Key Points
Plasma functional fibrinolysis correlates with concentrations of proinflammatory cytokines for children with COVID-19 infection.
Hypofibrinolysis, mediated, in part, via immunothrombosis, represents a key contributor to the prothrombotic state in this population.
Visual Abstract
The relationship between fibrinolysis, inflammation, and prothrombotic risk among children hospitalized for coronavirus disease 2019 (COVID-19)–related illness is ill defined. To investigate the association between plasma fibrinolytic capacity and proinflammatory cytokine concentrations among children hospitalized for primary COVID-19 infection and multisystem inflammatory syndrome in children (MIS-C), we hypothesized that cytokine concentrations differ by clinical phenotype and are associated with hypofibrinolysis. We analyzed banked plasma specimens serially collected from children aged <18 years admitted for primary COVID-19 or MIS-C and enrolled in the COVID-19 Anticoagulation in Children–Thromboprophylaxis multicenter trial, an open-label, multicenter, phase 2 clinical trial conducted between July 2020 and May 2021. Plasma coagulative and fibrinolytic function were measured via the clot formation and lysis (CloFAL) assay and modified mini-euglobulin clot lysis assay (ECLA). Interleukin-1β (IL-1β), IL-6, and IL-8, and tumor necrosis factor α were measured by the Meso Scale Discovery assay. Correlations were evaluated using Spearman rank testing. A total of 132 banked plasma specimens from 38 participants (COVID-19: n = 18; MIS-C: n = 20) were analyzed. Overall, increased coagulative function (ie, elevated CloFAL area under the curve) and impaired fibrinolytic function (ie, reduced CloFAL fibrinolytic index [FI] and elevated modified mini-ECLA clot lysis time ratio [CLTR]) were observed but most notably among those with MIS-C. Plasma cytokine concentrations correlated with assay indices of hypofibrinolysis (ie, modified mini-ECLA CLTR and CloFAL FI). In summary, among children hospitalized for COVID-19–related illness, hypercoagulability and hypofibrinolysis are mediated, in part, by inflammation that may contribute to prothrombotic risk. This trial was registered at www.ClinicalTrials.gov as #NCT04354155.
Introduction
The clinical phenotypes for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pediatrics include multisystem inflammatory syndrome in children (MIS-C) and primary respiratory coronavirus disease 2019 (COVID-19) infection.1 Both syndromes are characterized by a dysregulated host inflammatory response with marked coagulation activation (eg, elevated plasma D-dimer and fibrinogen levels).2 Among pediatric patients hospitalized for COVID-19–related illness, the cumulative incidence of hospital-acquired venous thromboembolism (HA-VTE) is estimated between 2.1% and 6.5%, substantively higher than that for the general critically ill pediatric population (estimated at <2%).3
Standard laboratory coagulopathy testing focuses on in vitro measures of clot formation, coagulation activation, and plasma markers of the hypercoagulable state. The coagulation and fibrinolytic systems, along with the cellular blood components and the endothelium, contribute to the physiological maintenance of hemostasis and development of a prothrombotic state. The plasma fibrinolytic system involves interactions among plasminogen activators, substrates (eg, plasminogen and fibrinogen), and inhibitors of plasminogen activation, and functions to degrade fibrin by means of the key enzyme, plasmin. In addition, inflammation and coagulation are closely linked, in which an exaggerated acute phase response, as described in acute presentations of COVID-19 infection, can result in an immune-mediated prothrombotic state (termed “immunothrombosis”) with life-threatening sequalae including VTE, myocardial infarction, and cerebral stroke.4-10
Although not fully understood, the prothrombotic state associated with COVID-19 infection has been associated with evidence of impaired fibrinolysis.11,12 Fibrinolysis has been measured in pediatric subpopulations using functional plasma assays such as the clot formation and lysis (CloFAL) assay and euglobulin clot lysis assay (ECLA).13 Furthermore, a modification of the ECLA requiring reduced plasma volume and increased automation and processing time (the modified mini-ECLA) has been described.14 These assays have distinct advantages over whole-blood point-of-care viscoelastic assays with fibrinolytic parameters, such as the thromboelastogram and rotational thromboelastometry, in that whole-blood viscoelastic assays lack established analytic sensitivity for physiological and pathologic changes in clinically-relevant fibrinolytic substrates, activators, and inhibitors. Immunothrombosis mediated by proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor α (TNF-α) are associated with plasminogen activator inhibitor-1 (PAI-1) concentrations, a critical regulator of fibrinolysis.15 Although CloFAL and ECLA have shown sensitivity to changes in PAI-1 and thrombin activatable fibrinolysis inhibitor levels in pediatric plasma ex vivo,9,13,16 the relationship between levels of proinflammatory cytokines, impairment in plasma fibrinolysis, and prothrombotic risk among children with COVID-19 infection has not been investigated to date.
To address these knowledge gaps related to overall coagulative function, fibrinolytic function, and their relationship with proinflammatory cytokine signaling, we analyzed banked plasma specimens from children previously enrolled in the COVID-19 Anticoagulation in Children–Thromboprophylaxis (COVAC-TP) multicenter trial (ClinicalTrials.gov identifier: NCT04354155).17 The primary aim was to investigate potential associations between measures of plasma fibrinolytic function (via the CloFAL and modified mini-ECLA assays) and proinflammatory cytokine concentrations (ie, IL-1β, IL-6, and IL-8, and TNF-α) after the acute presentation of COVID-19–related illness in children. We hypothesized that plasma concentrations of proinflammatory cytokines related to immunothrombosis are elevated and directly associated with impaired fibrinolysis. Additionally, we sought to determine, on an exploratory basis, whether differences in fibrinolytic indices and markers of immmunothrombosis exist between groups defined by clinical phenotype (ie, MIS-C vs primary COVID-19). We hypothesized that plasma fibrinolytic function (as measured by CloFAL fibrinolytic index [FI] and modified mini-ECLA clotting lysis time ratio [CLTR]) have a greater degree of impairment for those with MIS-C as compared with those with primary COVID-19.
Materials and methods
Study participants and specimen sampling
After written informed consent and assent, children hospitalized with primary COVID-19 and MIS-C were enrolled in the COVAC-TP trial, an open-label, multicenter, phase 2 clinical trial conducted between July 2020 and May 2021. This study was reviewed and approved by an institutional review board (institutional review board number 00247991; approved 13 May 2020) and the work described has been conducted in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Details regarding eligibility criteria, components of the clinical study protocol, and primary study analyses have been described elsewhere.16 Serial blood samples were collected (days 0, 1, 2, 3, 7, and 14 of hospitalization [dependent upon participant length of hospital stay]) for prespecified investigation of plasma coagulative and fibrinolytic function (ie, CloFAL and modified mini-ECLA assays) and measurement of proinflammatory cytokine concentrations (ie, IL-1β, IL-6, IL-8, and TNF-α). Day-0 samples were collected before the receipt of study anticoagulation as part of the COVAC-TP trial protocol. Clinical laboratory data at enrollment were recorded, including routine measures of coagulation (ie, D-dimer, prothrombin time, and partial thromboplastin time) and systemic inflammation (ie, cell counts), as available.
Plasma processing
Whole blood was collected via standard techniques into BD Vacutainer 3.2% buffered sodium citrate siliconized blood collection tubes, with the initial 1 mL of whole blood collected into a discard tube. Samples were then centrifuged at 2500g for 15 minutes at 4°C. The supernatant was isolated and subjected to repeat centrifugation for 15 minutes in the same manner. The resulting platelet-poor plasma supernatant was pooled, aliquoted into polypropylene tubes, and subsequently frozen at −80°C in the College of American Pathologists–accredited Johns Hopkins All Children’s (St Petersburg, FL) Pediatric Biorepository until time of assay. Before evaluation via CloFAL, plasma aliquots were pretreated with heparinase, as previously described,16 to remove any heparin (including subcutaneously administered low–molecular weight heparin administered given after enrollment).
CloFAL assay procedure
The procedure for the CloFAL assay has been described previously.16 Briefly, plasma samples were loaded in quadruplicate wells of a 96-well round-bottom Nunc microassay plate (Fisher Scientific, Santa Clara, CA). A solution of Tris-buffered saline, (pH 7.0) with calcium chloride for citrate reversal, tissue factor (TF) for coagulation activation, and tissue plasminogen activator (tPA) for acceleration of fibrinolysis, were added to 3 of 4 wells per sample; the remaining well included Tris-buffered saline alone, to serve as a blank. Final plasma concentrations were as follows: CaCl2, 17 mM; human recombinant lipidated TF (BioMedica Diagnostics Inc, Windsor, NS, Canada), 2 pM; and human recombinant double-chain tPA (Reprokine Ltd, Valley Cottage, NY), 450 ng/mL. Kinetic blanked dual-wavelength (450 and 605 nm) absorbance measurements were immediately obtained at 45-second intervals in a Cytation 5 microplate scanning spectrophotometer (BioTek, Winooski, VT) for 90 minutes, generating a waveform indicative of clot formation and subsequent lysis. Absorbance data and waveforms were exported to Microsoft Excel for further analysis. The assay standard was factor assay control plasma (George King Inc, Overland Park, KS). An intra-assay normal control run consisted of a separate pooled normal plasma (George King Inc), and abnormal controls consisted of severe factor VII–deficient plasma (George King Inc), antithrombin-deficient plasma (Haematologic Technologies Inc, Essex Junction, VT), and plasminogen-deficient plasma (Affinity Biologicals Inc, Hamilton, ON, Canada). CloFAL indices included: time to maximum amplitude (MA); (MA); coagulation index defined as area under the curve (AUC) through 30 minutes for the sample as a percent of the standard; cumulative AUC for the entire assay (as a percent of the standard); and the FI, defined as the area over the curve for the period of 20 minutes after time to MA, divided by MA of the sample.
Modified mini-ECLA procedure
In accordance with the recently described modified mini-ECLA,14 300 μL of 0.02% acetic acid (refrigerated at 4°C) was added to 25 μL of citrated plasma, in duplicate, in a 96-well microplate resting on wet ice. Plasma and acetic acid samples were mixed via pipetting and incubated on wet ice for 10 minutes. After incubation, the microplate was centrifuged at 2000g at 25°C for 5 minutes, inverted, and the supernatant decanted. The microplate containing the euglobulin fraction pellets was then inverted on an absorbent material (eg, Kimwipe) for 1 minute. A wash of the euglobulin fraction pellet was performed with 100 μL of milli-Q water and the microplate was centrifuged again as above, followed by decanting and inversion. The pellets were then resuspended using 100 μL of prewarmed (37°C) buffer containing 154 mM NaCl and 2.6 mM sodium borate in each well. After resuspension, 100 μL of prewarmed (37°C) buffer containing 0.025 M CaCl2, 2 pM TF (BioMedica, Windsor, NS, Canada), and 0.7 ng/mL tPA (Reprokine, Rehovot, Israel), was added to the second of the duplicate wells for each sample, resulting in a final well concentration of 1 pM TF and 0.35 ng/mL tPA. The first well served as a blank for each sample. The microplate was immediately placed in the spectrophotometer (Cytation 1; Agilent, Santa Clara, CA) programmed to run a 5-second double orbital shake followed by kinetic absorbance reading at 405 nm every 1 minute for 180 minutes. Instrument output consisted of absorbance optical density (OD) readings at each time point, minimum OD value, and maximum OD. The adjusted clot lysis time was defined as the time the absorbance reaches the median OD value between the minimum and maximum OD readings (50% clot lysis), minus the time at maximum OD reading (ie, lag time to maximal amplitude of clot formation). The CLTR was calculated by dividing the adjusted clot lysis time of the sample by the adjusted clot lysis time of the control plasma. An MA-indexed CLTR was calculated by multiplying the CLTR by the ratio of MA of the sample to the MA of the pooled plasma standard.
Plasma proinflammatory cytokines
Plasma concentrations of IL-1β, IL-6, IL-8, and TNF-α were measured using a V-Plex human biomarker panel (Meso Scale Discovery, Rockville, MD) according to manufacturer’s instructions. These cytokines were selected for analysis a priori based on prior studies demonstrating increased concentration in various settings of clinical prothrombotic states.18-23
Statistical analyses
Patient demographics and baseline laboratory characteristics were summarized with counts and percentages for categorical variables, and with means and standard deviations or medians and interquartile ranges (IQRs) for numeric variables, as appropriate. Intergroup comparisons by SARS-CoV-2 phenotype were performed using Fisher exact or Wilcoxon rank-sum tests for categorical and quantitative variables, respectively. Additionally, pooled comparisons between days of sample collection were assessed with Wilcoxon rank-sum tests. Spearman rank correlations (ρ) were calculated between cytokine concentrations and indices of coagulative and fibrinolytic function in plasma for the entire study sample and by subgroups of sample collection time points and SARS-CoV-2 clinical phenotypes. All statistical testing was 2-sided with an alpha level of 0.05. Missing data were not imputed. Data were analyzed independently of the study investigators by the Johns Hopkins All Children’s Hospital biostatistics and epidemiology shared resource. Analyses were conducted with SAS version 9.4 (SAS Institute Inc, Cary, NC) and data visualization was performed with either Stata /SE version 17.1 (StataCorp LLC, College Station, TX) or with R’s ggplot2 package (R version 4.3.2).
Results
Study sample and descriptive data
Among 38 patients enrolled in the COVAC-TP trial (n = 18 classified as COVID-19, and n = 20 as MIS-C), plasma samples were available from 132 research specimen collection time points, with a median of 4 (IQR, 3-5) samples per participant. Table 1 summarizes demographic and enrollment laboratory features of the overall study population, with comparisons made by SARS-CoV-2 clinical phenotype. For the overall study sample, the mean age was 11.7 ± 4.5 years, 47.4% were female, and median body mass index was 22.7 (IQR, 17.2-31.8). Enrollment D-dimer values were elevated (median, 2.2 μg/mL [IQR, 1-4.7]) and greater among those with MIS-C (3.4 μg/mL [IQR, 2.1-7.1]) as compared with primary COVID-19 (1.0 μg/mL [IQR, 0.7-2.2]; P < .01). Descriptive and trial-related outcomes have been reported fully elsewhere.7 Of note, 2 participants with primary COVID-19 developed central venous catheterization (CVC)–related HA-VTE on days 1 and 7, respectively, while on primary enoxaparin TP. No statistically significant differences in acute values of, or time trends in, of CloFAL AUC, modified mini-ECLA CLTR, or CloFAL FI, were detected between patients with COVID-19 vs MIS-C.
Demographic and laboratory features among participants of the COVAC-TP trial by SARS-CoV-2 clinical phenotype
| Study variable, statistic/unit . | Overall, N = 38 . | COVID-19, n = 18 . | MIS-C, n = 20 . | P value . |
|---|---|---|---|---|
| Age, y, mean (SD) | 11.7 (4.5) | 14 (3.9) | 9.6 (4.1) | <.01 |
| Sex assigned at birth, n (%) | ||||
| Female | 18 (47.4) | 11 (61.1) | 7 (35.0) | .1 |
| Male | 20 (52.6) | 7 (38.9) | 13 (65.0) | |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 8 (21.1) | 2 (11.1) | 6 (30.0) | .6 |
| Non-Hispanic Black | 12 (31.6) | 5 (27.8) | 7 (35.0) | |
| Hispanic | 12 (31.6) | 7 (38.9) | 5 (25.0) | |
| Other or mixed race | 3 (7.9) | 2 (11.1) | 1 (5.0) | |
| Unknown | 3 (7.9) | 2 (11.1) | 1 (5.0) | |
| BMI, median (IQR) | 22.7 (17.2-31.8) | 32.1 (23.1-43.4) | 20.1 (16.2-23.3) | <.01 |
| Complete blood count, median (IQR) | ||||
| Hemoglobin, g/dL | 11.4 (9.6-12.8) | 12.8 (11.9-13.4) | 9.8 (9.1-11.2) | <.01 |
| White blood cell count, × 103/μL | 6.4 (5.1-11.2) | 5.9 (3.6-10.3) | 9.2 (5.2-13.8) | .1 |
| Platelet count, × 103/μL | 153 (115-217) | 181 (125-253) | 145 (109-176) | .3 |
| Coagulation data at screening, median (IQR) | ||||
| Prothrombin time, s | 13.9 (12.7-15.2) | 13.4 (12.6-14.0) | 14.4 (13-16) | .08 |
| Partial thromboplastin time, s | 33.5 (31.6-40) | 32.1 (30-34) | 39 (33-43.5) | .01 |
| International normalized ratio | 1.1 (1-1.3) | 1.0 (0.9-1.2) | 1.2 (1.1-1.3) | .04 |
| Fibrinogen, mg/dL | 503 (374-573) | 487 (388-525) | 530 (367-580) | .8 |
| Serum creatinine, mg/dL | 0.6 (0.5-0.7) | 0.6 (0.5-0.7) | 0.6 (0.5-0.7) | .4 |
| D-dimer, μg/mL | 2.2 (1-4.7) | 1.0 (0.7-2.2) | 3.4 (2.1-7.1) | <.01 |
| Study variable, statistic/unit . | Overall, N = 38 . | COVID-19, n = 18 . | MIS-C, n = 20 . | P value . |
|---|---|---|---|---|
| Age, y, mean (SD) | 11.7 (4.5) | 14 (3.9) | 9.6 (4.1) | <.01 |
| Sex assigned at birth, n (%) | ||||
| Female | 18 (47.4) | 11 (61.1) | 7 (35.0) | .1 |
| Male | 20 (52.6) | 7 (38.9) | 13 (65.0) | |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 8 (21.1) | 2 (11.1) | 6 (30.0) | .6 |
| Non-Hispanic Black | 12 (31.6) | 5 (27.8) | 7 (35.0) | |
| Hispanic | 12 (31.6) | 7 (38.9) | 5 (25.0) | |
| Other or mixed race | 3 (7.9) | 2 (11.1) | 1 (5.0) | |
| Unknown | 3 (7.9) | 2 (11.1) | 1 (5.0) | |
| BMI, median (IQR) | 22.7 (17.2-31.8) | 32.1 (23.1-43.4) | 20.1 (16.2-23.3) | <.01 |
| Complete blood count, median (IQR) | ||||
| Hemoglobin, g/dL | 11.4 (9.6-12.8) | 12.8 (11.9-13.4) | 9.8 (9.1-11.2) | <.01 |
| White blood cell count, × 103/μL | 6.4 (5.1-11.2) | 5.9 (3.6-10.3) | 9.2 (5.2-13.8) | .1 |
| Platelet count, × 103/μL | 153 (115-217) | 181 (125-253) | 145 (109-176) | .3 |
| Coagulation data at screening, median (IQR) | ||||
| Prothrombin time, s | 13.9 (12.7-15.2) | 13.4 (12.6-14.0) | 14.4 (13-16) | .08 |
| Partial thromboplastin time, s | 33.5 (31.6-40) | 32.1 (30-34) | 39 (33-43.5) | .01 |
| International normalized ratio | 1.1 (1-1.3) | 1.0 (0.9-1.2) | 1.2 (1.1-1.3) | .04 |
| Fibrinogen, mg/dL | 503 (374-573) | 487 (388-525) | 530 (367-580) | .8 |
| Serum creatinine, mg/dL | 0.6 (0.5-0.7) | 0.6 (0.5-0.7) | 0.6 (0.5-0.7) | .4 |
| D-dimer, μg/mL | 2.2 (1-4.7) | 1.0 (0.7-2.2) | 3.4 (2.1-7.1) | <.01 |
BMI, body mass index; SD, standard deviation.
Plasma coagulative and fibrinolytic function
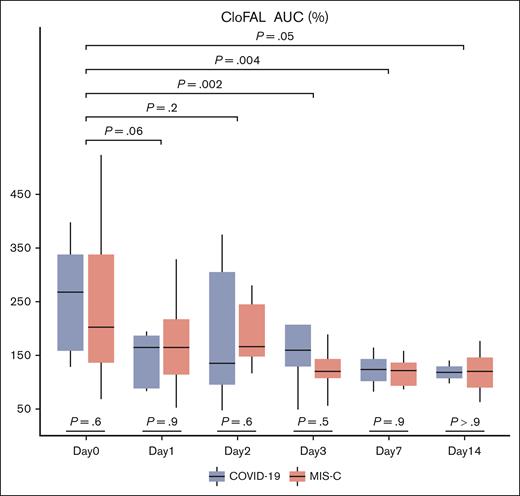
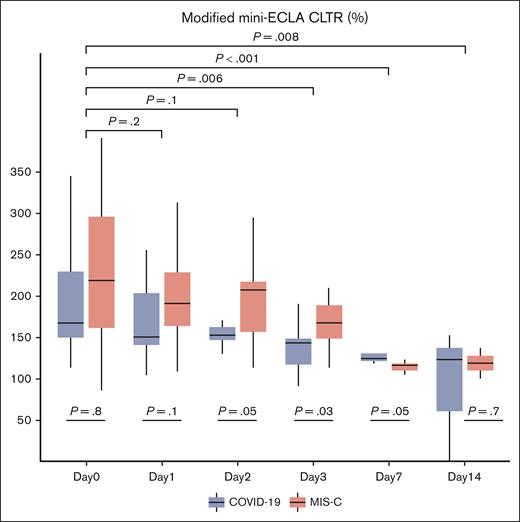
Serial values for CloFAL and modified mini-ECLA assay indices were summarized for the overall study sample and by SARS-CoV-2 clinical phenotype cohorts in supplemental Table 1A-B, respectively. For the study sample, a marked increase in coagulative function was demonstrated within 24 hours of enrollment (median CloFAL AUC: 230.2% [IQR, 146.1%-342.6%]) relative to healthy pooled plasma control (defined as 100%) that decreased significantly over time, as compared with baseline, (time trend toward normalization) through subsequent study days (Figure 1). As demonstrated in Figure 2, decreased fibrinolytic function was observed in the study sample within 24 hours of enrollment by modified mini-ECLA (median CLTR: 190% [IQR, 160%-310%]) relative to the healthy pooled plasma control (defined as 100%) and demonstrated a statistically significant decrease from baseline in values over time (most evident during study days 3 through 7). By contrast, the CloFAL FI signified less-markedly impaired fibrinolytic function acutely (median CloFAL FI: 69.4% [IQR, 47.3%-88.6%]) with a nonsignificant change in values throughout subsequent study days (Figure 3).
Box and whisker plot of CloFAL AUC for participants of the COVAC-TP trial with COVID-19 and MIS-C. The P values presented below plots for each day represent comparisons of values between those with COVID-19 and MIS-C. The P values with brackets above the plots represent comparison of pooled values for each day as compared with day 0. Total samples analyzed by study time point are as follows: day 0 (n = 20), day 1 (n = 24), day 2 (n = 19), day 3 (n = 17), day 7 (n = 8), and day 14 (n = 4).
Box and whisker plot of CloFAL AUC for participants of the COVAC-TP trial with COVID-19 and MIS-C. The P values presented below plots for each day represent comparisons of values between those with COVID-19 and MIS-C. The P values with brackets above the plots represent comparison of pooled values for each day as compared with day 0. Total samples analyzed by study time point are as follows: day 0 (n = 20), day 1 (n = 24), day 2 (n = 19), day 3 (n = 17), day 7 (n = 8), and day 14 (n = 4).
Box and whisker plot of modified mini-ECLA CLTR for participants of the COVAC-TP trial with COVID-19 and MIS-C. The P values presented below plots for each day represent comparisons of values between those with COVID-19 and MIS-C. The P values with brackets above the plots represent comparison of pooled values for each day as compared with day 0. Total samples analyzed by study time point are as follows: day 0 (n = 26), day 1 (n = 35), day 2 (n = 28), day 3 (n = 29), day 7 (n = 10), and day 14 (n = 4).
Box and whisker plot of modified mini-ECLA CLTR for participants of the COVAC-TP trial with COVID-19 and MIS-C. The P values presented below plots for each day represent comparisons of values between those with COVID-19 and MIS-C. The P values with brackets above the plots represent comparison of pooled values for each day as compared with day 0. Total samples analyzed by study time point are as follows: day 0 (n = 26), day 1 (n = 35), day 2 (n = 28), day 3 (n = 29), day 7 (n = 10), and day 14 (n = 4).
Box and whisker plot of CloFAL FI for participants of the COVAC-TP trial with COVID-19 and MIS-C. The P values presented below plots for each day represent comparisons of values between those with COVID-19 and MIS-C. The P values with brackets above the plots represent comparison of pooled values for each day as compared with day 0. Total samples analyzed by study time point are as follows: day 0 (n = 20), day 1 (n = 24), day 2 (n = 19), day 3 (n = 17), day 7 (n = 8), and day 14 (n = 4).
Box and whisker plot of CloFAL FI for participants of the COVAC-TP trial with COVID-19 and MIS-C. The P values presented below plots for each day represent comparisons of values between those with COVID-19 and MIS-C. The P values with brackets above the plots represent comparison of pooled values for each day as compared with day 0. Total samples analyzed by study time point are as follows: day 0 (n = 20), day 1 (n = 24), day 2 (n = 19), day 3 (n = 17), day 7 (n = 8), and day 14 (n = 4).
Plasma proinflammatory cytokines
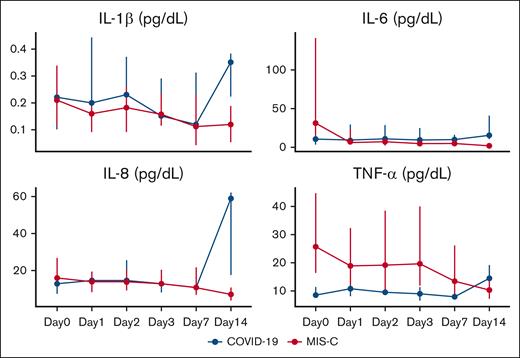
Serial concentrations of cytokines in plasma are summarized for the overall population and by SARS-CoV-2 clinical phenotype are listed in supplemental Table 2. For the study sample, median plasma concentrations of TNF-α (16.4 pg/mL [IQR, 7.9-32.1]), IL-6 (14.9 pg/mL [IQR, 5.2-55.8]), and IL-8 (15.7 pg/mL [IQR, 7.4-25.8]) were elevated within 24 hours of enrollment as compared with values reported among children who are critically ill and healthy controls.24-27 When comparing these values between SARS-CoV-2 clinical phenotype, significantly higher median concentrations of TNF-α were detected among children with MIS-C than those with primary COVID-19 (25.8 pg/mL [IQR, 16.3-44.5] vs 8.7 pg/mL [IQR, 7.4-11.4]; P = .01). Plasma TNF-α concentrations demonstrated a statistically significant decrease over time among patients, for both children with COVID-19 and MIS-C (Figure 4). Furthermore, among patients with MIS-C, elevated plasma TNF-α concentrations appeared to persist for at least 3 days after enrollment above concentrations of those with primary COVID-19. No other statistically significant differences in acute concentrations of, or time trends in, proinflammatory cytokine concentrations were detected between patients with primary COVID-19 vs MIS-C. The participants who developed HA-VTE despite enoxaparin TP exhibited impaired fibrinolysis (modified mini-ECLA CLTR of 340% and 480%; CloFAL FI of 66%) and elevated plasma cytokine concentrations (supplemental Table 3).
Proinflammatory cytokine concentrations among children hospitalized with COVID-19 and MIS-C enrolled into the COVAC-TP trial. Concentrations are depicted below as median with IQR. Total samples analyzed by study time point are as follows: day 0 (n = 28), day 1 (n = 35), day 2 (n = 28), day 3 (n = 28), day 7 (n = 11), and day 14 (n = 5).
Proinflammatory cytokine concentrations among children hospitalized with COVID-19 and MIS-C enrolled into the COVAC-TP trial. Concentrations are depicted below as median with IQR. Total samples analyzed by study time point are as follows: day 0 (n = 28), day 1 (n = 35), day 2 (n = 28), day 3 (n = 28), day 7 (n = 11), and day 14 (n = 5).
Association between plasma proinflammatory cytokines and fibrinolytic function
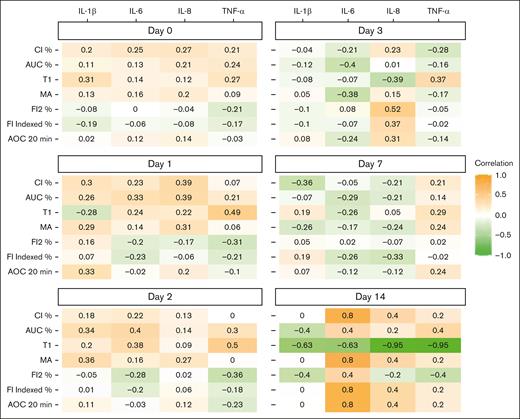
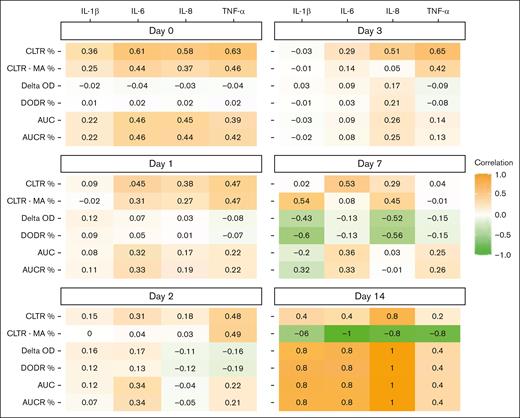
Figures 5 and 6 depict Spearman rank correlations as a heat map of plasma proinflammatory cytokine concentrations and CloFAL and modified mini-ECLA functional indices by study day. Notably, plasma IL-6, IL-8, and TNF-α concentrations were positively correlated (ρ = 0.61, 0.58, and 0.63, respectively) with modified mini-ECLA CLTR within 24 hours of enrollment. Overall, correlations were of greater magnitude among patients with MIS-C than those with primary COVID-19 (supplemental Table 4).
Spearman rank correlation heat map between plasma CloFAL indices and proinflammatory cytokines concentrations among children hospitalized with COVID-19 and MIS-C enrolled into the COVAC-TP trial. AOC, area over the curve; CI, coagulation index; T1, time to MA.
Spearman rank correlation heat map between plasma CloFAL indices and proinflammatory cytokines concentrations among children hospitalized with COVID-19 and MIS-C enrolled into the COVAC-TP trial. AOC, area over the curve; CI, coagulation index; T1, time to MA.
Spearman rank correlation heat map between plasma modified mini-ECLA indices and proinflammatory cytokine concentrations among children hospitalized with COVID-19 and MIS-C enrolled into the COVAC-TP trial. AUCR, area under the curve ratio; DODR, delta optic density ratio.
Spearman rank correlation heat map between plasma modified mini-ECLA indices and proinflammatory cytokine concentrations among children hospitalized with COVID-19 and MIS-C enrolled into the COVAC-TP trial. AUCR, area under the curve ratio; DODR, delta optic density ratio.
Discussion
In this analysis of banked plasma from the COVAC-TP trial of children hospitalized for primary COVID-19 infection and MIS-C receiving primary TP with subcutaneous enoxaparin, we observed increased coagulative function (ie, elevated CloFAL AUC) and impaired fibrinolysis (ie, reduced CloFAL FI and elevated modified mini-ECLA CLTR) compared with normal pooled plasma, and an association between elevated proinflammatory cytokine concentrations of IL-6, IL-8, and TNF-α, and impaired fibrinolysis. In particular, plasma fibrinolytic function as measured by ECLA CLTR was impaired among those with MIS-C and directly correlated with plasma proinflammatory cytokine concentrations such as TNF-α. Although additional validation is needed, these findings suggest that both hypercoagulability and hypofibrinolysis, mediated, in part, by proinflammatory cytokines, may contribute to the prothrombotic state in children hospitalized for COVID-19–related illness.28,29
In general, our study findings are consistent with recent observational reports that revealed elevated rates of VTE for children hospitalized for SARS-CoV-2–related illness. Whitworth et al performed a multicenter retrospective cohort study among children hospitalized in the general pediatric wards and intensive care units of 8 United States quaternary pediatric facilities and found the following clinical features to be associated with HA-VTE: age of ≥12 years, cancer, CVC, and the MIS-C phenotype.30 Similarly, Tehseen et al assessed an international multicenter database, the Picnic registry, and observed elevated rates of thrombosis among children with invasive mechanical ventilation, CVC, congenital heart disease, obesity, and MIS-C.31 Our data complement these inferred associations, because participants of the COVAC-TP trial, including those with and without MIS-C, exhibited increased coagulative capacity and reduced fibrinolytic function in plasma, which correlated with elevated plasma cytokine concentrations.
Suppressed fibrinolytic and intensified procoagulant function have been demonstrated among adults hospitalized with COVID-19–related illness. Blasi et al performed rotational thromboelastometry and assessed plasma levels of PAI-1, a key inhibitor of plasma fibrinolysis that is often elevated in proinflammatory conditions.32 Even in the presence of heparin, samples from adults with COVID-19 had both enhanced clot formation and decreased fibrinolytic function. In a similar analysis of 48 adults hospitalized for COVID-19, Nougier et al observed increased PAI-1 levels and impaired fibrinolysis using rotational thromboelastometry.33 Finally, Bouck et al compared the coagulative and fibrinolytic potential of adult samples using thrombin and plasmin generation assays among adults with COVID-19 as compared with those with non-COVID-19–related sepsis and noted both hypercoagulability and hypofibrinolysis among those with COVID-19 as compared with sepsis.34 These reports, in combination with our study findings in children and with the knowledge that 2 participants from COVAC-TP trial developed HA-VTE despite attaining their protocol-directed TP goal anti–factor Xa levels with enoxaparin, suggest that a subset of children with impaired fibrinolysis may benefit from intensified anticoagulation and adjunctive approaches directed at the fibrinolytic system or its proinflammatory mediators.
Consensus-based recommendations on the use of anticoagulant TP among children hospitalized with COVID-19–related illness have been published by the International Society on Thrombosis and Haemostasis.35 In the absence of contraindications, the administration of low–molecular weight heparin is recommended as primary TP for children hospitalized for COVID-19–related illness with markedly elevated D-dimer or coexisting HA-VTE risk factors (ie, CVC). The COVAC-TP trial provided evidence in support of the safety and preliminary clinical efficacy for enoxaparin for this indication. This analysis of stored samples from the COVAC-TP trial interrogates and adds to an existing framework that hypofibrinolysis contributes to a prothrombotic state and is mediated, in part, by immunothrombosis in children with SARS-CoV-2 infection. Both plasma IL-6 and TNF-α concentrations have been previously associated with PAI-1 dysregulation in patients with COVID-19.36,37 IL-6 trans-signaling induces PAI-1 secretion from endothelial cells, whereas TNF-α induces the expression of TF, a key initiator of coagulative activation.28,38 Inhibitors of TNF-α are commonly prescribed for hyperinflammatory conditions, such as inflammatory bowel disease, and, incidentally, are associated with reduced occurrence rates of VTE as compared with systemic corticosteroids.39 One clinical trial is actively enrolling children with MIS-C to assess the potential effect of TNF-α inhibitors as compared with other immunomodulatory treatments such as IL-1 receptor antagonists and systemic corticosteroids on laboratory measures of inflammation (eg, C-reactive protein).40 Presently, it is unknown whether immunomodulators for COVID-19 also affect the occurrence rate of HA-VTE in children and this represents an area of interest for future research.
Limitations
This study has several limitations, including its relatively small patient sample size. However, these data represent, to our knowledge, the largest prospective cohort of pediatric patients with diagnosed COVID-19 with serial plasma sampling available for analysis. Although study participants had blood samples drawn at standard intervals during hospitalization, those children who recovered more rapidly contributed fewer samples to later study time points (ie, days 7 and 14), introducing the potential for some sampling bias with regard to persistent inflammation or disease severity. Missing samples or inadequate stored samples contributed to missing data, which were not imputed. Despite these limitations, our study offers key insights into the mechanisms underlying the prothrombotic state in children hospitalized for COVID-19–related illness. Several substrates and modulators of fibrinolytic activity (eg, thrombin activatable fibrinolysis inhibitor, PAI-1, and plasminogen) were not analyzed as part of this work and should be evaluated in future research leveraging the COVAC-TP biobank.
Conclusions
Children hospitalized for COVID-19–related illness exhibit enhanced coagulation and impaired fibrinolysis in plasma, the latter which is moderately correlated with plasma concentrations of key proinflammatory cytokines IL-6, IL-8, and TNF-α. These data support the premise that hypofibrinolysis, mediated, in part, via proinflammatory signaling cytokines, is a key contributor to the prothrombotic state in children hospitalized for COVID-19, providing a rationale for future studies of adjunctive therapies to conventional anticoagulant TP in children who are at heightened risk of VTE.
Acknowledgment
This research was conducted with financial support from the Johns Hopkins All Children’s Hospital Foundation (grant number 6551388137) to the principal investigator (A.A.S.).
Authorship
Contribution: A.A.S. and A.R.S. provided substantial contributions to concept and design, analysis, and/or interpretation of data, critical writing or revising the intellectual content; and final approval of the version to be published; M.B., J.M.M., D.A., and J.L.F. participated in substantial interpretation of data, critical revising the intellectual content, and final approval of the version to be published; E.K.A. participated in substantial contribution to concept and design and analysis of data, critical revising the intellectual content, and final approval of the version to be published; S.B. participated in analysis and interpretation of data, revising the intellectual content, and final approval of the version to be published. V.I. participated in analysis and interpretation of data, revising the intellectual content, and final approval of the version to be published; and N.A.G. participated in substantial contribution to concept and design and interpretation of data, critical writing the intellectual content, and final approval of the version to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the COVID-19 Anticoagulation in Children–Thromboprophylaxis Trial Investigators appears in the supplemental Appendix.
Correspondence: Anthony A. Sochet, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, 501 6th St S, REB Room 3200, St. Petersburg, FL 33701; email: anthony.sochet@jhmi.edu.
References
Author notes
Original data are available on request from the corresponding author, Anthony A. Sochet (sochet@jh.edu).
The full-text version of this article contains a data supplement.