Key Points
Azathioprine remains a viable and safe treatment in relapsed/refractory ITP in the TPO-RA era.
Azathioprine offers clinicians a comparable drug response irrespective of prior TPO-RA exposure or splenectomy.
Visual Abstract
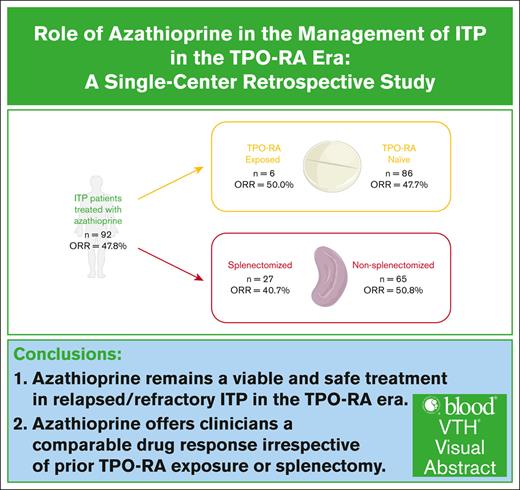
Access to modern therapeutics for immune thrombocytopenia (ITP), such as thrombopoietin-receptor agonists (TPO-RAs), remains a challenge, limiting clinicians’ options. We investigated azathioprine in relapsed/refractory ITP to determine its efficacy and safety, focusing on evaluating its utility in post–TPO-RA patients. We retrospectively reviewed all adult patients, aged ≥18 years, who were worked up for thrombocytopenia between 2009 and 2022 at a tertiary care center in Ontario, Canada. Only patients with ITP treated with azathioprine were included. We identified 92 patients with ITP who received azathioprine, with a mean age of 55.6 ± 22.3 years; 53 were females and 39 males, with 64 having primary ITP. The overall response rate (ORR) was 47.8% (44/92), with a sustained response rate of 77.3% (34/44) at 6 months. The median time to response was 6 weeks. Fourteen patients (31.8%) relapsed, with a median duration of response of 10 weeks. Most patients (73.9%) had documented side effects, with nausea/vomiting, infections, and myelosuppression being the most common. The majority of patients received azathioprine as third-line therapy; 6 patients after TPO-RA and 27 after splenectomy. ORR was 50.0% (3/6) and 40.7% (11/27) in each group, respectively. This is the largest retrospective study, to our knowledge, demonstrating benefit with azathioprine in relapsed/refractory ITP. Its efficacy remains consistent both after TPO-RA (P = .948) and after splenectomy (P = .259), offering clinicians a comparable drug response irrespective of prior TPO-RA exposure or splenectomy. We propose that azathioprine remains a viable option for relapsed/refractory ITP in the TPO-RA era.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease involving the formation of autoantibodies against platelets. This leads to accelerated platelet destruction via phagocytosis in the spleen, as well as impaired platelet production via inhibition of megakaryocytopoiesis.1,2 First-line therapies for ITP consist of corticosteroids and IV immunoglobulins, followed by rituximab, splenectomy, or the novel thrombopoietin-receptor agonists (TPO-RAs), as outlined in the American Society of Hematology (ASH) and International Consensus Report (ICR) 2019 guidelines.3,4 Modern therapeutics include spleen tyrosine kinase inhibitors and neonatal Fc receptor blockers that target alternative pathways.5,6
However, despite these recent advances, the disease’s refractory nature can be challenging to manage in some patients. Some of these emergent treatments are also difficult to access in certain countries, be it from drug availability or patient-related costs, limiting clinicians’ options.7,8 The scarcity of studies surrounding further lines of therapy has remained evident for years, especially in the age of modern standard-of-care therapeutic agents, such as TPO-RAs. Due to the lack of robust data, subsequent lines of treatment are often chosen based on patient and provider preferences. Recent guidelines highlight the importance of addressing this research gap, especially for patients who have failed recommended therapeutic modalities or for whom cost or availability precludes their use.3
Azathioprine is an immunosuppressive drug that has been widely used over the past 50 years in the treatment of autoimmune diseases, hematologic malignancies, and solid organ transplantation. It is metabolized from the prodrug to the active form, 6-mercaptourine, which blocks purine synthesis and thus DNA synthesis, with relative specificity to lymphocytes due to their lack of a salvage pathway.9 Azathioprine is given orally, and its common side effects include nausea, increased risk of infection, and myelosuppression.10 The active drug is metabolized via the enzyme thiopurine methyltransferase (TPMT); patients with deficient TPMT activity are at higher risk of accumulating cytotoxic metabolites, possibly leading to fatal myelotoxicity.11
Azathioprine has been used in the treatment of ITP since at least 1966,12 and it is included in current guidelines as a potential third-line agent.3 It is also affordable and easy to access in many countries. Despite >50 years of use, the paucity of robust data surrounding its use prevents the formulation of strong recommendations.3 A recent systematic review highlighted the many areas of unknown regarding azathioprine in ITP, with few quality studies having been published to date.13 Its efficacy remains uncertain, particularly after exposure to novel treatments, such as TPO-RAs. This study aims to investigate the role of azathioprine in relapsed/refractory ITP, with a focus on evaluating its utility in patients being treated after TPO-RA.
Methods
We performed a retrospective review of all adult patients, aged ≥18 years, who were worked up for thrombocytopenia between January 2009 and December 2022 at the London Health Sciences Centre, a single tertiary care center in Ontario, Canada. Patients diagnosed with ITP who received azathioprine during their disease course were included and subsequently analyzed. Secure electronic records and documentation were used for data gathering. Relevant data were entered in a password-protected Microsoft Excel sheet. This research was approved by the Western University Research Ethics Board, London, Ontario (no. 123200). This study was conducted in accordance with the Declaration of Helsinki.
Patients were diagnosed as per the International Working Group (IWG)–proposed definitions of disease.14 Demographics and previous lines of treatment were recorded. Azathioprine dosage, timing, and concurrent therapies were noted. All reported adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0,15 and their subsequent management were documented. Laboratory values, including platelet counts, were collected at various time points in the treatment course. Efficacy and quality of response were recorded as per the IWG criteria:
Complete response (CR) was defined as platelet count ≥100 × 109/L and absence of bleeding.
Response (R) was defined as platelet count ≥30 × 109/L and at least twofold increase from the baseline count and absence of bleeding.
No response was defined as platelet count <30 × 109/L or less than twofold increase of baseline platelet count or bleeding.
The overall response rate (ORR) was defined as the proportion of patients who achieved CR or R. Time to response was measured from a minimum of 4 weeks, which is the first time that a response to azathioprine could be reasonably expected.16 Late responses not attributable to azathioprine were not defined as CR or R. The cutoff applied for late responses was 6 months, based on the expected time to peak response with azathioprine and previous studies reporting time to response.14,17,18 Patients who developed major adverse effects leading to the discontinuation of azathioprine before reaching the minimum 4-week threshold were noted as intolerant. Patients who required initiation of another ITP treatment or who were corticosteroid and/or IV immunoglobulin dependent were considered nonresponders. Relapse was defined as the loss of CR or R while still on azathioprine therapy. Duration of response was measured from the achievement of CR or R to relapse. A subgroup analysis was conducted on patients who had received TPO-RA before starting azathioprine to determine whether there was a statistical difference in efficacy compared with patients who had not received TPO-RA previously. A similar analysis was also performed to compare azathioprine’s efficacy in splenectomized versus nonsplenectomized patients.
Statistical methods
Descriptive statistics were used to characterize the population. Continuous variables were summarized using means and standard deviations or medians with ranges as appropriate. To assess the significance of different variables, the χ2 test and Student t test were used when applicable, and a P value of <.05 was used. All analyses were conducted using Microsoft Excel (version 16.85).
Results
A total of 1199 patients were referred for thrombocytopenia at our institution between January 2009 and December 2022. The reason for consultation could include other cytopenias, such as anemia or leukopenia, in addition to thrombocytopenia. Of this cohort, 500 patients were diagnosed with ITP and underwent treatment. Patients who had possible ITP and who did not receive treatment were excluded. Among those treated, 92 patients were exposed to azathioprine during their disease course, and their outcomes were analyzed. Five additional patients had received azathioprine before the implementation of our institution’s electronic medical records and were excluded due to insufficient documentation and data.
Demographics and baseline parameters
Demographic profiles and azathioprine characteristics of these 92 patients are summarized in Table 1. The mean age of our study population was 55.6 (± 22.3) years; 53 patients were female (57.6%), and 39 were male (42.4%). As per the IWG-proposed definitions of disease, 64 patients were diagnosed with primary ITP (69.6%) and 28 with secondary ITP (30.4%). Secondary ITP was defined as all forms of ITP except primary ITP14; most patients had concomitant autoimmune diseases, such as systemic lupus erythematosus or Evans syndrome. Classification was also based on the treating physician’s documentation.
Demographic profiles and azathioprine characteristics for all 92 patients with ITP who received azathioprine
| Demographic profiles . | n (%) . |
|---|---|
| Sex | |
| Male | 39 (42.4%) |
| Female | 53 (57.6%) |
| Age at diagnosis, mean (± SD), y | 55.6 (± 22.3) |
| Diagnosis | |
| Primary ITP | 64 (69.6%) |
| Secondary ITP∗ | 28 (30.4%) |
| Baseline platelet count, median (range), × 109/L | 22 (4-217) |
| Duration of ITP before starting azathioprine, median (range), wk | 71 (1-2499) |
| Duration of follow-up, median (range), wk | 338 (7-2801) |
| Azathioprine characteristics | |
| Starting dose, median (range), mg/d | 100 (25-150) |
| Maintenance dose, median (range)†, mg/d | 100 (25-150) |
| Duration of treatment, median (range), wk | 20 (1-1787) |
| Concurrent therapies | |
| Corticosteroids | 38 (41.3%) |
| Other immunosuppressive drugs | 11 (12.0%) |
| TPO-RA | 7 (7.6%) |
| Demographic profiles . | n (%) . |
|---|---|
| Sex | |
| Male | 39 (42.4%) |
| Female | 53 (57.6%) |
| Age at diagnosis, mean (± SD), y | 55.6 (± 22.3) |
| Diagnosis | |
| Primary ITP | 64 (69.6%) |
| Secondary ITP∗ | 28 (30.4%) |
| Baseline platelet count, median (range), × 109/L | 22 (4-217) |
| Duration of ITP before starting azathioprine, median (range), wk | 71 (1-2499) |
| Duration of follow-up, median (range), wk | 338 (7-2801) |
| Azathioprine characteristics | |
| Starting dose, median (range), mg/d | 100 (25-150) |
| Maintenance dose, median (range)†, mg/d | 100 (25-150) |
| Duration of treatment, median (range), wk | 20 (1-1787) |
| Concurrent therapies | |
| Corticosteroids | 38 (41.3%) |
| Other immunosuppressive drugs | 11 (12.0%) |
| TPO-RA | 7 (7.6%) |
SD, standard deviation.
Defined as all forms of ITP except primary ITP; most patients had concomitant autoimmune diseases.
Defined as the dose at which patients were maintained on the longest to maintain the desired therapeutic effect.
The median baseline platelet counts immediately before the initiation of azathioprine was 22 × 109/L (range, 4 × 109/L to 217 × 109/L), and the median duration of ITP before starting azathioprine was 71 weeks (range, 1-2499). The median duration of follow-up, defined as the difference between the date of last follow-up in clinic and the date of ITP diagnosis, was 338 weeks (range, 7-2801). Dosage of azathioprine was determined by the treating physician and was mainly weight based. The median starting dose of azathioprine was 100 mg per day orally (range, 25-150). The median maintenance dose was also 100 mg per day (range, 25-150). The maintenance dose was defined as the dose at which patients were kept on the longest to retain the desired therapeutic effect. The median duration of azathioprine treatment was 20 weeks (range, 1-492). A total of 38 patients were taking azathioprine with concurrent corticosteroids, 11 with other immunosuppressive drugs (eg, danazol, mycophenolate mofetil, hydroxychloroquine, and cyclophosphamide, etc), and 7 with TPO-RA.
In our study population, azathioprine predominantly served as a third- or subsequent-line therapy. These data are presented in Table 2. The median number of lines of therapy before azathioprine exposure was 2 (range, 1-6), with 30 patients (32.6%) having received azathioprine after 2 lines of treatment. All 92 patients had received corticosteroids before azathioprine therapy; additionally, 27 patients (29.3%) in our study received it after splenectomy and 6 (6.5%) after TPO-RA.
Prior lines of therapy for all patients with ITP who received azathioprine
| Patients who received azathioprine (N = 92) . | n (%) . |
|---|---|
| No. of prior lines of therapy, median (range) | 2 (1-6) |
| 1 line | 22 (23.9%) |
| 2 lines | 31 (33.7%) |
| 3 lines | 26 (28.3%) |
| 4 lines | 9 (9.8%) |
| 5 lines | 3 (3.3%) |
| 6 lines | 1 (1.1%) |
| Individual treatments received before azathioprine | |
| Corticosteroids | 92 (100.0%) |
| IVIG | 54 (58.7%) |
| Rituximab | 22 (23.9%) |
| Splenectomy | 27 (29.3%) |
| TPO-RA | 6 (6.5%) |
| Other immunosuppressive drugs∗ | 17 (18.5%) |
| Patients who received azathioprine (N = 92) . | n (%) . |
|---|---|
| No. of prior lines of therapy, median (range) | 2 (1-6) |
| 1 line | 22 (23.9%) |
| 2 lines | 31 (33.7%) |
| 3 lines | 26 (28.3%) |
| 4 lines | 9 (9.8%) |
| 5 lines | 3 (3.3%) |
| 6 lines | 1 (1.1%) |
| Individual treatments received before azathioprine | |
| Corticosteroids | 92 (100.0%) |
| IVIG | 54 (58.7%) |
| Rituximab | 22 (23.9%) |
| Splenectomy | 27 (29.3%) |
| TPO-RA | 6 (6.5%) |
| Other immunosuppressive drugs∗ | 17 (18.5%) |
IVIG, IV immunoglobulin.
Includes danazol, mycophenolate mofetil, hydroxychloroquine, and cyclophosphamide, etc.
Efficacy of azathioprine
The data pertaining to the azathioprine’s efficacy are summarized in Table 3. Individual patients’ courses are documented in Table 4. In all patients with ITP who received azathioprine, the cumulative ORR was 47.8%, with 35 patients achieving CR (38.0%) and 9 achieving R (9.8%). There were 32 patients who had no response to azathioprine (34.8%), and 16 patients were intolerant (17.4%). In the 48 combined patients who were either nonresponders or intolerant, 13 had secondary ITP. The median time to response was 6 weeks (range, 4-27). Of the 44 patients who achieved CR or R, 14 patients relapsed (31.8%). The median duration of response for patients who relapsed was 10 weeks (range, 1-123).
Efficacy of azathioprine for all patients with ITP
| . | n (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Patients who received azathioprine (N = 92) . | Patients who did not receive TPO-RA before azathioprine (n = 86) . | Patients who received TPO-RA before azathioprine (n = 6) . | P value∗ . | Patients who did not receive splenectomy before azathioprine (n = 65) . | Patients who received splenectomy before azathioprine (n = 27) . | P value† . | |
| Response achieved | .948 | .259 | |||||
| ORR | 44 (47.8%) | 41 (47.7%) | 3 (50%) | 33 (50.8%) | 11 (40.7%) | ||
| CR | 35 (38.0%) | 33 (38.4%) | 2 (33.3%) | 26 (40.0%) | 9 (33.3%) | ||
| R | 9 (9.8%) | 8 (9.3%) | 1 (16.7%) | 7 (10.8%) | 2 (7.4%) | ||
| NR | 32 (34.8%) | 29 (33.7%) | 2 (33.3%) | 24 (36.9%) | 8 (29.6%) | ||
| Intolerant (I) | 16 (17.4%) | 16 (18.6%) | 1 (16.7%) | 8 (12.3%) | 8 (29.6%) | ||
| Time to response‡, median (range), wk | 6 (4-27) | 6 (4-27) | 5 (4-10) | .588 | 6 (4-13) | 6 (4-27) | .186 |
| Relapse§ | 14 (31.8%) | 13 (31.7%) | 1 (33.3%) | N/A | 13 (39.4%) | 1 (9.1%) | N/A |
| Duration of response||, median (range), wk | 10 (1-123) | 10 (1-87) | 123 | N/A | 10 (1-87) | 123 | N/A |
| . | n (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Patients who received azathioprine (N = 92) . | Patients who did not receive TPO-RA before azathioprine (n = 86) . | Patients who received TPO-RA before azathioprine (n = 6) . | P value∗ . | Patients who did not receive splenectomy before azathioprine (n = 65) . | Patients who received splenectomy before azathioprine (n = 27) . | P value† . | |
| Response achieved | .948 | .259 | |||||
| ORR | 44 (47.8%) | 41 (47.7%) | 3 (50%) | 33 (50.8%) | 11 (40.7%) | ||
| CR | 35 (38.0%) | 33 (38.4%) | 2 (33.3%) | 26 (40.0%) | 9 (33.3%) | ||
| R | 9 (9.8%) | 8 (9.3%) | 1 (16.7%) | 7 (10.8%) | 2 (7.4%) | ||
| NR | 32 (34.8%) | 29 (33.7%) | 2 (33.3%) | 24 (36.9%) | 8 (29.6%) | ||
| Intolerant (I) | 16 (17.4%) | 16 (18.6%) | 1 (16.7%) | 8 (12.3%) | 8 (29.6%) | ||
| Time to response‡, median (range), wk | 6 (4-27) | 6 (4-27) | 5 (4-10) | .588 | 6 (4-13) | 6 (4-27) | .186 |
| Relapse§ | 14 (31.8%) | 13 (31.7%) | 1 (33.3%) | N/A | 13 (39.4%) | 1 (9.1%) | N/A |
| Duration of response||, median (range), wk | 10 (1-123) | 10 (1-87) | 123 | N/A | 10 (1-87) | 123 | N/A |
CR was defined as platelet count ≥100 × 109/L and absence of bleeding; R, platelet count ≥30 × 109/L and at least twofold increase the baseline count and absence of bleeding; NR, platelet count <30 × 109/L or less than twofold increase of baseline platelet count or bleeding.Intolerant includes patients who discontinued azathioprine before reaching a minimum of 4 weeks, which is the first time that a response to azathioprine could be reasonably expected. N/A represents “not applicable” due to 1 group having an array size of n = 1.
NR, no response.
Calculated between patients who received TPO-RA before azathioprine and those who did not.
Calculated between patients who had a splenectomy before azathioprine and those who did not.
Defined as time from the start of azathioprine to CR or R.
Patients who lost CR or R while still on azathioprine.
Defined as time from the achievement of CR or R to relapse.
Individual patient courses for all patients with ITP who received azathioprine
| ID . | Treatments received before azathioprine . | Efficacy of azathioprine . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Corticosteroids . | IVIG . | Rituximab . | Splenectomy . | TPO-RA . | Other immunosuppressive drugs . | Response achieved . | Relapse∗ . | Sustained response† . | |
| 001 | Y | Y | N | Y | N | Danazol | I | ||
| 003 | Y | Y | Y | Y | N | NR | |||
| 009 | Y | Y | Y | N | N | CR | Y | N | |
| 012 | Y | Y | N | N | N | Fostamatinib | CR | Y | N |
| 019 | Y | Y | Y | N | N | NR | |||
| 022 | Y | N | N | Y | N | CR | N | Y | |
| 025 | Y | N | N | N | N | CR | Y | Y | |
| 026 | Y | Y | N | N | N | Danazol, cyclophosphamide | NR | ||
| 027 | Y | N | N | N | N | R | N | Y | |
| 054 | Y | N | N | N | N | CR | Y | N | |
| 055 | Y | Y | N | N | N | NR | |||
| 056 | Y | Y | Y | N | N | I | |||
| 057 | Y | N | Y | N | N | NR | |||
| 058 | Y | Y | N | N | N | NR | |||
| 059 | Y | Y | N | Y | N | Danazol | I | ||
| 061 | Y | Y | N | Y | Y | CR | N | Y | |
| 063 | Y | Y | N | Y | N | CR | N | Y | |
| 066 | Y | Y | N | Y | N | CR | N | Y | |
| 068 | Y | Y | Y | Y | N | CR | N | Y | |
| 069 | Y | Y | Y | Y | N | NR | |||
| 070 | Y | Y | N | Y | N | CR | N | Y | |
| 071 | Y | Y | N | Y | Y | Hydroxychloroquine | R | N | Y |
| 072 | Y | Y | N | N | N | R | Y | N | |
| 073 | Y | Y | N | Y | N | I | |||
| 074 | Y | Y | N | N | N | CR | N | Y | |
| 075 | Y | Y | N | N | N | CR | N | Y | |
| 077 | Y | Y | N | Y | N | Danazol | I | ||
| 082 | Y | Y | Y | Y | N | NR | |||
| 084 | Y | Y | Y | Y | N | Cyclophosphamide, cyclosporine | I | ||
| 085 | Y | Y | Y | Y | N | NR | |||
| 086 | Y | Y | Y | Y | Y | CR | Y | Y | |
| 087 | Y | Y | Y | Y | Y | Mycophenolate mofetil | NR | ||
| 093 | Y | Y | N | N | N | NR | |||
| 096 | Y | Y | N | N | N | NR | |||
| 102 | Y | Y | Y | N | N | NR | |||
| 103 | Y | Y | N | N | N | CR | Y | N | |
| 105 | Y | Y | N | N | N | NR | |||
| 106 | Y | N | N | N | N | CR | Y | N | |
| 109 | Y | Y | N | N | N | NR | |||
| 110 | Y | Y | N | N | N | CR | N | Y | |
| 111 | Y | Y | N | N | N | NR | |||
| 112 | Y | N | N | N | N | NR | |||
| 113 | Y | Y | N | N | N | Hydroxychloroquine | CR | Y | N |
| 118 | Y | Y | Y | N | N | NR | |||
| 151 | Y | Y | N | N | N | I | |||
| 164 | Y | N | N | Y | N | CR | N | Y | |
| 167 | Y | N | Y | Y | N | CR | N | Y | |
| 168 | Y | N | Y | Y | N | R | N | Y | |
| 170 | Y | N | N | N | N | NR | |||
| 180 | Y | N | N | N | N | Fostamatinib | NR | ||
| 181 | Y | N | N | N | Y | I | |||
| 183 | Y | Y | N | N | N | Fostamatinib | CR | N | Y |
| 184 | Y | N | N | N | N | I | |||
| 185 | Y | N | N | N | N | CR | N | Y | |
| 186 | Y | N | N | N | N | R | N | Y | |
| 191 | Y | N | Y | N | N | Hydroxychloroquine | CR | N | Y |
| 192 | Y | N | Y | N | N | I | |||
| 255 | Y | Y | N | Y | N | I | |||
| 258 | Y | N | N | N | N | CR | N | Y | |
| 259 | Y | Y | N | N | N | Danazol | NR | ||
| 263 | Y | Y | Y | N | N | CR | N | Y | |
| 320 | Y | Y | N | N | N | R | Y | N | |
| 322 | Y | Y | N | N | N | CR | Y | N | |
| 336 | Y | Y | N | N | N | I | |||
| 342 | Y | N | N | N | N | CR | N | Y | |
| 343 | Y | N | N | N | N | CR | Y | Y | |
| 347 | Y | N | N | N | N | CR | N | Y | |
| 359 | Y | N | N | Y | N | I | |||
| 371 | Y | N | Y | Y | N | NR | |||
| 373 | Y | N | N | N | N | NR | |||
| 380 | Y | N | N | N | N | CR | N | Y | |
| 389 | Y | N | N | N | N | CR | Y | Y | |
| 395 | Y | N | N | N | N | R | Y | N | |
| 442 | Y | Y | Y | N | N | NR | |||
| 447 | Y | Y | N | N | N | NR | |||
| 465 | Y | N | N | Y | N | NR | |||
| 511 | Y | N | N | N | N | I | |||
| 528 | Y | N | N | N | N | Hydroxychloroquine | CR | N | Y |
| 535 | Y | Y | N | N | N | CR | N | Y | |
| 641 | Y | N | Y | N | N | Cyclosporine, mycophenolate mofetil | CR | N | Y |
| 647 | Y | Y | N | N | Y | NR | |||
| 652 | Y | N | N | N | N | Fostamatinib | R | N | Y |
| 656 | Y | Y | N | N | N | I | |||
| 666 | Y | N | N | Y | N | I | |||
| 670 | Y | Y | N | Y | N | NR | |||
| 673 | Y | N | N | N | N | CR | N | Y | |
| 675 | Y | Y | N | N | N | Fostamatinib | R | N | Y |
| 682 | Y | N | N | N | N | NR | |||
| 683 | Y | Y | N | N | N | I | |||
| 704 | Y | N | N | N | N | NR | |||
| 705 | Y | N | N | N | N | NR | |||
| 706 | Y | Y | N | N | N | CR | N | Y | |
| ID . | Treatments received before azathioprine . | Efficacy of azathioprine . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Corticosteroids . | IVIG . | Rituximab . | Splenectomy . | TPO-RA . | Other immunosuppressive drugs . | Response achieved . | Relapse∗ . | Sustained response† . | |
| 001 | Y | Y | N | Y | N | Danazol | I | ||
| 003 | Y | Y | Y | Y | N | NR | |||
| 009 | Y | Y | Y | N | N | CR | Y | N | |
| 012 | Y | Y | N | N | N | Fostamatinib | CR | Y | N |
| 019 | Y | Y | Y | N | N | NR | |||
| 022 | Y | N | N | Y | N | CR | N | Y | |
| 025 | Y | N | N | N | N | CR | Y | Y | |
| 026 | Y | Y | N | N | N | Danazol, cyclophosphamide | NR | ||
| 027 | Y | N | N | N | N | R | N | Y | |
| 054 | Y | N | N | N | N | CR | Y | N | |
| 055 | Y | Y | N | N | N | NR | |||
| 056 | Y | Y | Y | N | N | I | |||
| 057 | Y | N | Y | N | N | NR | |||
| 058 | Y | Y | N | N | N | NR | |||
| 059 | Y | Y | N | Y | N | Danazol | I | ||
| 061 | Y | Y | N | Y | Y | CR | N | Y | |
| 063 | Y | Y | N | Y | N | CR | N | Y | |
| 066 | Y | Y | N | Y | N | CR | N | Y | |
| 068 | Y | Y | Y | Y | N | CR | N | Y | |
| 069 | Y | Y | Y | Y | N | NR | |||
| 070 | Y | Y | N | Y | N | CR | N | Y | |
| 071 | Y | Y | N | Y | Y | Hydroxychloroquine | R | N | Y |
| 072 | Y | Y | N | N | N | R | Y | N | |
| 073 | Y | Y | N | Y | N | I | |||
| 074 | Y | Y | N | N | N | CR | N | Y | |
| 075 | Y | Y | N | N | N | CR | N | Y | |
| 077 | Y | Y | N | Y | N | Danazol | I | ||
| 082 | Y | Y | Y | Y | N | NR | |||
| 084 | Y | Y | Y | Y | N | Cyclophosphamide, cyclosporine | I | ||
| 085 | Y | Y | Y | Y | N | NR | |||
| 086 | Y | Y | Y | Y | Y | CR | Y | Y | |
| 087 | Y | Y | Y | Y | Y | Mycophenolate mofetil | NR | ||
| 093 | Y | Y | N | N | N | NR | |||
| 096 | Y | Y | N | N | N | NR | |||
| 102 | Y | Y | Y | N | N | NR | |||
| 103 | Y | Y | N | N | N | CR | Y | N | |
| 105 | Y | Y | N | N | N | NR | |||
| 106 | Y | N | N | N | N | CR | Y | N | |
| 109 | Y | Y | N | N | N | NR | |||
| 110 | Y | Y | N | N | N | CR | N | Y | |
| 111 | Y | Y | N | N | N | NR | |||
| 112 | Y | N | N | N | N | NR | |||
| 113 | Y | Y | N | N | N | Hydroxychloroquine | CR | Y | N |
| 118 | Y | Y | Y | N | N | NR | |||
| 151 | Y | Y | N | N | N | I | |||
| 164 | Y | N | N | Y | N | CR | N | Y | |
| 167 | Y | N | Y | Y | N | CR | N | Y | |
| 168 | Y | N | Y | Y | N | R | N | Y | |
| 170 | Y | N | N | N | N | NR | |||
| 180 | Y | N | N | N | N | Fostamatinib | NR | ||
| 181 | Y | N | N | N | Y | I | |||
| 183 | Y | Y | N | N | N | Fostamatinib | CR | N | Y |
| 184 | Y | N | N | N | N | I | |||
| 185 | Y | N | N | N | N | CR | N | Y | |
| 186 | Y | N | N | N | N | R | N | Y | |
| 191 | Y | N | Y | N | N | Hydroxychloroquine | CR | N | Y |
| 192 | Y | N | Y | N | N | I | |||
| 255 | Y | Y | N | Y | N | I | |||
| 258 | Y | N | N | N | N | CR | N | Y | |
| 259 | Y | Y | N | N | N | Danazol | NR | ||
| 263 | Y | Y | Y | N | N | CR | N | Y | |
| 320 | Y | Y | N | N | N | R | Y | N | |
| 322 | Y | Y | N | N | N | CR | Y | N | |
| 336 | Y | Y | N | N | N | I | |||
| 342 | Y | N | N | N | N | CR | N | Y | |
| 343 | Y | N | N | N | N | CR | Y | Y | |
| 347 | Y | N | N | N | N | CR | N | Y | |
| 359 | Y | N | N | Y | N | I | |||
| 371 | Y | N | Y | Y | N | NR | |||
| 373 | Y | N | N | N | N | NR | |||
| 380 | Y | N | N | N | N | CR | N | Y | |
| 389 | Y | N | N | N | N | CR | Y | Y | |
| 395 | Y | N | N | N | N | R | Y | N | |
| 442 | Y | Y | Y | N | N | NR | |||
| 447 | Y | Y | N | N | N | NR | |||
| 465 | Y | N | N | Y | N | NR | |||
| 511 | Y | N | N | N | N | I | |||
| 528 | Y | N | N | N | N | Hydroxychloroquine | CR | N | Y |
| 535 | Y | Y | N | N | N | CR | N | Y | |
| 641 | Y | N | Y | N | N | Cyclosporine, mycophenolate mofetil | CR | N | Y |
| 647 | Y | Y | N | N | Y | NR | |||
| 652 | Y | N | N | N | N | Fostamatinib | R | N | Y |
| 656 | Y | Y | N | N | N | I | |||
| 666 | Y | N | N | Y | N | I | |||
| 670 | Y | Y | N | Y | N | NR | |||
| 673 | Y | N | N | N | N | CR | N | Y | |
| 675 | Y | Y | N | N | N | Fostamatinib | R | N | Y |
| 682 | Y | N | N | N | N | NR | |||
| 683 | Y | Y | N | N | N | I | |||
| 704 | Y | N | N | N | N | NR | |||
| 705 | Y | N | N | N | N | NR | |||
| 706 | Y | Y | N | N | N | CR | N | Y | |
CR was defined as platelet count ≥100 × 109/L and absence of bleeding; R, platelet count ≥30 × 109/L and at least twofold increase the baseline count and absence of bleeding; NR, platelet count <30 × 109/L or less than twofold increase of baseline platelet count or bleeding. I includes patients who discontinued azathioprine before reaching a minimum of 4 weeks, which is the first time that a response to azathioprine could be reasonably expected.
I, intolerant; ID, unique study patient identification number; IVIG, IV immunoglobulin; N, no; NR, no response; R, response; Y, yes.
Patients who lost CR or R while still on azathioprine.
Patients who maintained a safe platelet count (ie, >30 × 109/L) for a minimum of 6 months after initial response.
In our study population, 27 postsplenectomy patients subsequently received azathioprine treatment. In this cohort, the cumulative ORR was 40.7%, with 9 patients achieving CR (33.3%) and 2 patients achieving R (7.4%). Eight patients were nonresponders (29.6%), and 8 were intolerant (29.6%). Their median time to response was 6 weeks (range, 4-13). Only 1 patient relapsed after response, and their duration of response was 123 weeks.
Another subgroup of 6 patients had received TPO-RA (eltrombopag or romiplostim) before the initiation of azathioprine. In this cohort, the cumulative ORR was 50.0%, with 2 patients achieving CR (33.3%) and 1 patient achieving R (16.7%). Two patients had no response (33.3%), and 1 was intolerant (16.7%). Their median time to response was 5 weeks (range, 4-10), and only 1 patient in this cohort relapsed after 123 weeks. Notably, this patient was also the single case of relapse in the postsplenectomy group.
Safety of azathioprine
Side effects while on azathioprine were reported in 68 of 92 patients (73.9%); all adverse events with their grading and management are summarized in Table 5. The most common side effects reported were nausea/vomiting (30.4%), followed by concomitant infections (20.7%), fatigue (20.7%), and myelosuppression (15.2%). Myelosuppression was defined as any decrease in hemoglobin levels, neutrophil count, or lymphocyte count below the lower limits of the reference range at our institution, which were 115 g/L, 2.0 × 109/L, and 1.0 × 109/L, respectively; patients who had established cytopenias before azathioprine initiation were omitted. Hepatotoxicity was defined as any increase in aspartate aminotransferase or alanine aminotransferase above the upper limits of normal at our institution, which were 33 U/L and 32 U/L, respectively. Among the 19 patients (20.7%) who developed concomitant infections during azathioprine treatment, the infections were predominantly respiratory (10 cases), followed by genitourinary (4 cases) and skin/soft tissue (3 cases). The remaining 2 cases were of undetermined origin. Of note, 1 patient developed a malignancy (diffuse large B-cell lymphoma) while on azathioprine.
Safety of azathioprine for all patients with ITP
| Adverse events∗ . | All grades, n (%) . | Grade 3 or higher, n (%) . |
|---|---|---|
| Any side effects | 68 (73.9%) | 20 (21.7%) |
| Nausea/vomiting | 28 (30.4%) | 5 (5.4%) |
| Infections | 19 (20.7%) | 3 (3.3%) |
| Fatigue | 19 (20.7%) | 2 (2.2%) |
| Myelosuppression† | 14 (15.2%) | 6 (6.5%) |
| Diarrhea | 13 (14.1%) | 3 (3.3%) |
| Abdominal pain | 12 (13.0%) | 1 (1.1%) |
| Weight loss | 8 (8.7%) | 1 (1.1%) |
| Hepatotoxicity‡ | 7 (7.6%) | 3 (3.3%) |
| Fevers | 4 (4.3%) | 0 (0.0%) |
| Night sweats | 3 (3.3%) | 0 (0.0%) |
| Rash | 3 (3.3%) | 0 (0.0%) |
| Malignancy | 1 (1.1%) | 1 (1.1%) |
| Others | 25 (27.2%) | 5 (5.4%) |
| Pancreatitis | 2 | |
| Acute kidney injury | 2 | |
| Arrhythmia | 1 | |
| Management of side effects | n (%) | |
| Dose reduction | 9 (13.2%) | |
| Treatment of side effects | 1 (1.5%) | |
| Discontinuation | 31 (45.6%) |
| Adverse events∗ . | All grades, n (%) . | Grade 3 or higher, n (%) . |
|---|---|---|
| Any side effects | 68 (73.9%) | 20 (21.7%) |
| Nausea/vomiting | 28 (30.4%) | 5 (5.4%) |
| Infections | 19 (20.7%) | 3 (3.3%) |
| Fatigue | 19 (20.7%) | 2 (2.2%) |
| Myelosuppression† | 14 (15.2%) | 6 (6.5%) |
| Diarrhea | 13 (14.1%) | 3 (3.3%) |
| Abdominal pain | 12 (13.0%) | 1 (1.1%) |
| Weight loss | 8 (8.7%) | 1 (1.1%) |
| Hepatotoxicity‡ | 7 (7.6%) | 3 (3.3%) |
| Fevers | 4 (4.3%) | 0 (0.0%) |
| Night sweats | 3 (3.3%) | 0 (0.0%) |
| Rash | 3 (3.3%) | 0 (0.0%) |
| Malignancy | 1 (1.1%) | 1 (1.1%) |
| Others | 25 (27.2%) | 5 (5.4%) |
| Pancreatitis | 2 | |
| Acute kidney injury | 2 | |
| Arrhythmia | 1 | |
| Management of side effects | n (%) | |
| Dose reduction | 9 (13.2%) | |
| Treatment of side effects | 1 (1.5%) | |
| Discontinuation | 31 (45.6%) |
∗As per the National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0.
Defined as any increase in aspartate aminotransferase or alanine aminotransferase above the upper limits of the reference range at our institution, which were 33 U/L and 32 U/L, respectively.
Defined as any decrease in hemoglobin levels, neutrophil count, or lymphocyte count below the lower limits of the reference range at our institution, which were 115 g/L, 2.0 × 109/L, and 1.0 × 109/L, respectively; patients who had established cytopenias before azathioprine initiation were omitted.
Severity of all adverse events was graded as per the Common Terminology Criteria for Adverse Events. The highest number of grade 3 or higher adverse events were noted with myelosuppression and nausea/vomiting. No grade 5 events were recorded. Of the 68 patients having documented side effects, 9 patients decreased their dose of azathioprine, and 31 had to discontinue azathioprine due to adverse events and/or intolerance (45.6%). Only 1 patient underwent TPMT testing before the initiation of azathioprine. Although formal assessment of bleeding events was not included as part of the azathioprine monitoring protocol, all patients’ clinical records during their azathioprine treatment period were reviewed; no major bleeding events occurred from the initiation to the termination of azathioprine therapy.
Discussion
ITP is an autoimmune condition with a diverse, often relapsing disease course; treatment options are constantly evolving and now include the innovative TPO-RAs and spleen tyrosine kinase inhibitors, which have shown great efficacy.8,19-21 However, the availability of these novel therapies can be variable, which may result in health disparities and poor adherence to international standard of care.7,8 Azathioprine is an affordable, readily available immunosuppressive drug that has been used in relapsed/refractory ITP for >50 years.12,22 Despite its longevity, clinicians still lack the data to support the use of azathioprine in their patients with relapsed/refractory ITP, as highlighted in current guidelines and literature.3,13 Additionally, the scarcity of data surrounding its efficacy after TPO-RA limits clinicians’ abilities to make informed decisions and advise this subset of refractory patients on the use of azathioprine. This study aims to investigate the natural history of azathioprine use in relapsed/refractory ITP, with a focus on evaluating its utility in patients being treated after TPO-RA.
Our cohort consisted of 92 patients, with the majority being females (57.6%). Women are more commonly affected by autoimmune diseases,23 and other studies evaluating azathioprine in ITP had female-predominant cohorts as well.17,24-28 Our population was overall older, with a mean age at diagnosis of 55.6 years (± 22.3), than the existing literature’s younger cohorts; recent studies done in India and Egypt had cohorts with a median age of 27.8 and 28 years, respectively.18,27 This discrepancy can be expected due to our local geographical demographics; a study done in Germany described a mean age of 50.5 years.26 In our cohort, primary ITP was the leading diagnosis (69.6%), but our study did include patients with secondary ITP as well. Most patients had received 2 lines of therapy before the initiation of azathioprine, which is consistent with the ASH 2019 and ICR 2019 guidelines, as well as the existing literature.12,18,29
In our study, the cumulative ORR for all patients who received azathioprine was 47.8%, which is within the previously described ORRs of 38.1% to 83.8%.12,17,18,24-30 This wide range of reported efficacy may be due to the differences in azathioprine dosage, timing, as well as disease definitions across studies. There is unfortunately no official definition of sustained response in ITP from the IWG, ICR, or ASH; in our study, we defined sustained response as maintaining a safe platelet count (ie, >30 × 109/L) for a minimum of 6 months after the initial response. Among the 44 individuals who exhibited a positive clinical response (CR or R), 34 patients (77.3%) achieved a sustained response at 6 months. The median time to response in all of our patients was 6 weeks, which is in keeping with azathioprine’s expected time to initial response of 30 to 90 days.14 Unfortunately, time to response is not commonly reported in the current literature, with estimates from 2 studies ranging from 90 days to 6 months.17,18 Of the 44 patients with a positive clinical response (CR or R), 14 relapsed (31.8%). The median duration of response for those patients was 10 weeks (range, 1-123). These variables, relapse rates and durations of response with azathioprine in ITP, were also rarely documented in the existing literature.
From a safety standpoint, azathioprine’s most common reported adverse effects include nausea, an increased risk of infection, and myelosuppression.10 The majority of our patients experienced at least 1 side effect, as noted in Table 5; our overall rate of adverse events (73.9%) surpasses the rates noted in the literature (33%-44.7%).18,26 This disparity may stem from our study’s documentation of a wider spectrum of adverse events than what was reported in the existing literature, such as fatigue, which may occur due to other common conditions. Fatigue is notably among the most frequent and severe symptom associated with ITP, although most physicians assign it lower priority.31 In our study, the overall rate of myelosuppression with azathioprine (15.2%) aligns with the documented rates from prior studies using azathioprine in ITP (13.2%-29.7%).12,17,18,25,29,30 Hepatoxicity was observed in 7.6% of our azathioprine-treated patients, comparable with the reported literature (3.8%-16.7%).17,18,25,28
Notably, 20 patients (21.7%) experienced a grade 3 or higher adverse event; the highest proportion of grade 3 or higher adverse events occurred among patients who developed myelosuppression (6/14) and hepatotoxicity (3/7), suggesting that these side effects may be more important to monitor because they pose the highest risk of severe harmful outcomes. One patient developed diffuse large B-cell lymphoma on azathioprine therapy, necessitating concomitant treatment for both ITP and lymphoma. Interestingly, only 2 cases of malignancy (mantle cell lymphoma and endometrial carcinoma) were reported in the existing literature on the use of azathioprine in ITP.25,29 Although TPMT testing with azathioprine has been discussed in other autoimmune diseases,32 there is a lack of data regarding the utility of TPMT testing in ITP13; only 1 patient in our cohort was tested before azathioprine initiation. The implementation of routine TPMT testing could potentially have limited the incidence of myelotoxicity observed in our population.
Broadly, our findings pertaining to the efficacy and safety of azathioprine in relapsed/refractory ITP are compatible with the existing literature. Among our patients undergoing azathioprine therapy, most had failed multiple prior lines of treatment, contending with a challenging disease course; other studies echoed a similar narrative. Refractory ITP has classically been defined as patients who have failed splenectomy, although this may be subject to change given the lowering rates of splenectomy in the era of modern therapeutics.4,14 Our study population did include a subgroup of 27 postsplenectomy patients, which we subsequently analyzed. Using χ2 analysis and Student t test, no statistically significant differences were observed in the quality of response with azathioprine between splenectomized and nonsplenectomized individuals (P = .259). Time to response was also not statistically different between both groups (P = .186). The rate of relapse and duration of response could not be compared due to only 1 patient in the postsplenectomy group having relapsed (Table 3).
Interestingly, another subgroup analysis focused on the 6 patients who were exposed to TPO-RA before azathioprine. Similarly, there were no statistically significant differences in the response achieved with azathioprine in TPO-RA–naïve and –exposed patients (P = .948), nor in the time to response (P = .588). As with the splenectomy subgroup, only 1 patient relapsed, precluding a meaningful comparison of relapse rates and duration of response (Table 3).
These data suggest that azathioprine continues to be a viable and safe option in relapsed/refractory ITP. Its efficacy remains consistent in both splenectomized and nonsplenectomized patients, and across both TPO-RA–naïve and –exposed cohorts, offering clinicians a comparable drug response irrespective of prior TPO-RA exposure or splenectomy. To date, this is the largest retrospective study conducted, to our knowledge, on the use of azathioprine in relapsed/refractory ITP, and it is the first of its kind establishing its utility in post–TPO-RA patients. Additional strengths of our study include the long duration of follow-up, enabling better reporting of late adverse events and improved detection of delayed relapses. Although derived from a single center, our data are generalizable to many clinical environments; our patients’ demographic profiles mirror the diversity encountered in real-world settings, and azathioprine’s use in ITP at our institution is representative of the standard practice reported in the literature.
Typical limitations accompany the retrospective nature of our study, with sparse data and documentation availability at various time points, especially for patients who were followed during the early years of our electronic medical records. Another limitation of our study lies in the lack of a washout period before azathioprine initiation as well as the frequent use of concomitant therapies among patients receiving azathioprine. This concurrent treatment approach introduces potential confounding factors, because certain observed responses may be attributable to these additional therapies rather than to azathioprine alone. Upon further review, we identified 3 patients who, for at least 12 weeks before azathioprine initiation, were either off all ITP medications or maintained stable doses of an ITP drug without relapse. Among these 3 patients, 1 responded and achieved a sustained response. Azathioprine dosage was also not clearly defined in our study but was typically based on weight per the treating physician. Weight was not consistently available in this retrospective review to calculate the per kilogram dosing that was used. Avenues for future research may include performing a prospective randomized trial with azathioprine, assessing the role of predosage TPMT testing to limit toxicities, and evaluating the utility of other immunosuppressive drugs, such as mycophenolate mofetil, in the post–TPO-RA setting. To enhance our understanding of azathioprine's tolerability, patient self-assessments could be incorporated into future studies as well. Our data suggest that the current side effect profile may not fully capture the range of patient experiences, potentially underrepresenting azathioprine’s impact on quality of life.
Overall, our data suggest that azathioprine remains a viable and safe treatment in relapsed/refractory ITP in the era of TPO-RAs. Its efficacy is consistent in both splenectomized and nonsplenectomized patients, and across both TPO-RA–naïve and –exposed cohorts, offering clinicians a comparable drug response irrespective of prior TPO-RA exposure or splenectomy. Azathioprine’s ease of access and low cost render it an attractive option, especially in resource-constrained settings.
Authorship
Contribution: A.L.-N. designed the research, collected and analyzed data, and wrote the manuscript; S.M. collected data and revised the manuscript; C.C.H. designed the research, collected data, and revised the manuscript; and all authors have read the final manuscript and consented to submission of the manuscript.
Conflict-of-interest disclosure: C.C.H. has participated in advisory boards for Amgen, Medison, Novartis, and Sobi. The remaining authors declare no competing financial interests.
Correspondence: Cyrus C. Hsia, Division of Hematology, Department of Medicine, London Health Sciences Centre, Room E6-219A, Victoria Hospital, 800 Commissioners Rd East, London, ON N6A 5W9, Canada; email: cyrus.hsia@lhsc.on.ca.
References
Author notes
Original data are available from the corresponding author, Cyrus C. Hsia (cyrus.hsia@lhsc.on.ca), on request.