TO THE EDITOR:
The coronavirus disease 2019 (COVID-19) pandemic was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The clinical presentation is heterogeneous, ranging from asymptomatic diseases to those requiring mechanical ventilation.2 Venous thrombosis is a critical complication in many patients with diffuse lung injury, such as COVID-19. Of patients with COVID-19, ∼20% developed venous thrombosis, and 3% developed cerebrovascular accidents.3-5 The etiology of thromboembolic events in acute respiratory distress syndrome (ARDS), such as COVID-19, has been an active area of investigation.3,6-8
This manuscript examined plasma concentrations of podoplanin (PDPN) in patients with COVID-19 and its correlation with known laboratory parameters (D-dimer, ferritin, and C-reactive protein [CRP]) known to predict the severity of disease.9-11 PDPN is a membrane sialoglycoprotein12 that binds to the C-type lectin-like receptor 2 (CLEC-2) on platelets and activates platelets.13 In adult lungs, PDPN is predominantly expressed on alveolar epithelial type 1 (AT1) cells,14 covering the alveolar sac close to the alveolar capillaries. Severe COVID-19 pneumonia is associated with diffuse alveolar damage and disruption of normal lung architecture.15 It is plausible that PDPN on AT1 cells becomes exposed to platelets in the injured lung. We hypothesized that PDPN-platelet interaction in the damaged lung could result in intrapulmonary microthrombi (reported in patients with severe COVID-19). In addition, PDPN released from the damaged lung into circulation might contribute to extrapulmonary thrombosis.
All human studies were approved by the institutional review board of Baylor College of Medicine with a waiver of informed consent, because this study was a retrospective analysis of blood specimens collected for other diagnostic purposes. The blood specimens from patients with SARS-CoV-2 infection (n = 31) admitted from July to September 2020, and 10 simultaneously hospitalized patients without SARS-CoV-2 infection (controls) were analyzed. The diagnosis of COVID-19 was confirmed by reverse transcription polymerase chain reaction assay of nasopharyngeal swabs. Within a few hours after the blood draw, the leftover blood samples were centrifuged at 180g for 10 minutes to obtain platelet-rich plasma, and again for 10 minutes at 1000g to obtain platelet-poor plasma. Aliquots of plasma samples were stored at −80°C until testing. D-dimer, ferritin, and CRP were measured as routine laboratory workup. The imaging studies documented thrombotic events. The severity of SARS-COV2 infection was defined according to the National Institutes of Health criteria (https://www.covid19treatmentguidelines.nih.gov).
The PDPN level was measured using a commercially available enzyme-linked immunosorbent assay kit per the manufacturer's protocol (RayBiotech, Atlanta, GA). All plasma samples and standards were measured in duplicate. PDPN levels were calculated based on the standard curve generated with the standards.
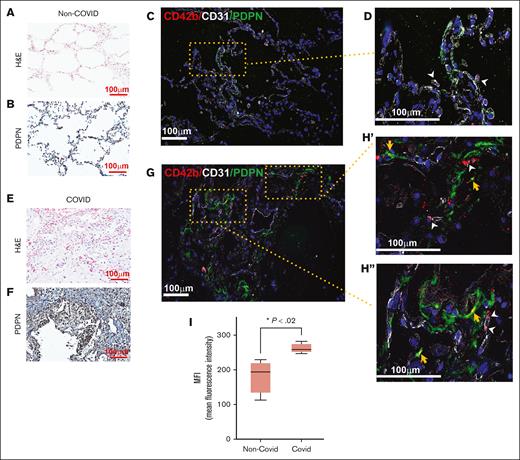
Slides from formalin-fixed paraffin-embedded lung specimens were deparaffinized and underwent antigen retrieval. For immunofluorescence staining, the slides were incubated with antiplatelet antibody (antibody to CD42b; sc-7070, 1:100 dilution; Santa Cruz), anti-PDPN (ab109059; 1:100 dilution; Abcam), and anti-CD31 (MA5-13188; 1:100 dilution; Thermo Fisher) overnight at 4°C. Slides were washed and incubated with the appropriate fluorophore secondary antibodies. For immunohistochemistry staining, the same anti-PDPN antibody was used with the VECTASTAIN ABC (avidin-biotin complex)-HRP (horseradish peroxidase) kit and hematoxylin counterstain (Leica Biosystems Inc, Buffalo Grove, IL).
PDPN levels were analyzed using the Mann-Whitney U test (nonparametric). A Welch t test was used to compare means, and the Spearman correlation test was used to assess correlations. Statistical analysis was completed using GraphPad Prism 9.1.0.
The mean plasma PDPN concentration was higher in patients with COVID-19 (275 ng/mL; 95% confidence interval, 89.34-461.1) than in control patients without COVID-19 (19.71 ng/mL; 95% confidence interval, 12.48-51.90; P = .0092; Figure 1). Median PDPN levels in patients with moderate disease (77.67 ng/mL; range, 32.6-535) and in 22 patients with severe disease (40 ng/mL; range, 4.0-234) were significantly higher than controls (median plasma levels, 0-20.9 ng/mL; Figure 1; P = .008 and .013, respectively). There was no difference in median PDPN levels in 3 patients with mild disease (2.0 ng/mL; range, 0-105) compared with controls (P = .45). Three episodes of venous thrombosis (11%) were documented in patients with severe disease despite receiving prophylactic anticoagulation. Four patients with severe disease and 1 with moderate COVID-19 had very high plasma concentrations of PDPN (>1000 ng/mL). In addition, we did not detect a significant correlation between PDPN levels and D-dimer, CRP, and ferritin level in our patients (data not shown). Increased PDPN in circulation may result from injury to alveolar epithelial cells or inflammation.15-17
PDPN plasma level in patients with COVID-19. PDPN plasma levels and the severity of COVID-19 as defined by the National Institutes of Health criteria.
PDPN plasma level in patients with COVID-19. PDPN plasma levels and the severity of COVID-19 as defined by the National Institutes of Health criteria.
We also examined the autopsy lung specimens of 2 patients with COVID-19 and 2 patients without COVID-19 with light and immunofluorescence microscopy (Figure 2). We detected disruption of alveolar architecture and colocalization of PDPN and platelets. We found that PDPN increased the lung parenchyma (2E-I) of patients with COVID-19. An increase in the expression of PDPN in vessel walls of thrombosed veins has been reported.18 Upon lung injury, the physical proximity of capillaries to AT1 cells and the presence of platelets and megakaryocytes in the lung interstitium19 provide ample opportunity for PDPN-platelet interactions. Our findings raise the possibility of PDPN-induced platelet aggregation playing a role in microthrombosis in injured lungs and increased risk of systemic thrombosis in patients with diffuse lung injury resulting in disruption of architecture of lung parenchyma.3,20,21
Expression of PDPN in the lung in COVID-19 pneumonia. Tissue specimens from the lungs of patients with COVID-19 pneumonia and patients without COVID-19 obtained during autopsy were examined. (A) Hematoxylin and eosin (H&E) and (B) immunohistochemistry stain for PDPN of the non–COVID-19 lung. Immunofluorescence staining of non–COVID-19 lungs with (C) lower original magnification (×100) and (D) higher original magnification (×200) showing few platelets in the lung parenchyma inside blood vessels (white arrowheads). (E) H&E and (F) immunohistochemistry stain for PDPN of COVID-19 lungs. Immunofluorescence staining of COVID-19 lungs at (G) lower original magnification (×100) and (H′-H′′) higher original magnification (×200). Platelets inside blood vessels are indicated with white arrowheads, and those colocalized with PDPN with yellow arrowheads. (I) Expressions of PDPN in COVID-19 and non–COVID-19 lungs are compared as mean fluorescence intensity (MFI) of immunofluorescence-stained slides. The box and whisker graph shows minimum to maximum values in each group (non–COVID-19: 182.5 ± 24.8, COVID-19: 261.7 ± 7.42; n = 4 images per group; P = .02, unpaired t test).
Expression of PDPN in the lung in COVID-19 pneumonia. Tissue specimens from the lungs of patients with COVID-19 pneumonia and patients without COVID-19 obtained during autopsy were examined. (A) Hematoxylin and eosin (H&E) and (B) immunohistochemistry stain for PDPN of the non–COVID-19 lung. Immunofluorescence staining of non–COVID-19 lungs with (C) lower original magnification (×100) and (D) higher original magnification (×200) showing few platelets in the lung parenchyma inside blood vessels (white arrowheads). (E) H&E and (F) immunohistochemistry stain for PDPN of COVID-19 lungs. Immunofluorescence staining of COVID-19 lungs at (G) lower original magnification (×100) and (H′-H′′) higher original magnification (×200). Platelets inside blood vessels are indicated with white arrowheads, and those colocalized with PDPN with yellow arrowheads. (I) Expressions of PDPN in COVID-19 and non–COVID-19 lungs are compared as mean fluorescence intensity (MFI) of immunofluorescence-stained slides. The box and whisker graph shows minimum to maximum values in each group (non–COVID-19: 182.5 ± 24.8, COVID-19: 261.7 ± 7.42; n = 4 images per group; P = .02, unpaired t test).
In another study, PDPN plasma level was lower in patients with COVID-19 (n = 30) than in control healthy individuals.22 The expression of PDPN messenger RNA in single-cell RNA sequencing of lung specimens from patients with COVID-19 showed a reduction compared with normal lungs. Considering the coexpression of angiotensin-converting enzyme 2 and PDPN on AT1 cells (pneumocyte type I) in the lung, it is possible that these cells, providing the binding sites for SARS-CoV-2 virus via angiotensin-converting enzyme 2, bear the brunt of the viral injury. Injury to AT-1 cells might cause a decrease in their PDPN messenger RNA levels. Our study found an increase in PDPN protein staining in lung specimens of patients with COVID-19 associated with the destruction of lung parenchyma. We found an elevation in PDPN plasma levels compared with that in patients without COVID-19, a gradient in PDPN plasma level correlated with COVID-19 severity and thrombotic events in patients with severe infection.
Our observed association between elevated PDPN plasma levels and the severity of COVID-19 is consistent with previous studies showing a role for the PDPN-CLEC-2 axis in inflammatory conditions, and inflammation- and cancer-induced thrombosis.12,17,23-29 In contrast, the study by Rayes et al on the murine models of sepsis showed that CLEC-2 deficiency reduced systemic inflammation and organ injury.30 Platelets and activation of platelets via the PDPN-CLEC2 axis may affect immunity differently in bacterial vs viral infections and in lungs vs other organs. An antibacterial role for platelets,31 particularly against Escherichia coli,32 the most common pathogen in the cecal ligation and puncture model, has been shown. However, the impact of platelets on viral infections might be different. Platelets increase inflammatory response to several strains of influenza viruses in mice.33 Accumulation of platelets in the lungs of influenza-infected mice increase inflammation, and antiplatelet reagents reduce mortality and lung injury.33
The small number of patients limited our retrospective and cross-sectional study. Another limitation of our study is the lack of clinical and demographic information (such as age, cause of death, and time from COVID-19 diagnosis to death) on the control non–COVID-19 cohort and patients who underwent autopsy. In controls, possible unmeasured confounders, such as inflammation, non–COVID-19 involvement of the lungs, or thrombocytopenia, may have influenced the results.
We speculate that PDPN may play a role in the pathogenesis of thrombosis in COVID-19 and other ARDSs. Additional studies are required to examine plasma PDPN as a predictive biomarker for thrombosis in COVID-19 and other disorders with diffuse lung injury.
Contribution: J.G. wrote the manuscript and performed the enzyme-linked immunosorbent assay and collection of clinical data; R.G.D., M.S.C., B.W., and H.L. assisted with manuscript preparation and immunostaining; A.V. assisted with statistical analysis; S.K.D. revised the manuscript; M.A.C. assisted with the conduct of the study and collecting the plasma samples; and V.A.-K. and P.T. conceptualized the project and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vahid Afshar-Kharghan, Benign Hematology, MD Anderson Cancer Center, 6565 MD Anderson Blvd, Houston, TX 77030; email: vakharghan@mdanderson.org; and Perumal Thiagarajan, Michael E. DeBakey Veterans Affairs Medical Center, MS #113, 2002 Holcombe Blvd, Houston, TX 77030; email: perumalt@bcm.edu.
References
Author notes
Data are available from the corresponding authors, Vahid Afshar-Kharghan (vakharghan@mdanderson.org) and Perumal Thiagarajan (perumalt@bcm.edu), on request.