Abstract
The prognosis for younger adults (≤ 55–60 years) with acute myeloid leukemia (AML) has improved during the last four decades. However, there has been little progress in the treatment of older adults. This disappointing observation is important because the median age of patients with AML is about 70 years. Approximately 60%–80% of younger adults with AML achieve complete remission (CR) with the cytotoxic agents cytarabine and an anthracycline such as daunorubicin or idarubicin or the anthracenedione mitoxantrone. However, only 30%–40% of such patients are alive and disease-free at 5 years. Among older adults, CR is achieved in 40%–55%, but there are very few long-term survivors. Many studies have evaluated the impact of alternative doses and schedules, as well as additional cytotoxic drugs, on the prognosis for this group of patients. The outcome has not improved substantially beyond that achieved with conventional doses of an anthracycline and cytarabine followed by high-dose cytarabine consolidation.
Several factors identified at diagnosis can predict outcome. The most important of these is the karyotype of the leukemic cells. Another critical factor is the presence of transmembrane transporter proteins, which confer multidrug resistance and mutations in or overexpression of specific genes such as WT1, C/EBPα, BAX, and BCL-2/BAX ratio, BAALC, EVI1, KIT and FLT3. The development of specific agents directed at gene mutations, signal transduction pathways and unique cell surface antigens provide the foundation for new therapeutic strategies. Such agents include the immunoconjugate gemtuzumab ozogamicin, multidrug resistance inhibitors, farnesyltransferase inhibitors, histone deacetylase and proteosome inhibitors, antiangiogenesis agents, FLT3 inhibitors, apoptosis inhibitors, and nucleoside analogs. All of these agents can potentially address the heterogeneous abnormalities in AML and significantly improve the outcome for patients.
Introduction
Epidemiology
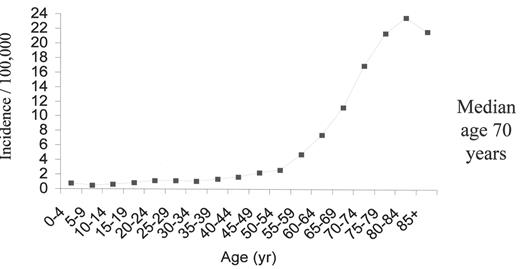
The opportunities to develop new strategies in the therapy of acute myeloid leukemia (AML) are abundant. Insights into the molecular pathogenesis of AML have led to increased recognition the disease’s substantial heterogeneity. An estimated 11,900 new cases of AML occurred in the United States in 2004 and 8900 patients died from the disease.1 The overall incidence is 3.4 cases per 100,000 population; 1.2 cases per 100,000 population at age 30 and > 20 cases per 100,000 population at age 80 years. The median age at diagnosis is approximately 70 years, and the striking increase in incidence with advancing age has important implications for therapy (Figure 1 ). The biology of AML in older adults differs considerably from that of younger adults. In older patients, the disease is more frequently associated with antecedent hematologic disorders such as myelodysplastic syndromes and myeloproliferative disorders, unfavorable karyotypes, and expression of multidrug resistance proteins.
Overview of current therapy
The differences in the biology of subtypes of AML provide both challenges and opportunities for drug development. Cytarabine and anthracyclines such as daunorubicin or idarubicin remain the gold standard for remission induction for the majority of patients of all ages with AML. Younger patients often benefit from multiple courses of intensive consolidation chemotherapy. Those patients with intermediate- or high-risk cytogenetics may be considered for hematopoietic stem cell transplantation (HSCT) to exploit potential graft-versus-leukemia effect. There is no standard post-remission strategy for older adults. Both standard and intermediate doses (1–1.5 g/m2) of cytarabine may be considered, but the benefits of any post-remission chemotherapy are not established.
Foundation for drug development
Recently identified gene mutations and perturbations in signal transduction pathways have led to the development of specific targeted therapies that provide a foundation for new drug development. This approach may better address the heterogeneity of AML and improve outcome beyond that achieved with conventional cytotoxic chemotherapy.
Prognostic Factors
The outcome for adults with AML varies based on a variety of well-defined factors including age of the patient, intensity of post-remission therapy (in younger adults), and biologic characteristics of the disease, the most important of which is the karyotype at diagnosis. The karyotype of the leukemic cell distinguishes three prognostic groups: favorable-, intermediate-, or poor-risk with respect to CR rate and overall survival (OS).2 Other factors that influence outcome include the presence of transmembrane transporter proteins, which confer multidrug resistance,3 and mutations in or overexpression of specific genes such as WT1,4 C/EBPα,5 BAX and BCL-2/BAX ratio,6 BAALC,7 EVI1,8 KIT9 and FLT3.10 Some of these are expressed in patients with normal karyotypes and may prove useful in determining prognosis and in directing treatment for patients in the intermediate-risk cytogenetic category. These proteins and genes provide targets for which new agents have been developed, and these agents are being integrated into new therapeutic strategies.
Evolving Strategies in Induction Therapy
Over the last four decades, a series of studies has established a common induction regimen that is widely accepted. Standard induction therapy includes the cell cycle-specific agent cytarabine 100 mg/m2, administered by continuous infusion for 7 days, combined with the non-cell cycle-specific anthracycline antibiotic daunorubicin 45–60 mg/m2/day, administered intravenously for 3 days. With this regimen, the CR rate for younger adults (≤ 55–60 years) is 60%–80% and the OS rate is approximately 30% (Table 1 ). Among older adults (> 55–60 years), the CR rate is 40%–55%, but there are few (10%–15%) long-term survivors (Table 2 ).
Strategies to improve the CR rate have included alternative and higher doses of anthracyclines or anthracenediones, higher doses of cytarabine,11 additional conventional cytotoxic agents such as etoposide, or novel agents with unique mechanisms of action such as the purine analog fludarabine or the camptothecin topotecan,11 sequential standard therapy followed by high doses of cytarabine12–13 timed sequential therapy, or the addition of growth factors for either hematologic support or priming to recruit leukemia cells into the cell cycle to render them more susceptible to cytotoxic chemotherapy.14 For the majority of patients, none of these approaches is definitively better than a standard 2-drug regimen. However, some subgroups may have an improved outcome with other specific strategies. For example, those with intermediate-risk cytogenetics may benefit from priming with cytokines,14 and those at high- risk (more than 40% blasts in the marrow at day 16 of induction, unfavorable karyotypes and elevated LDH level) may fare better with high-dose cytarabine.
Postremission Strategies
Increasing the intensity of postremission therapy is beneficial in younger adults, but not in older adults (Table 3 ). Patients under age 60 often benefit from intensive consolidation chemotherapy. The extent of benefit is based, in part, on the cytogenetics at diagnosis. For patients who have favorable-risk cytogenetics, data suggest that high-dose cytarabine for three or four courses may provide the highest likelihood of cure. Alternatively, one or two courses of high-dose cytarabine followed by an autologous HSCT are an acceptable strategy; however, the role of autologous HSCT in such patients is not established. A patient’s age, concomitant medical conditions and issues such as fertility may influence this choice. Patients with intermediate-risk cytogenetics also typically receive high-dose cytarabine for three to four courses or are considered for a matched sibling allogeneic HSCT. The presence of internal tandem duplications of the FLT3 gene confers an unfavorable prognosis and may be taken into account in developing a postremission strategy. If a matched sibling HSCT is to be pursued, the importance of pretransplant consolidation is not established. Patients with either poor-risk cytogenetics or an antecedent hematological disease often proceed to either a matched sibling or alternative donor HSCT, since the graft-versus-leukemia effect affords a lower relapse rate than is seen with consolidation chemotherapy (see “Role of allogeneic stem cell transplantation” by Cornelissen and Löwenberg in this volume.)
Integrating Novel Agents in Induction, Consolidation and Maintenance
Many new agents with diverse putative mechanisms of action are currently entering clinical trials (Table 4 ). The development of these agents, coupled with new insights into the molecular pathogenesis of the disease offers hope for significant progress in the treatment of AML.
Gemtuzumab ozogamicin
Gemtuzumab ozogamicin is an immunoconjugate of an anti-CD33 antibody chemically linked to a potent cytotoxic agent, calicheamicin. This agent has been approved by the FDA in the United States for older adults in first relapse who are not suitable candidates for intensive chemotherapy. It has also been licensed in Japan, but has not yet been approved by the regulation agencies in Europe. Complete remission by conventional criteria is achieved in approximately 15% of patients (overall CR 30%) with AML in first relapse.15 Occasional patients have developed a veno-occlusive disease-like syndrome (approximately 1% if given before other therapy). Caution is indicated for those patients proceeding to transplant within 3 to 4 months of exposure when the risk appears to be higher.16 Beyond this period, there appears to be little, if any, risk.
The results with gemtuzumab ozogamicin as a single agent for older adults as initial induction therapy have been modestly encouraging (CR + CRp of 8%–23%).17 However, three recent studies suggest that gemtuzumab ozogamicin may be associated with a high CR rate (approximately 85% in younger adults) when administered with intensive chemotherapy.17–19 Several cooperative groups are conducting phase III studies that incorporate gemtuzumab ozogamicin as part of standard induction chemotherapy in newly diagnosed patients with AML (Figure 2 ). The Southwest Oncology Group (SWOG) is carrying out a randomized study in which gemtuzumab ozogamicin is added to conventional cytarabine and daunorubicin. Patients then undergo a second randomization to receive or not receive gemtuzumab ozogamicin as maintenance. The Medical Research Council (MRC) is also testing the addition of gemtuzumab ozogamicin to a conventional 3-drug induction regimen in a randomized study. Patients in this trial are also randomized to receive or not receive gemtuzumab ozogamicin in consolidation. The European Organization for Research and Treatment of Cancer (EORTC) is currently randomizing older adults to induction chemotherapy with or without gemtuzumab ozogamicin and then randomizing patients again in consolidation with chemotherapy. The Eastern Cooperative Oncology Group (ECOG) is conducting a trial in which patients in CR after 2 cycles of intensive consolidation chemotherapy are randomized to a singe dose (6 mg/m2) of gemtuzumab ozogamicin prior to autologous HSCT. The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) is testing the benefits of gemtuzumab ozogamicin as maintenance. If randomized clinical trials confirm the improved outcomes observed in Phase II trials, this will be a major therapeutic advance, and conventional induction therapy for AML will change for the first time in more than three decades. Furthermore, data suggest that the effects of gemtuzumab ozogamicin may be potentiated by the use of a p-glycoprotein inhibitor, which lays the foundation for the integration of multiple novel agents.
Multidrug resistance inhibitors
P-glycoprotein is a cellular membrane protein encoded by the MDR1 gene, which serves as an efflux pump to extrude chemotherapy from the cell. It is particularly expressed in older adults and in those with relapsed and refractory AML and is associated with multidrug resistance.3 Inhibition or blockade of this efflux pump is an appealing therapeutic strategy. Several agents inhibit P-glycoprotein in vitro, but with the exception of a SWOG trial testing cyclosporine given with cytarabine and infusional daunorubicin in relapsed and refractory patients, randomized trials of MDR modulators such as cyclosporine and PSC-833 in both previously treated and untreated patients with AML have not shown benefit and, in some, have demonstrated excessive toxicity (Table 5 ). More potent second generation modulators such as Zosuquidar (formerly LY335979) are currently being studied.20 This agent does not require reduced doses of concomitant chemotherapy, a potential limitation of other multidrug resistance inhibitors such as PSC-833. The ECOG recently completed a prospective randomized trial of conventional induction with cytarabine and daunorubicin with or without Zosuquidar. Patients in this trial were also randomized to receive or not receive the drug combined with chemotherapy in consolidation.
Farnesyl transferase inhibitors
Mutations of the RAS gene have been associated with the development of myeloid leukemias. Farnesyltransferase inhibitors (FTI) interfere with RAS signaling by precluding farnesylation of RAS and transfer to the plasma membrane. However, in addition to RAS, other farnesylated proteins have been implicated as targets for FTIs including small GTPase proteins Rho B, the centromere proteins CENP-E and CENP-F, and the nuclear membrane structural lamins A and B. Farnesyltransferase inhibitors have activity both in refractory AML21 and in newly diagnosed patients.42 Responses do not correlate with the presence of mutations of RAS. Furthermore, responses do not necessarily correlate with the degree of farnesyltransferase inhibition, implicating other less defined targets of the drugs’ action. Preliminary data report a CR + PR rate of 34% and a CR rate of 18% with the oral agent Tipifarnib (Zarnestra) in 148 newly diagnosed older (median age 73 years) patients.22 These results have led to the activation of a randomized phase II US Intergroup trial (S0432) which evaluates Tipifarnib in older adults who are not felt to be suitable candidates for intensive conventional chemotherapy. A recent update (at FDA Oncology Drug Advisory Committee May, 2005) of the preliminary study suggested a lower CR rate than initially reported and therefore the results of the S0432 trial will be particularly important. Another US Intergroup trial is testing the benefits of Tipifarnib in a randomized trial of patients with AML in second or higher CR who are not candidates for HSCT, where the drug is used as maintenance after consolidation therapy (E2902). Both S0432 and E2902 address major unmet needs in the treatment of AML.
Histone deacetylase and proteosome inhibitors
Aberrant recruitment of nuclear co-repressor complexes by leukemogenic fusion proteins is a recurring theme in AML. Chromatin remodeling and consequent silencing of transcription involves posttranslational modification of his-tones through acetylation accomplished by histone acetyltransferase. Histone deacetylases (HDACs) promote the opposite effect and restore chromatin conformation. HDAC inhibitors induce differentiation of malignant cells. Several agents including suberoylanilide hydroxamic acid (SAHA), valproic acid (2-propylpentanoic acid), depsi-peptide and MS-275 are examples of such novel agents under evaluation alone and in combination with a variety of other agents. A US Intergroup study testing the benefits of MS-275 and low-dose 5-azacytidine in a randomized phase II trial for patients with MDS is under development.
The proteosome inhibitor bortezomib appears to have single-agent activity in leukemia and has in vitro synergistic activity with HDAC inhibitors.23 A phase I dose-escalating trial of bortezomib given on days 1, 4, 8, and 11 combined with conventional doses of idarubicin and cytarabine is underway.24
Antiangiogenesis agents
Bone marrow biopsies from patients with AML have increased angiogenesis compared to normal bone marrows, as demonstrated by an increased microvessel density. In addition, vascular endothelial growth factor (VEGF) stimulates growth and proliferation of leukemic cells and an increased endogenous level of VEGF is also associated with a poor prognosis. The use of receptor tyrosine kinase inhibitors of VEGF is another strategy under active study.25 Preliminary data suggest that SU5416, a small molecule inhibitor of phosphorylation of VEGF receptors 1 and 2, C-KIT, the SCF receptor and FLT3, has activity in AML.26 Bevacizumab, an anti-VEGF antibody, has been given after chemotherapy safely in patients with AML.27 In a phase II study of Bevacizumab given on day 8 following high-dose cytarabine (2 g/m2) and mitoxantrone in patients with relapsed and refractory AML, the CR rate was 33% (overall response 48%) with an induction mortality rate of 15%.
FLT3 and other tyrosine kinase inhibitors
Mutations that confer constitutive activation to the FLT3 receptor tyrosine kinase occur in ~30% of AML and confer a poor prognosis. These observations have prompted the development of FLT3-selective targeted tyrosine kinase inhibitors with in vitro cytotoxicity to leukemia cells. Four FLT3 inhibitors are currently undergoing investigation in clinical trials: PKC-412 (Novartis), CEP-701 (Cephalon), MLN518 (Millennium) and SU11248 (SuGen). They appear to be well tolerated at doses that successfully inhibit the target FLT3. These inhibitors have activity in relapsed AML patients with activating mutations. However, clinical responses have been modest. In general, only transient reduction in peripheral blood and bone marrow blasts have been observed. In one recent trial, a 50% reduction in peripheral blasts was observed in 40 of 57 (70%) patients and a 50% reduction was seen in marrow blasts in 6 patients.28 Unexpectedly, some patients without ITD or activation loop mutations have responded to FLT3 inhibitors, although mutations in other sites that activate FLT3 have been identified, which may account for this observation. Current clinical trials focus on the evaluation of FLT3 inhibitors in combination with chemotherapy. A preliminary study suggests that another tyrosine kinase inhibitor, Imatinib, may have activity in patients with C-KIT positive AML (CR in 2 of 21 patients refractory to chemotherapy, minor response in 2 patients and 1 without evidence of leukemia).29
Apoptosis inhibitors
Overexpression of the apoptosis inhibitor protein BCL-2 can render tumor cells resistant to induction of apoptosis. A high level of expression of BCL-2 in AML is associated with a poor prognosis. Downregulation of BCL-2 by antisense oligonucleotides in vitro sensitizes leukemic cells to chemotherapy in AML cell lines. A phase I trial of BCL-2 antisense oligonucleotide (Genasense [GNS], oblimersen sodium) showed a response in 8 of 20 patients with relapsed or refractory AML.30 In a subsequent phase I trial to determine feasibility of treatment in previously untreated older adults, the BCL-2 antisense oligonucleotide was administered with chemotherapy.31 Ten of 26 patients (45%) achieved CR without unexpected toxicities. A randomized phase III study conducted by the CALGB is currently evaluating the role of the BCL-2 antisense oligonucleotide both in induction and in consolidation.
Deoxynucleoside analogs
Clofarabine (2-chloro-2′fluoro-deoxy-9-β-D-arabino-furanosyladenine) was synthesized specifically to exploit the advantageous features of several other active nucleoside analogs, specifically fludarabine and cladribine. This agent is highly resistant to cleavage by bacterial purine nucleoside phosphorylase and is resistant to deamination by adenosine deaminase. Furthermore, this agent is not associated with the neurotoxicity observed with other analogs. In a phase II trial in relapsed and refractory patients with AML, and other hematologic malignancies, an overall response rate of 48% was achieved, including a CR rate of 32%.32 In a subsequent phase I–II trial in relapsed and refractory leukemias, predominantly AML, clofarabine was combined with cytarabine in an effort to modulate cytarabine triphosphase accumulation.33 The overall response rate was 38% with a CR rate of 22%. Two trials have explored this agent in previously untreated patients with AML and have demonstrated promising results.34–35 Burnett and colleagues treated 24 previously untreated older adults with AML who were not felt to be suitable for intensive chemotherapy with single agent clofarabine. A CR rate of 60% was observed. Although the majority of patients had intermediate-risk cytogenetics and therefore may have a more favorable response than other older adults, these results are encouraging. Troxacitabine is a β-L enantiomer nucleoside analog that is resistant to degradation by deoxycytidine deaminase. In a preliminary study this agent had a 26% response rate in patients with relapsed or refractory AML.36
Acute Promyelocytic Leukemia as a Paradigm for Integrating Novel Therapies
The changing approaches to the treatment of acute promyelocytic leukemia (APL) represent the best example of the rapid integration of novel therapies into leukemia treatment strategies. As a result, this subtype of AML is now highly curable. Strategies that combine all-trans retinoic acid (ATRA) with anthracycline-based chemotherapy for induction and consolidation followed by maintenance with ATRA, low-dose chemotherapy (methotrexate and 6-mercaptopurine) or, perhaps most effective, ATRA and chemotherapy in combination have increased the cure rate to 70%–85%, depending on prognostic factors. Several current controversies exist. First, the role of cytarabine in induction and consolidation is not clear. Second, two recent trials have suggested that patients who become molecularly negative for the PML-RARα fusion transcript after consolidation may not benefit from maintenance therapy.37–38 Patients can be classified as low-, intermediate- or high-risk for relapse based on the initial white blood cell count (> or < 10,000/μL) and platelet count (> or < 40,000/μL). The outcomes for the low- and intermediate-risk groups are excellent with current therapy. Therefore, new therapeutic strategies are focused on minimizing toxicities. The outcome for the high-risk group is less satisfactory. Given the remarkable activity of arsenic trioxide in patients with relapsed and refractory APL and its apparent synergy with ATRA, there has been interest in administering this agent earlier in the natural history of the disease such as in induction. 39 The current North American Intergroup trial (C9710), recently closed to accrual, tests the benefit of arsenic as an early consolidation in a randomized fashion and compares two maintenance regimens. A third controversy is whether arsenic trioxide combined with ATRA in induction and consolidation will permit significant reduction or even elimination of chemotherapy. Gemtuzumab ozogamicin is another very effective agent in APL and may be incorporated into future therapy. 40
Summary and Future Directions
A myriad of new agents are now available which, when administered either alone (ATRA or arsenic trioxide in APL) or in combinations with each other or with conventional cytotoxic chemotherapy, have the potential to change the standard of care for patients with AML. Combinations of several agents, targeting more than one gene mutation, signal transduction pathway or antigenic determinant may be the most effective. Many of these agents are currently under investigation in cooperative group trials (Table 6 ). As the molecular diversity of AML continues to be explored, it will become increasingly important to foster collaboration among institutions and cooperative groups to rapidly accrue patients with uncommon subtypes to clinical trials. The challenge will be to determine which strategy, agent or combination of agents will most effectively perturb the specific pathway responsible for the propagation of the leukemia cells from a specific clone.
Acute myeloid leukemia age-specific incidence rates: 1998–2002 (NCI-SEER Program).
Acute myeloid leukemia age-specific incidence rates: 1998–2002 (NCI-SEER Program).
Current cooperative group phase III studies of gemtuzumab ozogamicin (GO) in acute myeloid leukemia (AML).
Current cooperative group phase III studies of gemtuzumab ozogamicin (GO) in acute myeloid leukemia (AML).