Abstract
Active immunotherapy is a promising approach for the treatment of lymphomas. Immunization with the clonal tumor immunoglobulin, idiotype, expressed on the surface of B-cell malignancies was associated with induction of tumor-specific cellular and humoral immunity, molecular remissions, and prolonged disease-free survival in early clinical trials. Idiotype vaccination was also demonstrated to induce tumor-specific T-cell immunity in the absence of B cells following treatment with rituximab-containing chemotherapy, suggesting that vaccines may be used in combination with rituximab. Three double-blind randomized phase 3 idiotype vaccine trials are currently ongoing to definitively determine the clinical benefit of idiotype vaccination in patients with lymphoma. Novel second-generation lymphoma vaccines are in development to streamline the production of patient-specific cancer vaccines and show encouraging results in preclinical and pilot clinical studies. To enhance the clinical efficacy of active immunotherapy, future clinical trials are likely to use a combination strategy with the lymphoma vaccine to stimulate an antitumor T-cell response and the simultaneous suppression of immune regulatory pathways to augment the induced T-cell response.
Follicular lymphoma, the most common low-grade non-Hodgkin lymphoma (NHL) that comprises 22% of all NHL cases worldwide, is potentially curable with radiation therapy in the early stages.1 However, advanced disease is present in 85% of all patients at the time of diagnosis and is considered incurable with the available treatment options. Advanced-stage follicular lymphoma has generally an indolent course with a median survival of 8 to 10 years, but is characterized by repeated remissions and relapses with most patients eventually dying of their disease.1 The recent inclusion of rituximab, an anti-CD20 monoclonal antibody, in induction combination chemotherapy regimens has improved the response rates, duration of remission, progression-free survival, and overall survival of follicular lymphoma. However, these combinations do not appear to be curative for advanced-stage disease since no plateau in the survival curve has been demonstrable.2–4 Therefore, novel treatment options are needed to improve clinical outcome in these patients.
Active immunotherapy is a promising approach for the treatment of follicular lymphomas. Several lines of evidence suggest that follicular lymphomas may be highly sensitive to immunotherapy. First, spontaneous remissions lasting longer than 1 year have been observed in up to 23% of patients.5 Second, administration of single-agent rituximab monoclonal antibody to previously untreated follicular lymphoma patients has resulted in clinical responses in more than 70% of patients.6 Third, the survival of patients with follicular lymphoma appeared to correlate with the gene expression signatures of infiltrating non-malignant immune cells in the tumor.7 Taken together, these data support the development of novel immunotherapeutic strategies for the treatment of this lymphoma. Compared with passive immunotherapy with monoclonal antibodies, active immunotherapy with cancer vaccines may potentially induce polyclonal humoral and cellular immune responses with immunologic memory and minimize the emergence of tumor escape variants. Furthermore, cancer vaccines can target different tumor antigens and therefore can be complementary to monoclonal antibody therapy.
Vaccine strategies targeting NHL have largely focused on using the tumor immunoglobulin molecule expressed on the surface of malignant B cells as an antigen. The clonal tumor immunoglobulin molecule has many of the desirable characteristics of an ideal tumor antigen for active specific immunotherapy. First, the variable regions of the heavy and light chains of the tumor immunoglobulin contain unique determinants known as idiotype (Id) that are selectively expressed in tumor cells and therefore serve as a tumor-specific antigen. Second, the tumor immunoglobulin appears to be essential for tumor cell survival since immunoglobulin loss variants have rarely been described in follicular lymphoma.8,9 Third, idiotype vaccination can potentially induce polyclonal antibody and T-cell responses and therefore may reduce the appearance of immune escape variants.10
Preclinical Development of Idiotype Vaccines
Idiotype vaccination was first demonstrated to induce tumor resistance in the early 1970s by Lynch and Eisen in the mouse mineral-oil-induced plasmacytoma model.11 This phenomenon was reproduced subsequently in a number of lymphoma, myeloma, and leukemia models.12–14 Subsequently, Kaminski et al demonstrated that optimal immunization required conjugation to a strongly immunogenic carrier protein, such as keyhole-limpet hemocyanin (KLH).13 Further enhancement of the potency of the vaccine was demonstrated in a murine lymphoma model by Kwak et al with the addition of granulocyte-macrophage colony-stimulating factor (GM-CSF) as an adjuvant.14 GM-CSF facilitated the induction of tumor-specific CD8+ T cells, presumably by recruiting dendritic cells to the site of vaccination. These preclinical results, together with technical advances in Ronald Levy’s laboratory that made it feasible to isolate human idiotype proteins produced by heteromyeloma fusion, provided the rationale for testing autologous lymphoma-derived Id as a therapeutic “vaccine” in patients.
Clinical Trials of Idiotype Vaccines
Phase 1 clinical trial of idiotype vaccine
The first human study of Id vaccination was pioneered by Kwak et al, working in the Levy laboratory at Stanford, in patients with follicular lymphoma, which was a pilot study designed to determine whether it was possible to immunize against the Id portion of the protein.15 Patients with follicular lymphoma in clinical remission after chemotherapy were immunized with subcutaneous injections of autologous purified tumor-derived immunoglobulin conjugated to KLH along with a standard emulsion adjuvant. In all, 41 patients were treated on this pilot study; 41% demonstrated specific anti-Id antibody and 17% demonstrated cellular proliferative responses.16 These results demonstrated that patients with lymphoma could be induced to make sustained Id-specific immune responses by active immunization.
Phase 2 clinical trials of idiotype vaccines
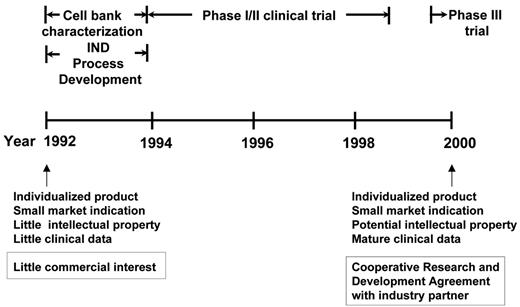
The technological advances that made it possible to pursue further clinical development (and eventually potential commercialization) were achieved by the Biological Resources Branch, Developmental Therapeutics Program of the National Cancer Institute (NCI) (Figure 1 ). These included, importantly, master and working heteromyeloma cell bank characterization, protein purification, and other process development according to good manufacturing practice (GMP) requirements and, eventually, successful filing of an Investigational New Drug (IND) application with the FDA. Then, based on the preclinical observation that the addition of GM-CSF as an adjuvant to the vaccine induced tumor-specific CD8+ T cells,14 Bendandi et al, working in the Kwak laboratory, conducted a phase 1/2 clinical trial where 20 previously untreated follicular lymphoma patients were treated with 5 monthly doses of autologous tumor-derived Id-KLH + GM-CSF vaccine following induction of clinical remission with a uniform chemotherapy regimen (prednisone, doxorubicin, cyclophosphamide, and etoposide [PACE]).17 The vaccine was well tolerated, with the main adverse effects being injection site reactions. Following vaccination, anti-Id antibody responses were induced in 15 out of 20 (75%) patients and Id-specific and/or tumor-specific CD4+ and CD8+ T-cell responses were observed in 19 out of 20 (95%) patients.17 Monitoring of the patients for minimal residual disease showed that 8 out of 11 patients with PCR-positive t(14;18) chromosomal translocation breakpoints converted to PCR negativity in their blood immediately after completing vaccination and sustained their molecular remissions for a median of 18+ months.17 These data provided evidence for antitumor effect of the Id vaccination in vivo. Furthermore, recent reports in the literature support the notion that achieving both clinical and molecular remission may correlate with longer freedom from disease recurrence in patients with follicular lymphoma.18,19 However, large-scale prospective studies are needed to confirm this observation prior to using molecular remission as a surrogate marker for therapeutic vaccination in follicular lymphoma. With a median follow-up of 9.2 years, median disease-free survival (DFS) is 8 years, and the overall survival rate is 95%.20 While definitive statements cannot be made, because this was not a randomized trial, the DFS appears superior to that of a historic, ProMACE chemotherapy-treated control group (median DFS, about 2.2 years).21
Additional clinical trials using Id-KLH + GM-CSF or Id-pulsed dendritic cell vaccinations also suggested a possible clinical benefit associated with idiotype protein vaccination in patients with follicular lymphoma when used alone or after cytoreductive therapy. Specifically, Timmerman and colleagues treated 35 patients with follicular lymphoma with dendritic cells pulsed with tumor-derived idiotype protein. Among 18 patients with residual tumor at the time of vaccination, 4 (22%) had tumor regression, and 16 of 23 patients (70%) remain without tumor progression at a median of 43 months after chemotherapy.22 In a recent clinical trial, Inoges and colleagues immunized 25 follicular lymphoma patients in first relapse with idiotype vaccine following second chemotherapy-induced remission. Idiotype-specific immune responses were observed in 20 of 25 (80%) patients. The median duration of second complete response among the immune responders was significantly prolonged compared with their first complete response.23
Idiotype vaccines in combination with rituximab
Understanding idiotype-induced tumor cell immunity has attained new clinical importance due to increased use of rituximab in the treatment of B-cell NHL. Rituximab is an anti-CD20 monoclonal antibody that depletes both normal and malignant B cells.24 Consequently, patients treated with rituximab are unlikely to have peripheral blood B cells and therefore unlikely to generate humoral immune responses following active immunotherapy. Furthermore, the role of B cells in the priming of naïve T cells has not been evaluated in humans. We recently evaluated the effects of B-cell depletion induced by rituximab on immune responses to idiotype vaccines in a pilot clinical trial where 26 previously untreated patients with mantle cell lymphoma received 5 monthly cycles of autologous tumor-derived Id-KLH + GM-CSF vaccination following induction of clinical remission with dose-adjusted rituximab, etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH-R).10 Unexpectedly, despite rituximab administration, antibody responses against the carrier molecule KLH and Id were detected in 17 out of 23 (74%) and 7 out of 23 (30%) evaluable patients, respectively. The humoral responses were delayed and correlated with B-cell recovery that began approximately 6 months after completion of EPOCH-R and returned to baseline by 1 year in most individuals. Thus, most humoral responses were detected after the fourth or fifth vaccination (7 to 8 months after completion of EPOCH-R), compared with after the first or second vaccinations in the follicular lymphoma study in which rituximab was not administered.10,25 Additionally, in several patients, humoral responses were delayed 4 to 10 months after the last vaccination, suggesting that priming may occur at low B-cell levels, whereas detectable antibody titers require adequate B-cell expansion. In contrast, vigorous CD4+ and CD8+ antitumor and KLH T-cell responses were not delayed and were induced in 20 out of 23 (87%) and 23 out of 23 (100%) patients, respectively, in the absence of circulating B cells.10 Professional antigen-presenting cells such as dendritic cells may effectively present antigen to T cells in the absence of B cells. Although we did not measure B cells within lymphatic tissues, other studies have shown that rituximab also depletes B cells in lymph nodes, spleen, and bone marrow in animals26,27 and humans.28 These results were consistent with the recent observation that long-lasting CD4+ memory T cells can be induced by hepatitis B vaccination in patients with X-linked agammaglobulinemia, a genetic disease characterized by the lack of circulating B cells.29
Taken together, these data indicated that idiotype vaccines could be administered in the setting of B-cell depletion induced by rituximab. Recently, this observation was independently confirmed in another clinical trial where induction of anti-KLH and anti-Id or tumor T-cell responses were observed in greater than 80% of follicular lymphoma patients vaccinated with autologous tumor-derived Id-KLH + GM-CSF vaccine following treatment with rituximab.30
Phase 3 clinical trials of idiotype vaccines
The encouraging immunological and clinical outcome of the Id-KLH + GM-CSF vaccine in the phase 1 and 2 clinical trials led to the initiation of three randomized double blind placebo controlled multicenter clinical trials to definitively answer the question of clinical benefit induced by idiotype vaccination.25,31 At least two of the three trials have recently completed accrual and the results are expected within the next 2 years.
Importance of Humoral and Cellular Immune Responses in Idiotype Vaccine Efficacy
Results from the phase 1 and phase 2 clinical studies suggest that both humoral and cellular immune responses may be important in inducing clinical benefit following idiotype vaccination. In a recent report, the induction of a specific anti-Id antibody response was associated with significantly prolonged progression-free survival (PFS) in patients with follicular lymphoma.32 Moreover, patients with a favorable FcγRIIIa polymorphism (158 valine/valine [V/V] genotype) that predicted for stronger binding of the Fc of antibodies to effector cells had a longer PFS than those with valine/ phenylalanine (V/F) or phenylalanine/phenylalanine (F/ F) genotypes. However, evidence in the literature also suggests that T-cell responses may induce tumor regression independent of a humoral response following idiotype vaccination. Evidence of clinical and molecular remissions was observed in various clinical trials following Id-pulsed DC vaccinations and/or idiotype vaccinations in combination with cytokines as adjuvants.10,17,,22,23,30,33,34 In particular, in the NCI phase 1/2 study, significant levels of HLA class I-restricted killing of autologous tumor targets were demonstrated in the majority of patients, suggesting the induction of a cytotoxic CD8+ T-cell response.17 Importantly, 3 patients who achieved molecular remissions did so in the absence of a detectable antibody response to Id. In addition, in the vast majority of vaccinated patients with mantle cell lymphoma (87%), lymphoma-specific T cells producing type 1 cytokines were detectable by state-of-the-art intracellular cytokine staining.10 Finally, definitive characterization of anti-idiotype cellular immune responses have defined the unique immunodominant peptide epitopes within the hypervariable complementarity-determining regions (CDR), but not framework regions of immunoglobulin heavy chain, recognized by lymphoma-specific T cells.35
Taken together, these results support the use of lymphoma vaccines in the setting of B-cell depletion induced by rituximab-based induction regimens. However, because the relative role of cellular versus humoral immunity for vaccine efficacy is uncertain, it may be advisable to administer booster vaccinations following B-cell recovery to optimize humoral responses.
Next generation lymphoma vaccines
The results of early phase clinical trials described above suggest that idiotype vaccination is safe, induces antitumor humoral and cellular immune responses, and is associated with long-term DFS in patients with follicular lymphoma. However, rapid progress in the development of active immunotherapeutic approaches for lymphoma has been hindered by the need to generate a custom-made product for each patient, by a process that is expensive, laborious, and time-consuming. Traditionally, idiotype was generated by hybridoma tissue culture technology for vaccine production.31 More recently, recombinant DNA technology has been used for idiotype protein production.36 Although faster, this process still requires 2 to 3 months to generate a vaccine for each patient.
DNA vaccines
An alternative to idiotype protein vaccination is to use DNA vaccines. Any delivery system that does not require protein expression holds tremendous potential for the goal of streamlining vaccine production. Immunoglobulin-variable genes specific for the B-cell malignancies can be readily cloned and combined into single-chain variable fragment (scFv) format, encoding a single polypeptide consisting solely of VH and VL genes linked together inframe by a short, 15–amino acid linker. Preliminary studies in mice and humans showed that the DNA vaccine is weakly immunogenic in most cases and needs to be used together with an adjuvant to render it immunogenic. King et al demonstrated that fusion of the gene encoding fragment C of tetanus toxin to scFv markedly enhances the anti-idiotypic antibody response and induced protection against B-cell lymphoma in mice.37 Biragyn and colleagues showed that the efficiency of DNA vaccination in vivo could be greatly increased by encoding a fusion protein consisting of idiotype (scFv) fused to a proinflammatory chemokine moiety that facilitates targeting of antigen-presenting cells for chemokine receptor–mediated binding, uptake, and processing of scFv antigen for subsequent presentation to CD4+ or CD8+ T cells, or both.38 Specifically, mice immunized by gene gun with plasmids encoding monocyte chemotactic protein 3 (MCP-3) or interferon inducible protein 10 (IP-10)–scFv fusions, but not scFv alone, induced protective antitumor immunity against a large tumor challenge (20 times the minimum lethal dose). Furthermore, the level of protection was equivalent or superior to that of the prototype Id-KLH protein vaccine. DNA vaccines using such fusion strategies have recently entered clinical trials.
Membrane proteoliposomal vaccine
Another alternative for streamlining the production of individualized tumor vaccines is to directly extract membrane proteins from the tumor cells and incorporate them into liposomes along with IL-2 to produce membrane-patched proteoliposomes (MPL) (Figure 2; see Color Figures, page 509).39 Surface immunofluorescent staining of the MPL vesicles showed the presence of IL-2, Id, and other proteins such as MHC and costimulatory molecules. IL-2 was previously shown to have a specific interaction with small unilamellar lipid vesicles leading to the formation of multilamellar coalescent vesicles used for vaccines. In addition, IL-2 can act as an adjuvant to expand activated T cells. Reports in the literature indicate that antigen encapsulated in liposomes is delivered into both the endosomal and cytosolic processing pathways of antigen-presenting cells, thereby generating both CD4+ and CD8+ T-cell responses. Testing in a mouse lymphoma model showed this formulation to be at least as potent as the prototype Id protein vaccine in inducing tumor protection.39 Protection experiments demonstrated that survival following tumor challenge was highly dependent upon having the membrane proteins and IL-2 in the same MPL vesicle. Liposomes containing only the tumor membrane extract or only IL-2 were ineffective, and administering these formulations as a mixture resulted in partial protection.39
The major advantage of the MPL vaccine is that it would circumvent the expensive and time-consuming steps of preparing Id protein vaccines by hybridoma or recombinant DNA technologies. Manufacturing such a vaccine would require only 24 hours, and the vaccine would be ready for administration immediately following standard release and sterility testing. Specifically, after an excisional biopsy of at least a 2-cm lymph node, the tumor sample is processed into a single-cell suspension, and proteins from whole-cell membranes are directly extracted from 2 × 109 lymph node biopsy cells with detergent. The membrane proteins are incorporated into liposomes along with IL-2 to produce the MPL (Oncoquest-L) vaccine. Each vaccine is formulated on a per-milliliter basis, with membrane proteins obtained from approximately 1.6 × 108 biopsy cells, 4 × 106 IU of IL-2, and 80 mg of dimyristoylphosphatidyl-choline (DMPC), which is used to generate liposomes.39,40
In a pilot clinical trial, the novel membrane proteoliposomal vaccine was safe, induced autologous tumor-specific type 1 cytokine responses in 5 out of 10 patients with advanced-stage follicular lymphoma (Figure 3; see Color Figures, page 209), and was associated with induction of a sustained complete response in 1 patient.40 Other patients had large tumor burdens and progressed after a median duration of 8 months. Despite the use of total membrane proteins as antigenic material in the vaccine formulation, there was no clinical or laboratory evidence of autoimmunity in any patient on this trial.40 Although this novel vaccine formulation requires the generation of a custom-made product for each patient, it offers several advantages over patient-specific idiotype vaccines. First, this formulation can be produced rapidly within a single day, in contrast to the 2 to 6 months required to manufacture idiotype vaccine for each patient. Second, in addition to the membrane idiotype protein, this vaccine formulation may induce immune responses against other unrecognized tumor-associated antigens. Finally, this novel formulation may serve as a model for vaccine development against other human malignancies, including certain leukemias, lymphomas, and solid tumors, where tumor-associated antigens have not been defined. The encouraging results in this pilot study of patients with follicular lymphoma with bulky disease, suggests that additional testing of this formulation may be warranted, particularly in the setting of low tumor burden or minimal residual disease.
Other individualized tumor vaccine formulations
Novel vaccine formulations in development for NHL include GM-CSF-transduced tumor/bystander cells,41 CD40-activated tumor cells,42 T-cell receptor vaccines,43 tumor lysate–pulsed dendritic cells,44 and tumor-derived heat shock protein–peptide complexes (Table 1 ).45 However, similar to idiotype protein vaccination, these vaccine formulations also require generating custom-made product for each patient, which may limit the broad applicability of this approach. Identification of universally expressed lymphoma-specific antigens will be necessary in the future to develop vaccine formulations that can be used in all lymphoma patients and are therefore easier and less costly to produce.
Future Directions
Enhancement of the potency of lymphoma vaccines is also required to improve the clinical benefit induced by active immunotherapy. With the increased use of rituximab for the treatment of B-cell NHL, improvement in the potency of the vaccines would require strategies to enhance the T-cell responses, since rituximab depletes normal B cells and impairs the generation of antibody responses following vaccination. Recent studies in animal models suggest that the T-cell immune responses against foreign or self-antigens are regulated by several immunoregulatory pathways and/or peripheral tolerance mechanisms.46 Therefore, disruption of the immunoregulatory pathways such as CD4+CD25+ regulatory T cells (Treg), cytotoxic T lymphocyte–associated antigen (CTLA)–4, programmed cell death 1 (PD-1), B7-H1, and B7-H4 that modulate the magnitude and duration of the T-cell immune responses may enhance the potency of cancer vaccines. Data in the literature suggest that these regulatory pathways may be important in B-cell lymphomas. For example, Treg appear to be actively recruited into the tumor microenvironment and inhibit the function of intratumoral CD4+ and CD8+ T cells.47,48 In a pilot clinical trial, administration of anti-CTLA–4 monoclonal antibody was associated with regression of tumors in patients with lymphoma.49 These results suggest that the combined use of lymphoma vaccines together with simultaneous suppression of immune regulatory pathways may lead to enhanced clinical efficacy of active immunotherapeutic strategies in the future.
Vaccine formulations in development for non-Hodgkin lymphomas.
| Vaccine formulation . | Starting material . | Production time . | Target tumor antigen(s) . | Phase of clinical development . | Potential applicability . | References . |
|---|---|---|---|---|---|---|
| Idiotype-based | FNA or core biopsy | Tumor-specific Well-defined | B-cell malignancies | |||
| Id protein-KLH | > 2 mo | Phase 3 | 10,15–17,23,30 | |||
| Id protein-DC | > 2 mo | Phase 2 | 22 | |||
| DNA | < 1 mo | Phase 1 | 37,38,50 | |||
| Membrane proteoliposome | Excisional biopsy | < 1 mo | Multiple Undefined | Phase 1 | Multiple malignancies | 39,40 |
| Tumor-cell based | Excisional biopsy | Multiple Undefined | Multiple malignancies | |||
| GM-CSF transduced | < 1 mo | Phase 2 | 41 | |||
| CD40 activated | < 1 mo | Phase 1 | 42 | |||
| HSP-96 | < 1 mo | Phase 1 | 45 |
| Vaccine formulation . | Starting material . | Production time . | Target tumor antigen(s) . | Phase of clinical development . | Potential applicability . | References . |
|---|---|---|---|---|---|---|
| Idiotype-based | FNA or core biopsy | Tumor-specific Well-defined | B-cell malignancies | |||
| Id protein-KLH | > 2 mo | Phase 3 | 10,15–17,23,30 | |||
| Id protein-DC | > 2 mo | Phase 2 | 22 | |||
| DNA | < 1 mo | Phase 1 | 37,38,50 | |||
| Membrane proteoliposome | Excisional biopsy | < 1 mo | Multiple Undefined | Phase 1 | Multiple malignancies | 39,40 |
| Tumor-cell based | Excisional biopsy | Multiple Undefined | Multiple malignancies | |||
| GM-CSF transduced | < 1 mo | Phase 2 | 41 | |||
| CD40 activated | < 1 mo | Phase 1 | 42 | |||
| HSP-96 | < 1 mo | Phase 1 | 45 |
One model for translational development of a home-grown therapeutic agent from academic laboratories. Timeline for the National Cancer Institute (NCI) development of lymphoma idiotype protein vaccines, with prolonged initial development (phase 1/2, initial phase 3) in academia before transfer of technology for potential commercialization. Abbreviations: IND, investigational new drug.
One model for translational development of a home-grown therapeutic agent from academic laboratories. Timeline for the National Cancer Institute (NCI) development of lymphoma idiotype protein vaccines, with prolonged initial development (phase 1/2, initial phase 3) in academia before transfer of technology for potential commercialization. Abbreviations: IND, investigational new drug.
References
Author notes
Assistant Professor, Department of Lymphoma/Myeloma, M.D. Anderson Cancer Center, Houston, TX
Professor and Chairman, Department of Lymphoma/Myeloma, M.D. Anderson Cancer Center, Houston, TX