives to Related Donor Stem Cells
One of the problems noted early in patients who have leukemia and are being considered for allogeneic transplantation is the identification of a donor. As patients age, although they may have siblings, some of whom are matched, the overall physical condition of the donor may preclude either bone marrow harvest or peripheral blood stem cell collection. In many cases, the older patient may, in fact, be the youngest of his or her siblings and, thus, more older patients are being considered for unrelated donor transplant, using stem cells from a much younger donor. However, the identification of a donor sometimes takes approximately 3 months. Given the high-risk nature of the disease, many patients actually never come to transplant because of early relapse. Thus, one way to improve the likelihood of proceeding to transplant while in remission is to perform HLA typing at the time of diagnosis in order to shorten the time to transplant utilizing an unrelated donor. Relapse prior to transplantation usually results in the patient not going to transplant at all as there is a low likelihood of achieving a second remission of the leukemia that is inherently resistant and has shown early relapse.
Unrelated cord blood has provided another source of immediately available stem cells. Initial experience in children showed a clear association between cell dose and survival, and more recent results on unrelated cord blood transplantion in adults using high-dose conditioning regimens have now shown comparable outcomes when adequate cell dose is used. Two retrospective studies now show that such results compare favorably to those obtained with a matched unrelated donor, but these reports have been limited to younger adults with high-dose conditioning regimens.27,28 Studies of double cord transplants have been published, demonstrating that this approach may lessen the risk of graft rejection and high early mortality and that such an approach can be used effectively in the reduced-intensity regimen. Data is now emerging that such an approach can be used in AML, thus expanding the possibilities for a patient who is older with AML undergoing a reduced-intensity transplant regimen from either a related donor, unrelated donor or cord blood. A recent report showed a comparable result for patients undergoing transplantation from either cord blood or unrelated donor, 30% versus 34%, progression-free survival at 3 years, respectively.29
Suggested Strategy for the Older Patient with AML
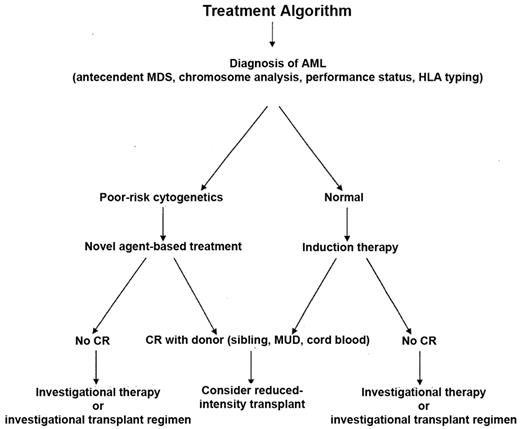
Given the optimistic results for those older patients who are able to undergo successful induction chemotherapy, achieve remission or near remission, have a donor and are able to proceed to transplantation, the strategy for such patients should include the option of transplant.30 HLA typing should be initiated at the time of diagnosis in order to know what the options might be for transplantation within the family and the typing can be used to at least determine the possibility of transplant from either an unrelated or cord blood donor. If a patient achieves a remission, then proceeding to transplant in a timely way would reduce the chances of an early relapse. Some, but not all, studies have validated the potential usefulness of a co-morbidity analysis, in addition to the Karnofsky performance scale (KPS), to help physicians and patients understand the risks of transplant and can be used to help make the decision about proceeding to transplant.31,32 For those patients who achieve a partial remission, then transplant on protocols that are specifically designed to treat such patients with an augmented regimen would be appropriate. Given that the disease is likely to increase as the population ages and people are now often in reasonably good physical condition at the time of diagnosis, even in their 70s, transplant may afford them an opportunity of cure. Figure 6 shows a suggested algorithm for the treatment approach for older patients with AML.
Overall survival among 348 patients older than 55 years with previously untreated AML entered onto a recent ECOG trial. Reprinted with permission from Rowe JM et al. Blood. 2004;103:479–485.7
Overall survival among 348 patients older than 55 years with previously untreated AML entered onto a recent ECOG trial. Reprinted with permission from Rowe JM et al. Blood. 2004;103:479–485.7
Survival of 122 patients with AML in CR1, CR2, or with more advanced disease treated with reduced-intensity conditioning using the Seattle regimen. Reprinted with permission from Hegenbart U et al. J Clin Oncol. 2006;24:444–453.12
Survival of 122 patients with AML in CR1, CR2, or with more advanced disease treated with reduced-intensity conditioning using the Seattle regimen. Reprinted with permission from Hegenbart U et al. J Clin Oncol. 2006;24:444–453.12
Probabilities of overall survival (A) and relapse (B) by the donor type. Reprinted with permission from Nakamura R et al. Bone Marrow Transplant. 2007;40:843–850.13
Probabilities of overall survival (A) and relapse (B) by the donor type. Reprinted with permission from Nakamura R et al. Bone Marrow Transplant. 2007;40:843–850.13
Impact of chronic graft-versus-host disease (GVHD) on the outcome of the patients: (A) overall survival; (B) disease-free survival; and (C) relapse. (——) Patients who developed chronic GVHD. (•••) Patients who did not develop chronic GVHD. Reprinted with permission from Valcarcel D et al. J Clin Oncol. 2008;26:577–584.16
Impact of chronic graft-versus-host disease (GVHD) on the outcome of the patients: (A) overall survival; (B) disease-free survival; and (C) relapse. (——) Patients who developed chronic GVHD. (•••) Patients who did not develop chronic GVHD. Reprinted with permission from Valcarcel D et al. J Clin Oncol. 2008;26:577–584.16
Survival after reduced-intensity conditioning allogeneic stem cell transplantation (RIC-allo-SCT) for acute myeloid leukemia (AML). (a) Comparison of leukemia-free survival (LFS) between the “donor” (solid line) and “no donor” (dashed line) groups; (b) Comparison of LFS between the “transplant” (solid line) and “no transplant” (dashed line) groups; (c) Comparison of overall survival (OS) between the “transplant” (solid line) and “no transplant” (dashed line) groups; (d) Comparison of OS between the “donor” (solid line) and “no donor” (dashed line) groups. Probabilities of LFS and OS were estimated from the time of diagnosis using the Kaplan-Meier product-limit estimates. Reprinted with permisson from Mohty M et al. Leukemia. 2009;23:194-195.20
Survival after reduced-intensity conditioning allogeneic stem cell transplantation (RIC-allo-SCT) for acute myeloid leukemia (AML). (a) Comparison of leukemia-free survival (LFS) between the “donor” (solid line) and “no donor” (dashed line) groups; (b) Comparison of LFS between the “transplant” (solid line) and “no transplant” (dashed line) groups; (c) Comparison of overall survival (OS) between the “transplant” (solid line) and “no transplant” (dashed line) groups; (d) Comparison of OS between the “donor” (solid line) and “no donor” (dashed line) groups. Probabilities of LFS and OS were estimated from the time of diagnosis using the Kaplan-Meier product-limit estimates. Reprinted with permisson from Mohty M et al. Leukemia. 2009;23:194-195.20
Suggested treatment strategy for older patients with newly diagnosed acute myelogenous leukemia.
Suggested treatment strategy for older patients with newly diagnosed acute myelogenous leukemia.
Disclosures Conflict-of-interest: The author is employed by the City of Hope Comprehensive Cancer Center. Off-label drug use: None disclosed.
References
Author notes
Department of Hematology and Hematopoietic Cell Transplantation, City of Hope Comprehensive Cancer Center, Duarte, CA