Abstract
The adoptive transfer of antigen-specific T cells has been used successfully to treat experimental tumors in animal models and viral infections in humans, but harnessing the exquisite specificity and potency of T cells to treat human malignancy has proven challenging. The efforts to use T cells to treat patients with cancer have often been informative in identifying limitations that must be overcome to improve therapeutic efficacy, and a clearer picture of the requirements for successful adoptive T-cell transfer is gradually emerging. Indolent and a subset of aggressive B-cell lymphomas in humans have been shown to be susceptible to eradication by T cells in clinical settings where highly immunogenic minor histocompatibility or viral antigens are presented by tumor cells. In this article, we will review how recent advances in our understanding of the properties of antigen-specific T cells that facilitate their long-term persistence in vivo and reversion to the memory pool after in vitro culture, combined with approaches to molecularly engineer T cells with receptors that target molecules expressed by B-cell lymphoma, are providing opportunities to broaden the application of T-cell therapy and improve its efficacy for this disease.
The development of immunotherapy for human malignancies has long held conceptual appeal because of the exquisite sensitivity and specificity of antibody and T-cell recognition, which could allow eradication of tumor cells without collateral damage to normal tissues. The ability of antibody or T-cell therapy to cure experimental tumors in animal models has been demonstrated repetitively, but clinical translation has not been straightforward. Early clinical trials in which murine monoclonal antibodies specific for tumor cell surface molecules were passively transferred revealed many obstacles, including immune recognition of murine determinants and inefficient recruitment of effector mechanisms, which were subsequently overcome by engineering of human and chimeric antibodies.1 With these advances, several monoclonal antibodies, including rituximab and CAMPATH-1 used in therapy of B-cell lymphomas, have been shown to improve the outcome for patients with cancer and become a component of standard therapy. T cells recognize peptide fragments of intracellular proteins displayed associated with class I and class II MHC molecules at the cell surface, and could specifically target tumor cells for destruction by secretion of cytolysins and recruitment of phagocytes and other nonspecific effector cells. Because T cells are capable of proliferation, the adoptive transfer of tumor-reactive T cells has the additional advantage of the potential for amplification of the anti-tumor response in vivo. However, the development of effective adoptive T-cell therapy for human malignancies has encountered many obstacles and, with a few exceptions, has not yet had a major impact in cancer treatment.2–4 The accelerating discovery of tumor-associated antigens, insights into the biology of T cells that enable selection and expansion of tumor-reactive T cells with the capacity to persist in vivo, and the ability to engineer tumor-reactive T cells by gene transfer provide opportunities to improve the efficacy and utility of adoptive T-cell therapy.
Requirements for Effective Adoptive T-Cell Therapy
Two fundamental principles for the deployment of adoptive T-cell therapy are the need to isolate and expand T cells specific for antigens expressed selectively or preferentially by the tumor, and the requirement that transferred tumor-reactive T cells persist in vivo after adoptive transfer.2–4 It is now clear that many human tumors, including B-cell malignancies, express potentially immunogenic proteins (Table 1 ). In B-cell malignancies, these may include tumor-specific proteins such as the immunoglobulin idiotype in B-cell lymphomas; overexpressed proteins such cyclin D1 and survivin that may play a role in proliferation and/or survival of mantle cell lymphoma and CLL, respectively; and viral proteins such as those encoded by Epstein-Barr virus (EBV), which is present in the malignant cells of a subset of patients with Hodgkin disease (HD) and in B-cell lymphomas that develop in immunocompromised individuals.5–8 In the setting of allogeneic hematopoietic stem cell transplantation (HCT), minor histocompatibility (H) antigens that are expressed in tumor cells provide an additional source of antigens that could serve as targets for T-cell therapy.9
The isolation of T cells with high avidity for tumor-associated antigens from tumor-bearing patients has proven to be difficult, and this may in part reflect local and systemic mechanisms that tumors use to render potentially reactive T cells that are present in the repertoire tolerant to tumor antigens.10 The development of efficient methods for selectively isolating T cells that are specific for tumor-associated antigens in vitro has been an area of significant progress. Stimulation of T cells with autologous or artificial antigen-presenting cells (APC) pulsed with antigenic peptides or transfected with genes that encode tumor or viral antigens has been used to enrich polyclonal T cells for a desired specificity.11 Peptide-MHC tetramers, bispecific antibodies, and antibodies that bind to T-cell surface molecules that are upregulated after antigen stimulation have also been developed and enable the use of flow cytometry or immunomagnetic selection for isolating T cells of a defined specificity.12,13 In circumstances where tumor-reactive T cells can be isolated from tumor-bearing patients, it is usually necessary to propagate these cells in vitro to derive sufficient numbers to augment the ineffective response in the host. Culture techniques for efficiently expanding T cells with retention of antigen specificity are now readily available, but the individualized nature of adoptive T-cell therapy and the specialized facilities that are required for culturing cells for human use remain significant obstacles. The ability of T cells to persist in vivo has correlated with therapeutic efficacy in most studies, and the process of culturing T cells is fraught with the potential to alter their properties such that they are unable to survive in vivo. The duration that cultured T-cell clones or polyclonal populations of T cells persist in vivo after infusion has been short in most trials of adoptive therapy, even when interleukin-2 (IL-2) is administered after cell transfer to support cell survival.14,15 Some factors that contribute to poor engraftment of transferred T cells have been identified, including the use of high concentrations of IL-2 during culture, which conditions T cells for death with subsequent activation or cytokine withdrawal; and a long duration of culture, which could drive T cells to a terminally differentiated state. The engraftment of adoptively transferred virus or tumor-specific T cells and therapeutic efficacy has been superior in lymphopenic hosts, such as when the T cells are administered after T-cell–depleted allogeneic HCT or after iatrogenic lymphodepletion with cytoxan and fludarabine.5,16 This may result from elevated levels of IL-7 and IL-15 that maintain normal lymphocyte homeostasis, or deletion of subsets of regulatory or suppressor cells.4 However, even in lymphodepleted hosts, the improved persistence of transferred T cells results from proliferation and/or survival of only a minor subset of the transferred population,16 suggesting that intrinsic properties of individual T cells dictate their ability to persist in vivo.
Isolating T Cells with the Intrinsic Capacity to Persist in Vivo for Immunotherapy
The T-cell subset from which tumor-specific CD8+ or CD4+ T cells are isolated for adoptive therapy is rarely known with certainty. The T-lymphocyte pool contains naïve (TN), central memory (TCM), and effector memory (TEM) subsets that differ in phenotype, function, and homing.17 After T-cell receptor (TCR) engagement by antigen in vivo, TN cells undergo rapid proliferation and programmed differentiation, resulting in the generation of large numbers of effector T cells (TE), most of which die as antigen is cleared, leaving a small pool of phenotypically distinct TCM and TEM cells. Memory T cells persist for life and respond to antigen re-exposure in vivo or in vitro by differentiating again into TE cells. The life-long maintenance of T-cell memory suggests that at least some cells in the memory pool must be capable of both self-renewal and differentiation, and recent studies have provided evidence that a subset of memory T cells have stem cell–like properties.18
In situations where tumor-reactive TE cells are generated from the blood of tumor-bearing patients, the cells could be derived from T cells already differentiated towards a memory phenotype by in vivo exposure to tumor antigen, if these cells have not been deleted or rendered dysfunctional.19 However, it is likely that tumor-reactive TE cells are often derived in culture by in vitro priming of TN precursors.20 Studies in murine models have suggested that the conditions under which TN cells are differentiated into effector cells in vitro can have a profound influence on their ability to persist in vivo and mediate antitumor effects. A single in vitro stimulation with antigen and IL-15 of CD8+ TN cells from transgenic mice with a tumor-specific TCR transgene generated T cells that retained CD62L expression, migrated to lymph nodes after cell transfer, and were more effective in tumor therapy than TN cells stimulated with antigen and IL-2.21 These results suggest that culture in IL15 elicits TE cells with some characteristics of memory T cells. However, it is unknown if the superior properties of T cells cultured short term in IL-15 would be retained through the long duration of culture necessary to generate a sufficient number of T cells for clinical immunotherapy.
In some situations, including those where viral antigens are the targets of tumor therapy or where T cells are engineered to have tumor specificity by gene transfer, T cells could be derived from fully functional TCM or TEM precursors, rather than from TN cells. In a nonhuman primate model, we have recently studied adoptive T-cell therapy by using cell sorting to purify CD8+ T cells into TCM or TEM subsets from which antigen-specific TE clones were generated, and we observed profound differences in the capacity of these cells to persist in vivo and revert to the memory pool after adoptive transfer. T-cell clones derived from TCM and TEM cells had a similar phenotype, avidity, lytic capacity, and telomere length before infusion, but only those derived from TCM cells persisted long-term, reacquired phenotypic and functional properties of memory cells, and occupied memory T-cell niches in vivo after adoptive transfer (C. Berger, S.R. Riddell, manuscript submitted). These results demonstrate that some of the progeny of clonally derived TE cells retain intrinsic programming of the parental cell of origin and may explain the inconsistent persistence of transferred T cells when unselected T cells are used as the source to generate tumor-specific T cells for adoptive immunotherapy. Of perhaps greater significance, adoptively transferred TCM-derived T-cell clones could be induced to proliferate in hosts with a full lymphoid compartment by antigen stimulation, illustrating the potential to amplify the magnitude of transferred T-cell immunity in vivo. Additional insights into the properties of T cells that dictate their ability to survive and function in vivo are likely to be derived and should improve our ability to select T cells with the desired fitness for adoptive therapy.
Adoptive Transfer of T Cells to Treat B-Cell Malignancies
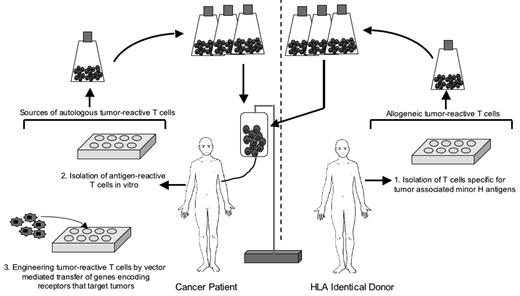
The most concentrated effort to use adoptive T-cell therapy for human cancer has been in malignant melanoma.22 However, the potential for T cells to contribute to the eradication of B-cell malignancies in humans is illustrated by the ability of allogeneic HCT to cure advanced lymphoma, which can be attributed in part to a T-cell–mediated graft-versus-tumor (GVT) effect; by the transfer of T cells specific for EBV antigens to treat EBV-associated B-cell lymphoproliferative disease and Hodgkin disease; and by the development of approaches using genetic modification to engineering T cells to have specificity for tumors, including B-cell malignancies (Figure 1 ).
The GVT effect of allogeneic HCT results from T cells in the donor graft that recognize minor H antigens expressed on tumor cells, and applies to a wide variety of hematopoietic neoplasms.9 There is compelling clinical evidence that a GVT effect contributes to the efficacy of HCT for B-cell malignancies, particularly follicular lymphoma, chronic lymphocytic leukemia (CLL), and multiple myeloma.23–25 However, because of toxicity of allogeneic HCT, including that resulting from graft-versus-host disease (GVHD), this approach is generally reserved for patients with advanced disease who have failed conventional chemotherapy and autologous HCT. To reduce toxicity and allow allogeneic HCT to be used in older patients, reduced-intensity conditioning regimens have been developed that alone are not myeloablative and only modestly reduce the tumor burden, but combined with the GVT effect can induce durable complete remissions in patients with lymphoma.24
The use of nonmyeloablative allogeneic HCT has also provided an opportunity to study the evolution of an effective antitumor immune response. Our group has focused on analysis of patients with CLL to dissect the immunologic basis for GVT activity and derive insights for the development of specific adoptive T-cell therapy to enhance the GVT effect. There are advantages to analyzing patients undergoing HCT for CLL, including the ease in obtaining and cryopreserving tumor cells from the patient’s peripheral blood prior to HCT and the ability to enhance the antigen-presenting capacity of CLL by stimulating the CD40 receptor on the tumor cells with its ligand. CD40L-stimulated CLL cells have been used as APC to evaluate the kinetics, phenotype, and specificity of tumor-reactive T cells that develop following allogeneic nonmyeloablative HCT. These studies show that patients who experience sustained tumor regression after HCT develop CD8+ and CD4+ T-cell responses directed against recipient minor H antigens expressed by CLL. These tumor-reactive T cells often develop very early after transplantation and may not be associated temporally with GVHD. In responding patients, the T-cell responses are typically directed against multiple minor H antigens expressed on CLL cells and persist long term, which may explain the low risk of subsequent relapse in patients that achieve remission after transplantation.26 A striking finding is that patients with progressive disease after HCT fail to develop T-cell responses that recognize the patient’s CLL, even though they may develop GVHD, suggesting that the failure to eradicate the tumor in these patients is because the alloreactivity is singularly focused on minor H antigens that are not expressed by the tumor (T. Nishida, S.R. Riddell, manuscript in preparation).
Adoptive T-cell transfer of in vitro–expanded T cells that are specific for individual minor H antigens could provide a strategy to selectively augment the GVT effect without GVHD, but several issues have impeded clinical translation. One problem is that most minor H antigens are expressed on both recipient hematopoietic and epithelial cells, and T cells specific for these antigens can cause GVHD in addition to GVT effects. A focus of work in the field is to identify minor H antigens that are limited in their expression to hematopoietic lineage cells, including tumor cells, and could serve as targets for a selective GVT response.9 Several antigens in this category that are applicable to therapy of B-cell malignancies have been identified and include HA-1, HB-1, PANE-1, bcl2A1, and P2X5.9,27–29 At the present time, only a subset of patients would be candidates for therapy targeting one of these minor H antigens, since the patient must have the correct HLA-restricting allele and be appropriately discordant for the minor H antigen with their donor.30 Thus, discovery of additional hematopoietic lineage-restricted minor H antigens is clearly needed. A second problem is that immunosuppressive drugs are required to prevent GVHD after allogeneic HCT with T-cell–replete stem cell products, and these drugs interfere with the function and/or survival of adoptively transferred T cells. Research in murine models has refined our view of GVHD pathogenesis and identified novel approaches to HCT that might diminish GVHD and the need for intensive immunosuppression, thereby enabling adoptive T-cell transfer to target alloreactivity to malignant cells after allogeneic HCT.31 A focus of future work will be to determine if the strategies that are effective for reducing GVHD in mice can be translated to the clinic.
Adoptive T-Cell Therapy of EBV-Associated B-Cell Malignancies
A subset of B-cell malignancies contain the EBV genome and express EBV-encoded proteins that can serve as targets for T-cell therapy. Severely immunocompromised patients who received transplants can develop a lymphoproliferative disease (EBV-LPD) consisting of EBV-infected B cells that express a subset of EBV genes, which include the highly immunogenic EBNA-3A, -3B, and -3C proteins.5 Heslop, Rooney and colleagues have performed studies in which EBV-specific T cells were isolated from allogeneic stem cell transplant donors and adoptively transferred to the respective immunocompromised HCT recipients who had developed EBV-LPD or were at high risk for EBV-LPD. In this setting, adoptively transferred T cells promoted regression of established EBV-driven lymphomas and prevented the development of tumors when administered prophylactically.5
A subset of Burkitt lymphoma (BL) and HD that occur in immunocompetent individuals also contain the EBV genome. BL express only the EBNA-1 protein, while HD express EBNA-1, LMP-1, and LMP-2.32 EBNA-1, LMP-1, and LMP-2 are weakly immunogenic, and these tumors evade immune recognition by a variety of mechanisms. Despite these obstacles, EBV-reactive T cells, including those specific for the weakly immunogenic LMP-2 protein, can be isolated and expanded from patients with EBV-associated lymphomas, and after adoptive transfer augment T-cell responses, migrate to tumor deposits, and promote tumor regression in a subset of patients.33,34 These results illustrate the potential efficacy of autologous T-cell therapy in B-cell malignancies, providing sufficiently immunogenic tumor antigens can be identified.
Engineering T Cells to Recognize Tumors by T-Cell Receptor Gene Transfer
With the exception of EBV and minor H antigens, the isolation of T cells specific for candidate antigens on B-cell lymphomas from tumor-bearing patients still presents a substantial hurdle, as it does for most human cancers. For tumors that express antigens that are shared among many patients, the requirement to isolate tumor-reactive T cells from each patient could be overcome if a patient’s T cells were engineered to have the specificity of a T-cell clone that was effective in other patients. This can be accomplished by transferring the TCR α and β genes derived from a tumor-specific T cell into T lymphocytes of the patient. Gene modification of T cells to confer specificity using retroviral vectors has been successful for targeting T cells to melanoma, viruses, minor histocompatibility antigens, and oncoproteins.35 Such engineered T cells acquire the antigen specificity of the introduced TCRs, including the ability to lyse antigen-bearing target cells and to eliminate tumors in vivo in animal models and in a small number of patients with melanoma.36
The preclinical and initial clinical studies of TCR gene transfer have shown promise but have also revealed limitations. Expression of the transferred TCR α and β chains must be sustained at sufficient levels for tumor recognition, and this is not always accomplished with currently available vectors. The introduced TCR chains can also cross-pair with the endogenous TCR chains, resulting in formation of hybrid receptors with unknown and potentially deleterious specificity. This problem may be resolved using murine rather than human constant regions in the introduced TCR chains, or by incorporating cysteine residues in the human constant regions of both the α and β chains to allow disulphide bond formation and promote preferential pairing of the introduced TCRs.37 Even with improved pairing and expression of the TCRs, the avidity of the receptors may still be low if the TCR genes were cloned from a T cell specific for a tumor-associated self-antigen. Introducing mutations in TCRs to confer higher avidity for the target antigen may be useful for further engineering of optimized receptors.
Engineering T cells to Recognize B-Cell Tumors by Expression of Chimeric Immunoreceptors
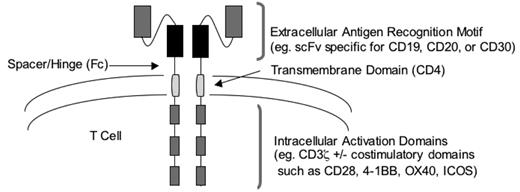
Genetic modification can also be used to render T cells capable of tumor recognition without the requirement for MHC-restricted recognition of peptide antigens by the TCR. This approach relies on the introduction into T cells of a chimeric antigen receptor (CAR) specific for a tumor cell surface molecule. Typically, CARs contain an extra-cellular domain that recognizes a tumor cell molecule fused to an intracellular signaling domain that triggers T-cell activation (Figure 2 ).38 The extracellular domain of most CARs is a single-chain antibody fragment (scFv) that incorporates the heavy and light variable chains (VH and VL, respectively) of a monoclonal antibody. This strategy seems ideal for targeting B-cell malignancies, since CD20 has been validated as a target in follicular, diffuse large-cell, and mantle cell lymphomas, and monoclonal antibodies specific for CD19 could be used to engineer T cells to target B-cell leukemias.38,39 The intracellular domain of CARs usually consists of the ζ-chain of the TCR complex and serves to activate T-cell effector functions and proliferation after engagement of the extracellular domain of the CAR by its target. Preclinical studies in murine models have demonstrated that T cells modified to express CARs can efficiently eliminate tumors in vivo, and phase 1 clinical trials of adoptively transferred CAR-modified T cells are in progress in patients with lymphoma and leukemia.
The use of genes encoding CARs to target T cells to tumors resolves the need to isolate or engineer MHC-restricted tumor-specific T cells. Additional preclinical and clinical evaluation will determine limitations of this approach that may interfere with efficacy. One issue identified in preclinical studies is that the stoichiometry of antibody binding to tumor cells will be different from that of the TCR/MHC interaction and might not provide for optimal T-cell activation.40 Furthermore, T-cell activation normally involves signaling through both TCR and costimulatory molecules, and CARs that only encode the CD3-ζ signaling domain would fail to provide a costimulatory signal. This latter issue might be overcome by the addition of costimulatory signaling domains to the ζ-chain of the CAR.38 An alternative strategy to promote full activation of CAR-modified T cells is to use T cells of a known antigen specificity, such as EBV or CMV, for genetic modification and take advantage of additional signaling that could be provided through the endogenous TCR. Many CARs are derived from murine antibodies, which could be recognized by host immune responses to the murine VH and VL fragments that may limit the in vivo persistence of the transferred cells. Thus, CAR antibody domains may need to be humanized to reduce immunogenicity. Finally, several of the molecules that are being targeted by CARs, including CD19 and CD20, are expressed by normal cells in addition to tumor cells, and the adoptive transfer of CAR-modified T cells poses a potential risk of injury to normal tissues.
Conclusions
A title for this manuscript suggested by the Chair of the session was “T-cell adoptive immunotherapy: hype or hope,” perhaps to reflect the sometimes tortuous path that the development of adoptive T-cell therapy has endured. It is understandable that investigators may feel unrestrained optimism after observing the dramatic regression of advanced tumors in otherwise incurable patients by the adoptive transfer of T cells or allogeneic HCT. The harsh reality is that the responses are too few and unpredictable. There are practical and scientific obstacles that must be overcome before T-cell therapy can be broadly and effectively used for human cancer, including B-cell malignancies. Murine models have been useful for elucidating principles of T-cell biology but have been much less successful in guiding the development of adoptive T-cell therapy for cancer. Many of the most important insights have come from careful analysis of patients enrolled on clinical trials, and it is critical these efforts continue. The array of tools that are now available to manipulate and study the T cells, the tumor, and the host is unprecedented, and provides hope that adoptive T-cell therapy will become a more refined and effective modality.
Strategies for deriving autologous or allogeneic T cells for adoptive therapy of B-cell lymphoma.
Strategies for deriving autologous or allogeneic T cells for adoptive therapy of B-cell lymphoma.
Design of chimeric antigen receptors using antibody domains for targeting T-cell specificity to B-cell malignancies.
Design of chimeric antigen receptors using antibody domains for targeting T-cell specificity to B-cell malignancies.
Program in Immunology, Fred Hutchinson Cancer Research Center, Seattle, WA