Key Points
Local and systemic test methods highlight the heterogeneity and the systemic nature of human endothelial dysfunction before cell therapy.
Our findings reinforce the utility of EASIX as a clinically practical, endothelial-related biomarker.
Visual Abstract
Endothelial dysfunction contributes to mortality after cellular therapies, yet its clinical assessment remains challenging. In this prospective observational study, we evaluated 169 patients undergoing allogeneic stem cell transplantation or chimeric antigen receptor T-cell therapy and 102 healthy controls to determine whether a comprehensive endothelial profile, including the endothelial activation and stress index (EASIX), glycocalyx thickness (via sublingual GlycoCheck microscopy), digital perfusion (Tivita hyperspectral imaging), endothelial serum markers (angiopoietin-2, soluble thrombomodulin [CD141], chemokine (C-X-C motif) ligand 8, chemokine (C-X-C motif) ligand 9, interleukin-18, enzyme-linked immunosorbent assays), and platelet aggregation (flow cytometry), correlates with clinical outcomes. We observed significant intercorrelations among EASIX, perfused boundary region (PBR), tissue perfusion, and endothelial serum markers. Importantly, elevated EASIX, angiopoietin-2, impaired PBR, and reduced digital perfusion were significantly associated with early sepsis, whereas EASIX also independently predicted nonrelapse mortality. These findings highlight the heterogeneity of endothelial responses to systemic insult and reinforce the need for multimodal assessment in a larger study. EASIX, as a simple and routinely available marker, emerges as a valuable tool to stratify endothelial risk and guide monitoring in patients undergoing cellular therapy. This trial was registered at www.ClinicalTrials.gov as #NCT05502887.
Introduction
The vascular endothelium plays a central role in regulating vascular tone, coagulation, vessel wall permeability, cellular adhesion and migration, as well as a wide range of inflammatory processes.1,2 Uninterrupted capillary flow is essential for delivering oxygen and nutrients to all organs and for removing potentially harmful substances such as carbon dioxide, amyloid-β, and drug metabolites.3 Acute disruptions of microcirculation, such as in capillary leak syndrome during sepsis, can lead to circulatory shock and organ failure. However, chronic microcirculatory impairment is observed in several conditions. For example, diabetes mellitus leads to tissue hypoxia without acute ischemia,4 whereas Alzheimer disease is associated with capillary dysfunction and restricted flow, contributing to cerebral hypoperfusion and progressive neurodegeneration.5 Similarly, patients with cardiovascular diseases or acute ischemic stroke exhibit systemic endothelial dysfunction extending beyond the primary target organs, such as the heart and brain.6,7 In coronary heart disease, systemic endothelial dysfunction measured 6 months after coronary intervention has been shown to strongly predict long-term survival.8
Despite its clinical significance, the quantification of systemic endothelial dysfunction in humans remains a challenge. Existing diagnostic methods are often invasive, operator-dependent, or resource-intensive,9 and no routinely available tool currently exists. Compounding this challenge is the intrinsic phenotypic and functional heterogeneity of endothelial cells (ECs), which likely explains why a universal prognostic marker has yet to be identified among endothelial-derived factors.3,10
Endothelial damage is a known adverse effect of many antineoplastic and immunotherapies, with the potential to injure ECs or worsen preexisting dysfunction.9 In particular, allogeneic hematopoietic stem cell transplantation (SCT) and chimeric antigen receptor T-cell (CAR-T) therapy are frequently complicated by severe endothelial syndromes, the risk of which can be predicted using biomarkers assessed before conditioning.9
To address this, we developed the endothelial activation and stress index (EASIX), based on 3 widely available laboratory parameters that reflect features of serious endothelial complications such as transplant-associated thrombotic microangiopathy.10,11 The EASIX formula is: EASIX = (LDH × creatinine) ÷ platelet count.5 Here, lactate dehydrogenase (LDH) reflects endothelial activation and cellular damage12; creatinine indicates kidney function often compromised by endothelial injury13; and platelet count captures the state of endothelial activation, vascular damage, and microthrombus formation.14
EASIX has proven predictive across a range of clinical scenarios, including allogeneic hematopoietic SCT, CAR-T therapy, and COVID-19,11,15,16 and was recently identified as a novel predictor of mortality risk in coronary heart disease.8 A modified version of EASIX (mEASIX), which substitutes C-reactive protein (CRP) for creatinine, has demonstrated similar or superior predictive value for endothelial complications after CAR-T therapy.17
We hypothesized that the morphological correlate of EASIX may be a reduced endothelial glycocalyx thickness and related microvascular changes. The Endothelial Cell Dysfunction and Outcome, Hematology (EndoCDO-H) study was designed to prospectively test this hypothesis. Using sublingual video microscopy (GlycoCheck) and hyperspectral imaging (HSI) (Tivita) of the fingers and palms, we assessed microcirculatory parameters in both healthy individuals (HIs) and patients undergoing cellular therapies. In parallel, we measured serum markers of endothelial dysfunction, including angiopoietin-2 (ANG2),18-20 chemokine (C-X-C motif) ligand 8 (CXCL8),21 interleukin-18 (IL-18),22,23 and soluble thrombomodulin (sCD141),24 as well as quantified platelet aggregates using flow cytometry.14,25 We examined intercorrelations among these diverse assessments and evaluated their associations with the risk of endothelial complications and mortality after cellular therapy (“Graphical abstract”).
Materials and methods
HIs and patient characteristics
All HIs, and all patients scheduled to undergo cellular therapy, were eligible for inclusion in the prospective observational EndoCDO-H study (ClinicalTrials.gov identifier: NCT05502887), provided they gave informed consent. Study end points were intercorrelations between the different endothelial markers in HIs and patients, and association of endothelial markers with nonrelapse mortality (NRM) and endothelial complications (eg, sepsis) after cellular therapy. Patients were consecutively enrolled between May 2022 and January 2024 if they were scheduled to receive allogeneic SCT (allo-SCT) or CAR-T therapy. For patients undergoing cellular therapy, all analyses were performed within 14 days before starting conditioning therapy. Living participants were followed-up for a median duration of 540 days (range, 313-912). Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. The study protocol, including sample and data collection procedures, was approved by the local ethics committee (ethics vote S-273/2022). Detailed patient characteristics are presented in Table 1.
Patient characteristics
| . | HIs N = 102 . | Patients all N = 169 . | AML/MDS/ALL N = 92 . | Lymphoma/myeloma N = 56 . | Other N = 21 . |
|---|---|---|---|---|---|
| Age, y (range) | 30 (17-70) | 61 (24-84) | 59 (19-73) | 62 (25-77) | 65 (37-72) |
| Sex, n (%) | |||||
| Female | 69 (68) | 62 (37) | 37 (40) | 14 (25) | 11 (56) |
| Male | 33 (32) | 107 (63) | 55 (60) | 42 (75) | 10 (44) |
| Disease status | |||||
| Complete remission | — | 93 (55) | 79 (86) | 12 (21) | 2 (10) |
| MRD positive | 20 (22) | 2 (100) | |||
| MRD negative | 49 (53) | 0 | |||
| Partial remission | — | 20 (12) | 1 (1) | 15 (27) | 4 (19) |
| Stable disease | — | 11 (7) | 0 | 10 (18) | 1 (5) |
| Progressive disease/refractory | — | 28 (17) | 6 (7) | 18 (32) | 4 (19) |
| Untreated | — | 16 (9) | 6 (7) | 0 | 10 (48) |
| LDH (U/L), median (range) | 235 (153-309) | 241 (80-1103) | 230 (80-839) | 254 (163-624) | 282 (159-1103) |
| Creatinine (mg/dL), median (range) | 0.75 (0.11-1.35) | 0.85 (0.5-1.4) | 0.81 (0.63-1.78) | 0.90 (0.6-1.4) | 0.9 (0.5-1.3) |
| Platelet count (×109/L), median (range) | 244 (122-326) | 162 (4-774) | 162 (11-475) | 167 (9-345) | 103 (9-774) |
| EASIX (LDH × creatinine/platelets) | 0.68 (0.1-1.5) | 1.4 (0.3-31) | 1.3 (0.3-31) | 1.5 (0.4-30) | 3.4 (0.4-31) |
| PBR 4-25 μm (μm) | 2.6 (1.8-2.9) | 2.6 (1.8-3.1) | 2.9 (2.1-3.8) | 2.7 (1.9-3.3) | 3.0 (2.4-3.4) |
| NIR (U) | 61 (50-74) | 66 (48-83) | 61 (35-76) | 61 (49-75) | 59 (53-66) |
| . | HIs N = 102 . | Patients all N = 169 . | AML/MDS/ALL N = 92 . | Lymphoma/myeloma N = 56 . | Other N = 21 . |
|---|---|---|---|---|---|
| Age, y (range) | 30 (17-70) | 61 (24-84) | 59 (19-73) | 62 (25-77) | 65 (37-72) |
| Sex, n (%) | |||||
| Female | 69 (68) | 62 (37) | 37 (40) | 14 (25) | 11 (56) |
| Male | 33 (32) | 107 (63) | 55 (60) | 42 (75) | 10 (44) |
| Disease status | |||||
| Complete remission | — | 93 (55) | 79 (86) | 12 (21) | 2 (10) |
| MRD positive | 20 (22) | 2 (100) | |||
| MRD negative | 49 (53) | 0 | |||
| Partial remission | — | 20 (12) | 1 (1) | 15 (27) | 4 (19) |
| Stable disease | — | 11 (7) | 0 | 10 (18) | 1 (5) |
| Progressive disease/refractory | — | 28 (17) | 6 (7) | 18 (32) | 4 (19) |
| Untreated | — | 16 (9) | 6 (7) | 0 | 10 (48) |
| LDH (U/L), median (range) | 235 (153-309) | 241 (80-1103) | 230 (80-839) | 254 (163-624) | 282 (159-1103) |
| Creatinine (mg/dL), median (range) | 0.75 (0.11-1.35) | 0.85 (0.5-1.4) | 0.81 (0.63-1.78) | 0.90 (0.6-1.4) | 0.9 (0.5-1.3) |
| Platelet count (×109/L), median (range) | 244 (122-326) | 162 (4-774) | 162 (11-475) | 167 (9-345) | 103 (9-774) |
| EASIX (LDH × creatinine/platelets) | 0.68 (0.1-1.5) | 1.4 (0.3-31) | 1.3 (0.3-31) | 1.5 (0.4-30) | 3.4 (0.4-31) |
| PBR 4-25 μm (μm) | 2.6 (1.8-2.9) | 2.6 (1.8-3.1) | 2.9 (2.1-3.8) | 2.7 (1.9-3.3) | 3.0 (2.4-3.4) |
| NIR (U) | 61 (50-74) | 66 (48-83) | 61 (35-76) | 61 (49-75) | 59 (53-66) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
GlycoCheck measurements and HSI of digits and palms
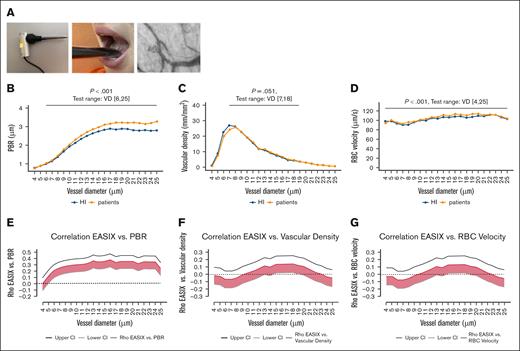
Sublingual video microscopy using the GlycoCheck system analyzes capillaries ranging from 4 to 25 μm in diameter. It provides quantitative measurements of vascular density, red blood cell (RBC) velocity, and, most notably, the perfused boundary region (PBR), a surrogate marker for glycocalyx thickness. The PBR reflects the deviation of RBCs from the ideal axial flow path, which is normally maintained by the repulsive forces of an intact negatively charged glycocalyx present on both erythrocytes and ECs.26,27 A higher PBR value indicates reduced glycocalyx thickness, suggesting endothelial dysfunction (Figure 1A). For each participant, 24 (minimum of 10) repetitive video sequences, each lasting 3 to 8 minutes, were recorded and analyzed to calculate these parameters. GlycoCheck measurements were successfully performed in 169 patients and 102 HIs.
HSI is a spectral imaging technique that captures data across a broad range of wavelengths, enabling detailed analysis of tissue composition and function beyond conventional “red, green, and blue” imaging. Unlike standard color images, which are limited to 3 color channels, HSI records spectral information for each pixel across dozens to hundreds of wavelengths, allowing precise characterization of structural and biochemical tissue properties. Tivita collects tissue reflectance images in the visible and near-infrared light and thereof calculates 4 parameter images: the near-infrared perfusion (NIR) assesses deeper (up to ∼5 mm) tissue perfusion, the tissue water index (TWI) represents vascular permeability, the tissue hemoglobin index associates with superficial tissue hyperemia, and tissue oxygen saturation assesses superficial tissue oxygenation.28 In this study, Tivita HSI was conducted in parallel with GlycoCheck on the left fourth fingertip pulp and the left palm. The average values from both regions were used for analysis (Figure 2A). HSI was performed in 72 patients and 28 healthy volunteers alongside sublingual microscopy.
Approximation of platelet aggregates (proxy measurement)
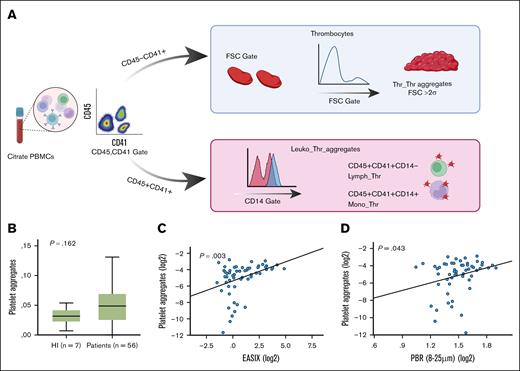
Platelet aggregates have been associated with poor prognosis in patients with COVID-19, serving as a marker of endothelial dysfunction and platelet activation.14,25 Given the established link between EASIX and clinical outcomes in COVID-19,29 we adopted the method developed by Nishikawa et al14 to quantify platelet aggregates in patients before cellular therapy and assess correlations with both EASIX values and local endothelial function parameters (Figure 3A).
Peripheral blood samples were collected from 63 individuals on the same day as EASIX assessment. Platelet aggregates were quantified by flow cytometry using diluted, citrate-treated peripheral blood mononuclear cells.16 Samples were stained for CD45, CD14, and CD41, then fixed in paraformaldehyde. Data analysis was performed using Kaluza analysis software.
Platelet aggregates, referred to as the “platelet aggregates proxy,” were identified as events negative for CD45 and CD14 but positive for CD41, and larger than the mean platelet size plus 2 standard deviations, as determined by forward scatter characteristics. In addition, leukocyte-platelet aggregates and monocyte-platelet aggregates were identified by coexpression of CD41 with CD45 and CD14, respectively (Figure 3A).
Serum markers
Routine laboratory measurements of LDH, creatinine, platelet counts, and CRP were performed on the day of GlycoCheck analysis by a certified clinical laboratory. The EASIX was calculated as follows: EASIX = (LDH [U/L] × creatinine [mg/dL]) ÷ platelet count [per nL].10 In addition, mEASIX was calculated by substituting CRP for creatinine: LDH (U/L) × CRP (mg/dL) ÷ platelets (per nL).17
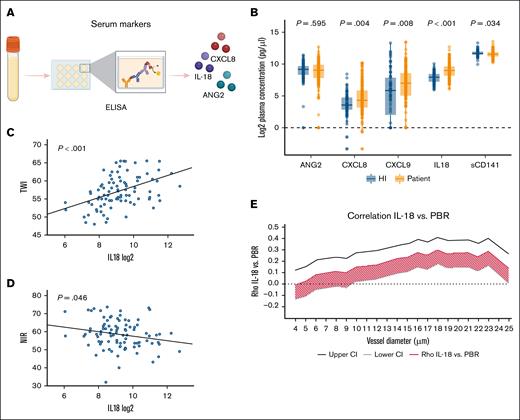
Blood samples for cytokine analysis were collected and frozen on the same day as GlycoCheck measurement. Serum levels of the following markers were quantified using R&D DuoSet enzyme-linked immunosorbent assay kits (Figure 4A): IL-18, n = 239; chemokine (C-X-C motif) ligand 9 (CXCL9) (monokine induced by gamma interferon, n = 242; CXCL8 [IL-8], n = 242; ANG2, n = 238; and sCD141, n = 242).
Clinical outcome parameters
All patients were monitored for a minimum of 50 days after cellular therapy. Sepsis was evaluated within the period from day 0 to day 50 after allo-SCT or T-cell infusion. The classification of sepsis was based on the modified sepsis-3 criteria, as defined by the Infectious Diseases Working Party and the Intensive Care Working Group of the German Society for Hematology and Medical Oncology, specifically adapted for patients with neutropenic cancer, as previously described.30 Survival and NRM were assessed with a median follow-up of 540 days (range, 313-912) among surviving patients.
Statistical analyses
Spearman ρ correlation coefficients and their 95% confidence intervals (CIs) were calculated for continuous variables. Wilcoxon rank test for dichotomous variables and Kruskal-Wallis test otherwise were applied for categorical vs continuous variables. For some tests, HIs and patients were age-matched by defining subgroups of younger/older, with ages less/greater than the median age of the entire cohort (HIs and patients). EASIX was log-transformed for multivariable regression analyses, whereas NIR, TWI, PBR, vascular density, and RBC velocity were parametric variables. Multivariable logistic regression analyses (complete case analyses) were calculated to test whether the association of PBR and EASIX was stable in the context of the covariables vascular density and RBC velocity, and the forced-in variables age, sex, and body mass index (BMI). For outcome analysis, all patients were evaluable for early sepsis through day +50 after cell therapy (n = 8 events). We performed receiver operating characteristic (ROC) curve analysis based on a logistic regression model and calculated the C-statistic, corresponding to the area under the curve (AUC), with a bootstrap-derived 95% CI. The model included CAR-T therapy vs allo-SCT as a forced-in variable. A Fine-Gray competing risks model was used to analyze NRM, treating relapse as a competing event. CAR-T therapy vs allo-SCT was included as a covariate in the model.
Spearman rank correlation coefficients (Spearman ρ) and their corresponding 95% CIs were calculated for continuous variables. For comparisons between categorical and continuous variables, the Wilcoxon rank-sum test was used for dichotomous variables, and the Kruskal-Wallis test was applied for variables with >2 categories.
To address age-related differences, HIs and patients were age-matched by stratifying the cohort into younger and older subgroups, defined as being below or above the median age of the entire cohort (HIs and patients combined).
EASIX values were log-transformed for multivariable regression analyses. Other continuous variables, including NIR, TWI, PBR, vascular density, and RBC velocity, were treated as parametric.
Multivariable logistic regression analyses (complete-case analysis) were performed to evaluate whether the association between PBR and EASIX remained significant when adjusting for vascular density and RBC velocity, as well as the forced-in covariates: age, sex, and BMI.
For clinical outcome analysis, all patients were evaluable for early sepsis through day +50 after cellular therapy (n = 8 events). ROC curve analysis was conducted based on a logistic regression model. The C-statistic (AUC) and its bootstrap-derived 95% CI were calculated. The model included therapy type (CAR-T vs allo-SCT) as a forced-in variable.
To analyze NRM, a Fine-Gray competing risks model was used, treating relapse as a competing event. Therapy type (CAR-T vs allo-SCT) was again included as a covariate in the model.
Results
Sublingual microscopy (GlycoCheck) reveals a significant association between glycocalyx integrity and EASIX
The GlycoCheck sublingual microscopy system, including laser-based imaging, measurement procedures, and video acquisition, is illustrated in Figure 1A and further detailed in the supplemental Video. The system computes 3 key parameters: PBR, vascular density, and RBC velocity, for each vessel diameter between 4 and 25 μm, separately (Figure 1B-D).
GlycoCheck parameters in HIs and patients, and correlation with EASIX. (A) Schematic representation of the GlycoCheck sublingual microscope, including the measurement setup, sublingual positioning, and an example image showing RBC flow through capillaries (see also supplemental Video). (B-D) Comparison of HIs and patients with hematological disease (preconditioning) across key GlycoCheck endothelial parameters stratified by VD between 4 and 25 μm. Median values were calculated for each individual and plotted by vessel size; (B) PBR (μm); (C) vascular density (mm/mm2); (D) RBC velocity (μm/s). P values from Wilcoxon rank-sum tests comparing HIs and patients are indicated for each diameter range. (E-F) Spearman ρ correlation coefficients (with 95% CIs) for EASIX and GlycoCheck parameters, calculated by individual VD. (E) Correlation between EASIX and PBR; (F) correlation between EASIX and vascular density; (G) correlation between EASIX and RBC velocity. VD, vessel diameter.
GlycoCheck parameters in HIs and patients, and correlation with EASIX. (A) Schematic representation of the GlycoCheck sublingual microscope, including the measurement setup, sublingual positioning, and an example image showing RBC flow through capillaries (see also supplemental Video). (B-D) Comparison of HIs and patients with hematological disease (preconditioning) across key GlycoCheck endothelial parameters stratified by VD between 4 and 25 μm. Median values were calculated for each individual and plotted by vessel size; (B) PBR (μm); (C) vascular density (mm/mm2); (D) RBC velocity (μm/s). P values from Wilcoxon rank-sum tests comparing HIs and patients are indicated for each diameter range. (E-F) Spearman ρ correlation coefficients (with 95% CIs) for EASIX and GlycoCheck parameters, calculated by individual VD. (E) Correlation between EASIX and PBR; (F) correlation between EASIX and vascular density; (G) correlation between EASIX and RBC velocity. VD, vessel diameter.
We first compared these endothelial parameters between HIs and patients with hematological disease. Across all vessel diameters, PBR values were significantly higher in patients, indicating a reduction in glycocalyx integrity (Figure 1B), and this difference was independent of age (supplemental Figure 1A-C) and sex (supplemental Figure 2A-C). Vascular density did not differ significantly between HIs and patients in the full cohort without age matching (Figure 1C); however, a significant difference emerged in the older subgroup (supplemental Figure 1C). For RBC velocity, a small significant difference was observed between HIs and patients across the full range of vessel diameters (Figure 1D) irrespective of sex (supplemental Figure 2), although this effect was driven by differences in the younger age subgroup (supplemental Figure 1B).
The correlation between GlycoCheck parameters and EASIX is presented in Figure 1E-G, analyzed by individual vessel diameter. EASIX positively correlated with PBR across a wide range of diameters, suggesting that higher EASIX reflects reduced glycocalyx integrity. Additionally, in vessels measuring 14 to 18 μm, EASIX also showed a positive correlation with vascular density and RBC velocity (Figure 1E–G).
HSI (Tivita) of digits and palms crossvalidates sublingual microscopy parameters and EASIX
The Tivita HSI system, as well as the measurement procedures for the left fourth fingertip and left palm, are illustrated in Figure 2A. We observed a positive correlation between NIR and tissue oxygen saturation, reflecting deep and superficial tissue perfusion (Spearman ρ = 0.40; 95% CI, 0.22-0.55; P < .001). In contrast, NIR was negatively correlated with TWI, a marker of vascular permeability (Spearman ρ = −0.28; 95% CI, −0.45 to −0.10; P < .001). Among all hyperspectral parameters, NIR and TWI showed the strongest associations with endothelial markers and were therefore selected for further analysis.
Tivita parameters in HIs and patients, and correlation with GlycoCheck parameters and EASIX. (A) Overview of the Tivita HSI system, including measurement positions (left palm and left fourth fingertip) and representative spectral images showing: (top) Tissue oxygen saturation; (middle) NIR; (bottom) TWI. (B) Box plots comparing age-matched HIs and patients for Tivita parameters (NIR and TWI, n = 100). Wilcoxon rank-sum tests were used to assess significance (P values shown). No significant differences were found between younger and older HI subgroups, whereas patients significantly differed from older HI subgroups (NIR, TWI) and younger HI subgroups (TWI). (C) Correlation analyses between GlycoCheck parameters and Tivita measures. (Left) Correlation between vascular density and NIR; (right) correlation between PBR and TWI. Spearman ρ correlation coefficients (red line) with 95% CIs (gray and black lines) are shown by VD (4-25 μm). A correlation was considered significant if the entire CI lay on 1 side of the y-axis. (D) Linear regression analysis between EASIX and Tivita parameters. (Left) Negative correlation between EASIX and NIR (reduced perfusion); (right) positive correlation between EASIX and TWI (increased vascular permeability).
Tivita parameters in HIs and patients, and correlation with GlycoCheck parameters and EASIX. (A) Overview of the Tivita HSI system, including measurement positions (left palm and left fourth fingertip) and representative spectral images showing: (top) Tissue oxygen saturation; (middle) NIR; (bottom) TWI. (B) Box plots comparing age-matched HIs and patients for Tivita parameters (NIR and TWI, n = 100). Wilcoxon rank-sum tests were used to assess significance (P values shown). No significant differences were found between younger and older HI subgroups, whereas patients significantly differed from older HI subgroups (NIR, TWI) and younger HI subgroups (TWI). (C) Correlation analyses between GlycoCheck parameters and Tivita measures. (Left) Correlation between vascular density and NIR; (right) correlation between PBR and TWI. Spearman ρ correlation coefficients (red line) with 95% CIs (gray and black lines) are shown by VD (4-25 μm). A correlation was considered significant if the entire CI lay on 1 side of the y-axis. (D) Linear regression analysis between EASIX and Tivita parameters. (Left) Negative correlation between EASIX and NIR (reduced perfusion); (right) positive correlation between EASIX and TWI (increased vascular permeability).
Compared with HIs, patients exhibited higher TWI and lower NIR values. The difference in NIR was statistically significant only in the older age subgroup, whereas TWI was significantly elevated in patients across both age subgroups (Figure 2B).
Higher digital perfusion (NIR) was positively correlated with sublingual vascular density (Figure 2C, left panel), whereas increased TWI was negatively correlated with glycocalyx integrity, as reflected by higher PBR values (Figure 2C, right panel). Furthermore, EASIX showed a negative correlation with NIR (ie, reduced perfusion) and a positive correlation with TWI (ie, increased vascular leakage) in fingers and palms (Figure 2D).
Increased platelet aggregates (proxy) in patients with high EASIX values and elevated PBR
Platelet-platelet aggregates, as well as leukocyte-platelet and monocyte-platelet aggregates, were analyzed via flow cytometry, as illustrated in Figure 3A. No significant associations were found between leukocyte-platelet or monocyte-platelet aggregates and any of the endothelial markers assessed in this study.
Thrombocyte-thrombocyte aggregates (proxy) in HIs and patients, and correlation with EASIX and PBR. (A) Gating strategy for identifying thrombocyte-thrombocyte aggregates (Thr_Thr, proxy) and leukocyte-thrombocyte aggregates (Leuko_Thr) by flow cytometry. Thr_Thr aggregates (proxy), identified as CD45−CD41+ platelets exceeding 2 standard deviations (>2σ) above the mean FSC, indicating larger platelet aggregates. Leuko_Thr aggregates, defined as CD45+CD41+ double-positive cells, further classified by CD14 expression to differentiate monocyte-platelet subtypes. (B) Box plots comparing Thr_Thr aggregate levels (proxy) between HIs and patients, showing a trend toward higher levels in patients (P = value from Kruskal-Wallis test). (C-D) Linear regression analyses showing correlations between (C) platelet aggregates (proxy) and EASIX (C) and platelet aggregates (proxy) and PBR (mean values across VDs 8-25 μm) (D). These findings suggest a link between platelet activation, systemic endothelial stress, and glycocalyx degradation. FSC, forward scatter; PBMCs, peripheral blood mononuclear cells.
Thrombocyte-thrombocyte aggregates (proxy) in HIs and patients, and correlation with EASIX and PBR. (A) Gating strategy for identifying thrombocyte-thrombocyte aggregates (Thr_Thr, proxy) and leukocyte-thrombocyte aggregates (Leuko_Thr) by flow cytometry. Thr_Thr aggregates (proxy), identified as CD45−CD41+ platelets exceeding 2 standard deviations (>2σ) above the mean FSC, indicating larger platelet aggregates. Leuko_Thr aggregates, defined as CD45+CD41+ double-positive cells, further classified by CD14 expression to differentiate monocyte-platelet subtypes. (B) Box plots comparing Thr_Thr aggregate levels (proxy) between HIs and patients, showing a trend toward higher levels in patients (P = value from Kruskal-Wallis test). (C-D) Linear regression analyses showing correlations between (C) platelet aggregates (proxy) and EASIX (C) and platelet aggregates (proxy) and PBR (mean values across VDs 8-25 μm) (D). These findings suggest a link between platelet activation, systemic endothelial stress, and glycocalyx degradation. FSC, forward scatter; PBMCs, peripheral blood mononuclear cells.
In contrast, platelet-platelet aggregates (proxy) were increased in patients compared with HIs, showing a trend toward significance (Figure 3B). Moreover, platelet-platelet aggregates showed a significant positive correlation with both EASIX (Spearman ρ = 0.38; 95% CI, 0.14-0.57; Figure 3C) and PBR (Spearman ρ = 0.26; 95% CI, 0.10-0.49; Figure 3D), suggesting that elevated platelet aggregation is associated with endothelial activation and glycocalyx degradation.
Serum endothelial markers: only IL-18 associates with EASIX, GlycoCheck, and Tivita parameters
Serum endothelial markers were measured on the same day as the GlycoCheck analysis, before the initiation of conditioning therapy. Compared with HIs, patients exhibited significantly elevated levels of CXCL8, CXCL9, and IL-18, whereas sCD141 levels were significantly lower, and ANG2 levels were comparable between the 2 groups (Figure 4B).
Serum cytokines in HIs and patients, and correlation with GlycoCheck and Tivita. (A) Cytokine levels were measured from frozen serum samples using R&D DuoSet ELISA kits. (B) Box plots comparing serum levels of endothelial-associated cytokines between HIs and patients, with Kruskal-Wallis tests used for statistical analysis (P values shown). Markers analyzed include ANG2 (n = 238); CXCL8 (n = 242); CXCL9 (n = 242), IL-18 (n = 239); and sCD141 (n = 242). (C-D) Linear regression analyses of IL-18 with Tivita parameters. (C) Positive correlation with TWI; (D) negative correlation with NIR; (E) Spearman ρ correlation analysis between IL-18 and GlycoCheck PBR. Correlation coefficients (red line) with 95% CIs (gray and black lines) are shown for each VD between 4 and 25 μm. A correlation was considered significant if the entire CI lay on 1 side of the y-axis. ELISA, enzyme-linked immunosorbent assay.
Serum cytokines in HIs and patients, and correlation with GlycoCheck and Tivita. (A) Cytokine levels were measured from frozen serum samples using R&D DuoSet ELISA kits. (B) Box plots comparing serum levels of endothelial-associated cytokines between HIs and patients, with Kruskal-Wallis tests used for statistical analysis (P values shown). Markers analyzed include ANG2 (n = 238); CXCL8 (n = 242); CXCL9 (n = 242), IL-18 (n = 239); and sCD141 (n = 242). (C-D) Linear regression analyses of IL-18 with Tivita parameters. (C) Positive correlation with TWI; (D) negative correlation with NIR; (E) Spearman ρ correlation analysis between IL-18 and GlycoCheck PBR. Correlation coefficients (red line) with 95% CIs (gray and black lines) are shown for each VD between 4 and 25 μm. A correlation was considered significant if the entire CI lay on 1 side of the y-axis. ELISA, enzyme-linked immunosorbent assay.
Correlation analyses between serum cytokines and other endothelial markers were conducted. However, no significant associations were found for CXCL8, CXCL9, sCD141, or ANG2. In contrast, IL-18 demonstrated significant associations across multiple parameters. Specifically, IL-18 positively correlated with EASIX (as previously reported,11,31 Spearman ρ = 0.37; 95% CI, 0.25-0.47; P < .001) as well as with the TWI (Figure 4C), and negatively correlated with NIR (Figure 4D). Furthermore, IL-18 positively correlated with PBR, indicating an association with reduced glycocalyx integrity (Figure 4E).
Endothelial markers in nontarget organs remain stable over time
To assess longitudinal stability of endothelial markers in nontarget organs, we repeated GlycoCheck measurements in 14 patients and Tivita imaging in 21 patients on day +28 and/or day +100 after allo-SCT. No significant changes were observed in PBR (supplemental Figure 3A-B), NIR, or TWI (P > .05, paired Wilcoxon tests), indicating temporal stability of endothelial parameters in nontarget tissues. This observation aligns with our previous findings of stable IL-18 levels before and after allo-SCT.23 In contrast, EASIX values were significantly elevated on day +28, likely reflecting incomplete platelet reconstitution at this time point (supplemental Figure 3C). Notably, the correlation between EASIX and PBR remained significant on day +28 (supplemental Figure 3D), supporting the robustness of this relationship over time.
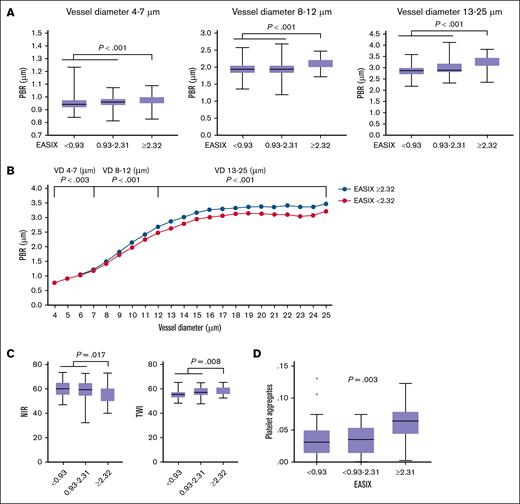
Validated EASIX preconditioning cutoff (≥2.32) stratifies endothelial marker levels
We previously validated an EASIX preconditioning cutoff of ≥2.32 as a predictor of early sepsis risk after allo-SCT32,33 and mortality in patients with coronary artery disease.8,32 In HIs, the third interquartile range for EASIX was 0.93 (n = 102), which is shown in Figure 5 for reference.
Validated EASIX cutoff (≥2.32) is associated with endothelial marker profiles. (A) Box plots showing EASIX values stratified into 3 subgroups: <0.93, 0.93 to 2.31, and ≥2.32 (validated cutoff for risk stratification). Median PBR values are shown across 3 VD categories: 4 to 7 μm, 8 to 12 μm, and 13 to 25 μm. A significant increase in PBR was observed in the EASIX ≥2.32 subgroup across all diameter ranges. P values from Kruskal-Wallis tests are shown (n = 261). (B) Median PBR values (μm) across individual VDs (4-25 μm) for patients with hematological disease (n = 169) stratified by EASIX of <2.32 and of ≥2.32. P values represent Kruskal-Wallis tests comparing low vs high EASIX subgroups. (C) Box plots of Tivita parameters, NIR and TWI, in the same EASIX subgroups. Significantly reduced NIR (impaired perfusion) and increased TWI (higher vascular permeability) were restricted to the EASIX ≥2.32 subgroup. P values from Kruskal-Wallis tests are shown (n = 100). (D) Box plots of platelet-platelet aggregates (proxy) by EASIX subgroup. A significant increase in platelet aggregation was observed in patients with EASIX ≥2.32. P value from Kruskal-Wallis test, n = 62.
Validated EASIX cutoff (≥2.32) is associated with endothelial marker profiles. (A) Box plots showing EASIX values stratified into 3 subgroups: <0.93, 0.93 to 2.31, and ≥2.32 (validated cutoff for risk stratification). Median PBR values are shown across 3 VD categories: 4 to 7 μm, 8 to 12 μm, and 13 to 25 μm. A significant increase in PBR was observed in the EASIX ≥2.32 subgroup across all diameter ranges. P values from Kruskal-Wallis tests are shown (n = 261). (B) Median PBR values (μm) across individual VDs (4-25 μm) for patients with hematological disease (n = 169) stratified by EASIX of <2.32 and of ≥2.32. P values represent Kruskal-Wallis tests comparing low vs high EASIX subgroups. (C) Box plots of Tivita parameters, NIR and TWI, in the same EASIX subgroups. Significantly reduced NIR (impaired perfusion) and increased TWI (higher vascular permeability) were restricted to the EASIX ≥2.32 subgroup. P values from Kruskal-Wallis tests are shown (n = 100). (D) Box plots of platelet-platelet aggregates (proxy) by EASIX subgroup. A significant increase in platelet aggregation was observed in patients with EASIX ≥2.32. P value from Kruskal-Wallis test, n = 62.
In the combined cohort of HIs and patients, the EASIX cutoff of ≥2.32 was significantly associated with elevated PBR values across all vessel diameters, indicating reduced glycocalyx integrity (Figure 5A). When restricting the analysis to patients only (n = 169), we observed a similar and statistically significant increase in PBR across the full range of vessel diameters (4-25 μm) in those with EASIX preconditioning of ≥2.32 (Figure 5B).
Likewise, reduced digital perfusion (NIR) and increased vascular permeability (TWI) were observed only in patients with EASIX of ≥2.32 (Figure 5C). This cutoff also stratified cytokine levels: patients with EASIX of ≥2.32 had significantly higher plasma concentrations of IL-18 (P < .001), CXCL9 (P < .001), and CXCL8 (P = .050), as assessed by Kruskal-Wallis tests.
Finally, elevated platelet-platelet aggregates (proxy) were found exclusively in patients with EASIX of ≥2.32 (Figure 5D), further supporting the utility of this threshold in identifying patients with systemic endothelial activation and dysfunction.
Multivariable regression analyses with end points: EASIX ≥ 2.32, PBR > median, and NIR > median
To explore the relationship between EASIX, PBR, and digital perfusion (NIR) in a multivariable framework, we performed logistic regression analyses using 3 separate end points: EASIX ≥ 2.32, PBR (4-25μm) > median (2.71 μm) and NIR > median (58.5 U). For each model, we included PBR or EASIX, vascular density, and RBC velocity as independent variables, along with age, sex, and BMI as forced-in covariates to control for potential confounding.
As shown in Table 2, the association between EASIX and PBR remained statistically significant in the multivariable context. Tables 3 and 4 provide similarly consistent findings for the full cohort, whereas supplemental Tables 1-3 present analogous results for the patient subgroup only (excluding HIs).
Multivariable logistic regression analysis
| . | OR . | 95% CI . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| Median PBR 4-25 (2.7 μm) | 5.65 | 2.64 | 12.12 | <.001 |
| Median VascD 4-25 (6.6 mm/mm2) | 0.88 | 0.45 | 1.72 | .711 |
| Median RBC veloc. 4-25 (107 μm/s) | 1.26 | 0.65 | 2.46 | .500 |
| Age per y | 1.03 | 1.01 | 1.06 | |
| Gender female = ref | 1.63 | 0.83 | 3.22 | |
| BMI (per kg/m2) | 1.03 | 0.97 | 1.10 | |
| . | OR . | 95% CI . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| Median PBR 4-25 (2.7 μm) | 5.65 | 2.64 | 12.12 | <.001 |
| Median VascD 4-25 (6.6 mm/mm2) | 0.88 | 0.45 | 1.72 | .711 |
| Median RBC veloc. 4-25 (107 μm/s) | 1.26 | 0.65 | 2.46 | .500 |
| Age per y | 1.03 | 1.01 | 1.06 | |
| Gender female = ref | 1.63 | 0.83 | 3.22 | |
| BMI (per kg/m2) | 1.03 | 0.97 | 1.10 | |
Dependent variable is EASIX of >2.32; healthy controls and patients (n = 268; missing values, n = 8). Age, gender, and BMI are forced-in variables in the parsimonious model.
Median PBR 4-25, PBR, median of all vessel sizes (4-25 μm); OR, odds ratio; RBC veloc. 4-25, RBC velocity, median of all vessel sizes (4-25 μm); ref, reference; VascD, vascular density, median of all vessel sizes (4-25 μm).
Multivariable logistic regression analysis
| . | OR . | 95% CI . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| EASIX per log2 | 1.43 | 1.17 | 1.75 | <.001 |
| Median VascD 4-25 (6.6 mm/mm2) | 0.96 | 0.56 | 1.64 | .884 |
| Median RBC veloc. 4-25 (107 μm/s) | 1.11 | 0.65 | 1.89 | .694 |
| Age per y | 1.02 | 1.01 | 1.04 | |
| Gender female = ref | 0.53 | 0.30 | 0.92 | |
| BMI (kg/m2) | 1.00 | 0.95 | 1.05 | |
| . | OR . | 95% CI . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| EASIX per log2 | 1.43 | 1.17 | 1.75 | <.001 |
| Median VascD 4-25 (6.6 mm/mm2) | 0.96 | 0.56 | 1.64 | .884 |
| Median RBC veloc. 4-25 (107 μm/s) | 1.11 | 0.65 | 1.89 | .694 |
| Age per y | 1.02 | 1.01 | 1.04 | |
| Gender female = ref | 0.53 | 0.30 | 0.92 | |
| BMI (kg/m2) | 1.00 | 0.95 | 1.05 | |
Dependent variable was PBR (vessel diameter, 4-25 μm) of more than the median (2.71 μm). Healthy controls and patients (n = 268; missing values, n = 8).
OR, odds ratio; RBC veloc. 4-25, RBC velocity, median of all vessel sizes (4-25 μm); ref, reference; VascD, vascular density, median of all vessel sizes (4-25 μm).
Multivariable logistic regression analysis
| . | Odds ratio . | 95% CI . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| EASIX ≥ 2.32 | 0.23 | 0.07 | 0.74 | .014 |
| Median VD 4-25 (6.6 mm/mm2) | 2.63 | 0.98 | 7.07 | .055 |
| Median RBC veloc. 4-25 (107 μm/s) | 1.65 | 0.64 | 4.26 | .3 |
| Age per y | 0.97 | 0.94 | 1.00 | |
| Gender female = ref | 3.80 | 1.45 | 9.98 | |
| BMI (kg/m2) | 1.08 | 0.96 | 1.20 | |
| . | Odds ratio . | 95% CI . | P . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| EASIX ≥ 2.32 | 0.23 | 0.07 | 0.74 | .014 |
| Median VD 4-25 (6.6 mm/mm2) | 2.63 | 0.98 | 7.07 | .055 |
| Median RBC veloc. 4-25 (107 μm/s) | 1.65 | 0.64 | 4.26 | .3 |
| Age per y | 0.97 | 0.94 | 1.00 | |
| Gender female = ref | 3.80 | 1.45 | 9.98 | |
| BMI (kg/m2) | 1.08 | 0.96 | 1.20 | |
Dependent variable was NIR greater than the median (58.5 U). Healthy controls and patients (n = 99; missing values, n = 1).
RBC veloc. 4-25, RBC velocity, median of all vessel sizes (4-25 μm); ref, reference; VD, vascular density, median of all vessel sizes (4-25 μm).
Endothelial markers associate with early sepsis (day +50) and NRM
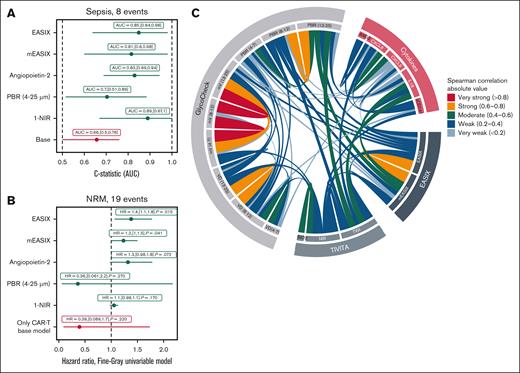
All patients reached day +50 after cellular therapy and were evaluable for early sepsis. We conducted ROC curve analyses and calculated the AUC, also known as C-statistic, to assess predictive performance, including allo-SCT vs CAR-T therapy as a covariate. CAR-T therapy vs allo-SCT is also considered as a base model. High EASIX, elevated ANG2, and low digital perfusion (NIR) were significantly associated with sepsis within the first 50 days after therapy. An association was defined by an AUC of ≥0.70 and a 95% CI lower bound of >0.50. We can also see that the endothelial markers outperform the base model in predicting sepsis risk (Figure 6A).
Endothelial markers and clinical outcomes after cellular therapy. (A) ROC curve analysis for prediction of sepsis by day +50 after cellular therapy. The C-statistic (AUC) is shown for each model. The base model includes only the therapy type (CAR-T therapy vs allo-SCT = reference) as a covariate. Extended models incorporate 1 endothelial-associated variable (eg, EASIX, ANG2, or NIR) in addition to the base model. A marker was considered predictive with AUC of >0.70 and the lower bound of the 95% CI of >0.50. (B) Fine-Gray competing risks model for NRM after cellular therapy, with relapse as a competing event. Subdistribution HRs are shown. As in panel A, the base model includes CAR-T therapy vs allo-SCT = ref., while extended models evaluate the added contribution of single endothelial markers with CAR-Ts as confounder. (C) Chord diagram illustrating the absolute values of significant Spearman rank correlations between endothelial-related observables, grouped into 4 categories: GlycoCheck parameters; Tivita HSI parameters; serum cytokines; and EASIX-related variables. Each chord represents a significant correlation (P < .05, univariable Spearman rank correlation test). The color of each chord corresponds to the effect size category, reflecting the strength of the association. Only pairwise correlations meeting the significance threshold are displayed, emphasizing robust interrelationships between local and systemic markers of endothelial function. HR, hazard ratio.
Endothelial markers and clinical outcomes after cellular therapy. (A) ROC curve analysis for prediction of sepsis by day +50 after cellular therapy. The C-statistic (AUC) is shown for each model. The base model includes only the therapy type (CAR-T therapy vs allo-SCT = reference) as a covariate. Extended models incorporate 1 endothelial-associated variable (eg, EASIX, ANG2, or NIR) in addition to the base model. A marker was considered predictive with AUC of >0.70 and the lower bound of the 95% CI of >0.50. (B) Fine-Gray competing risks model for NRM after cellular therapy, with relapse as a competing event. Subdistribution HRs are shown. As in panel A, the base model includes CAR-T therapy vs allo-SCT = ref., while extended models evaluate the added contribution of single endothelial markers with CAR-Ts as confounder. (C) Chord diagram illustrating the absolute values of significant Spearman rank correlations between endothelial-related observables, grouped into 4 categories: GlycoCheck parameters; Tivita HSI parameters; serum cytokines; and EASIX-related variables. Each chord represents a significant correlation (P < .05, univariable Spearman rank correlation test). The color of each chord corresponds to the effect size category, reflecting the strength of the association. Only pairwise correlations meeting the significance threshold are displayed, emphasizing robust interrelationships between local and systemic markers of endothelial function. HR, hazard ratio.
In competing risk analysis for NRM, with relapse as the competing event and allo-SCT vs CAR-T therapy as covariable, EASIX was significantly associated with increased NRM. There was also a trend toward significance for ANG2 and NIR. Associations in the competing risk model were defined by a subdistribution hazard ratio of >1 and P < .05 (Figure 6B).
Similar effects of mEASIX
As shown in supplemental Figure 4, mEASIX demonstrated significant correlations with key endothelial parameters, including PBR, NIR, and TWI. Furthermore, as illustrated in Figure 6A-B, mEASIX was also associated with the risk of early sepsis and NRM, showing a comparable predictive strength to the original EASIX.
Individual EASIX parameters independently correlate with PBR
To assess the contribution of individual EASIX components to endothelial dysfunction, we separately correlated LDH, creatinine, and platelet count with PBR in vessels ranging from 13 to 25 μm in diameter. As shown in supplemental Figure 5, all 3 parameters exhibited significant correlations with PBR, following the expected direction: positive correlations for LDH and creatinine, and a negative correlation for platelet count. These findings support the relevance of each EASIX component as an independent marker of glycocalyx degradation.
Discussion
This prospective study systematically evaluates and compares noninvasive local and systemic approaches for assessing endothelial dysfunction in humans. The primary objective of the EndoCDO-H trial was to explore the interrelationships among diverse endothelial parameters, including EASIX, serum cytokines, platelet aggregates (proxy), glycocalyx integrity (assessed via sublingual microscopy), and vascular perfusion indices (measured by digital HSI); and to determine their association with early posttherapy endothelial complications.
Our results demonstrate significant intercorrelations between systemic and local measures of endothelial function, suggesting that these independent techniques offer mutual crossvalidation. Among all variables, EASIX, PBR, NIR, and ANG2 showed the strongest associations with clinical outcomes.
Notably, the methods evaluated in this study have previously been investigated in isolation. By applying sublingual microscopy, we observed that EASIX correlates with PBR, indicating a link between systemic endothelial stress and glycocalyx degradation. Each of the 3 individual EASIX components (LDH, creatinine, and platelets) contributed significantly to this association.
Moreover, significant correlations were identified between Tivita HSI and GlycoCheck microscopy, particularly for vascular density and perfusion (NIR), as well as between PBR and TWI, representing glycocalyx integrity and vascular permeability (Figure 6C). These findings illustrate a functional convergence between the processed outputs of 2 technically distinct imaging modalities. That these interrelations were detectable in distinct anatomical regions (sublingual, palmar/digital) supports the hypothesis of systemic endothelial dysfunction before conditioning therapy, which may predispose patients to adverse outcomes.9 At the same time, the moderate strength of these correlations underscores the physiological heterogeneity of the endothelium across different tissue compartments.34
Interestingly, independent research efforts involving sublingual microscopy, HSI, and EASIX have all demonstrated their utility in distinguishing patients with and without sepsis,27,30,33,35 and in predicting severe COVID-19 outcomes.15,36,37 Mortality prediction is a well-established application of EASIX,11 and PBR has also been associated with mortality in sepsis and COVID-19.36
Consistent with previous findings35 EASIX predicted early clinical complications after cellular therapy in our study. Importantly, PBR, NIR, and ANG2 were also independently associated with early endothelial events, although further validation in larger cohorts is warranted to develop a combined endothelial risk score.
Our findings reinforce the clinical relevance of the prevalidated EASIX cutoff of ≥2.32,35 which reliably stratified patients by risk of glycocalyx thinning (elevated PBR), increased vascular permeability (TWI), impaired perfusion (NIR), higher cytokine levels (IL-18, CXCL8, and CXCL9), and increased platelet aggregates. These results support the use of EASIX of ≥2.32 as a threshold for endothelial dysfunction before cellular therapy.
Although EASIX is known to rise during acute stress events such as sepsis, chemotherapy, or vascular complications, earlier studies and our data show that baseline differences between high- and low-risk patients (based on EASIX) persist beyond transplantation.35,38 Similarly, PBR values remained stable between baseline and day +28 after allo-SCT, suggesting that the sublingual glycocalyx in nontarget organs may be relatively insensitive to short-term systemic changes. This supports the hypothesis that elevated PBR in patients who are prone to sepsis may reflect a preexisting endothelial condition, rather than an immediate response to acute inflammation.39 Comparable temporal stability of EASIX has also been observed in patient with coronary artery disease, for whom values remained similar between 2 months before and 6 months after intervention.8
Among cytokines, IL-18 was the only marker to correlate significantly with EASIX, TWI, and PBR, confirming previous reports.11,23 The lack of association between EASIX or imaging parameters and classical acute endothelial markers (eg, ANG2, sCD141) may reflect the relatively homeostatic baseline state of our patient cohort and healthy controls before cellular therapy.
Study limitations include a moderate sample size, although it exceeds most published studies using GlycoCheck or Tivita. Additionally, analyses of outcome and mortality across subgroups and combinations of endothelial markers require confirmation in larger, independent cohorts with extended follow-up.
Conclusions
This study provides a comprehensive, multimodal assessment of systemic and local endothelial dysfunction, linking EASIX, serum biomarkers, platelet aggregates, sublingual microscopy, and digital HSI in patients undergoing cellular therapy (Figure 6C). Our findings reinforce the utility of EASIX as a clinically practical, endothelial-related biomarker, particularly when applying the validated cutoff of ≥2.32. The results support its integration into interventional trials and risk stratification frameworks for endothelial complications in hematological settings.
Acknowledgments
The authors acknowledge the excellent work of Michael Heß (medical research assistant). Samples were processed and provided by National Center for Tumor Diseases Cell and Liquid Biobank, a member of BioMaterialBank Heidelberg.
Authorship
Contribution: T.L. designed the project; L.S., R.F.S., and T.L. designed the prospective Endothelial Cell Dysfunction and Outcome, Hematology trial; L.S., T.L., M.Q., I.I., J.B., and F.S. performed GlycoCheck measurements; H.V. assisted with GlycoCheck measurements; and all authors provided clinical data and wrote the manuscript.
Conflict-of-interest disclosure: H.V. is the cofounder and chief science officer of Microvascular Health Solutions and developed the GlycoCheck technology system. The remaining authors declare no competing financial interests.
Correspondence: Thomas Luft, Department of Internal Medicine V, Hematology, Oncology and Rheumatology, University Hospital Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; email: thomas.luft@med.uni-heidelberg.de.
References
Author notes
Data are available on request from the corresponding author, Thomas Luft (thomas.luft@med.uni-heidelberg.de).
The full-text version of this article contains a data supplement.