Key Points
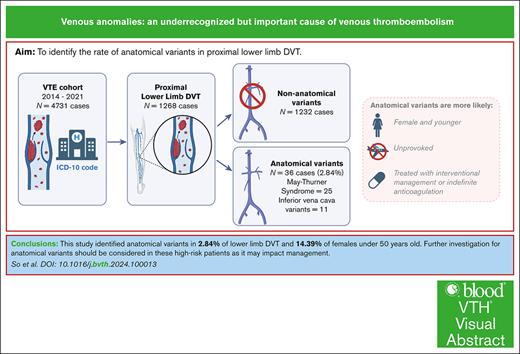
Venous anomalies were found in 2.8% of proximal lower limb DVT cases in our study, highlighting their underrecognized role in DVT.
In females <50 years old, 14% had venous anomalies; thus, further workup should be considered for at-risk groups, as it can affect management.
Visual Abstract
Anatomical variants, such as May-Thurner syndrome (MTS) and inferior vena cava (IVC) variants, are underrecognized causes of deep venous thrombosis (DVT), despite affecting management. We aimed to identify the proportion of anatomical variants in proximal lower limb DVT. A retrospective cohort study was performed with cases of acute proximal DVT from 2014 to 2021 identified from ICD-10 codes. We identified 4731 DVTs and included 1268 proximal DVTs. Thirty-six (2.84%) had an anatomical variant (25 MTS and 11 IVC variants), with a rate of 14.39% in females <50 years old. Compared with nonvariant DVTs, they were more likely to be unprovoked (81% vs 23%), younger (median age, 37 vs 63 years), female (67% vs 37%), and have postthrombotic syndrome (22% vs 9%). Variants frequently received thrombolysis (58% vs 1%) or angioplasty (47% vs 0%) and indefinite anticoagulation (83% vs 40%). Further investigation for variants should be considered for high-risk patients, as variants affect management.
Introduction
Venous thromboembolism (VTE) is associated with considerable morbidity and mortality.1 Unprovoked VTE is often further investigated for thrombophilia and malignancy screening2,3; however, anatomical variants are less commonly considered.4,5
Anatomical variants associated with deep vein thrombosis (DVT) include May-Thurner syndrome (MTS), defined as radiology findings of left vein external compression between the right common iliac artery and vertebral body with a left leg iliofemoral DVT4,6 and inferior vena cava (IVC) variants (congenital agenesis, hypoplasia, or malformation).5 Both are considered rare; however, increasing evidence suggests otherwise, with over 30% of iliac vein thrombosis with imaging consistent with MTS7 and studies of IVC variants observing rates as high as 8.7%.8
The identification of anatomical variants affects management, potentially benefiting from long-term anticoagulation and endovascular intervention to manage postthrombotic syndrome (PTS), a form of chronic venous insufficiency.9,10
This study aimed to identify the proportion of anatomical variants in lower limb DVT at a major metropolitan tertiary center. Secondary end points included identifying risk factors and longer-term outcomes for anatomical variants (recurrence and PTS) and the rate of investigation for anatomical variants in at-risk cohorts.
Study design
Patients were retrospectively identified from our institutional electronic medical records using discharge ICD-10 codes (I800, I801, I802, I803, I808, I809, and I82.40) between January 2014 and December 2021. Patients with acute proximal lower limb thrombosis (IVC/iliac/femoral/popliteal/bilateral) were included based on radiological findings from our radiology database. MTS or IVC variants were identified based on the radiologist’s report and verified by an interventional radiologist. Congenital vascular disorders such as Klippel-Trenaunay syndrome were excluded, as the focus was on common anatomical variants in adults that may change management at the time of DVT diagnosis.
From a database search and chart review, demographic and clinical data were collected, including background patient demographics, provoking factors, imaging, management, and complications. Provoking factors and risk factors were defined based on guidance from the International Society on Thrombosis and Haemostasis.11 Thrombophilia as a risk factor was defined as factor V Leiden mutation, prothrombin gene mutation, physiological anticoagulant (protein C, protein S, and antithrombin) deficiency, antiphospholipid syndrome, and myeloproliferative neoplasm, and identified from documented clinical history or subsequent testing.12 The complications observed were radiologically confirmed VTE recurrence and PTS, as documented by a hematologist using Villalta score in clinical documentation. Censoring occurred at discharge or the last outpatient visit at our institution.
Statistical analyses were performed using GraphPad Prism 9.0.0 and R 4.3.1 software, with the Fisher exact test for categorical variables, t test for continuous data and reverse Kaplan-Meier analysis for median follow-up. A P value <.05 was considered statistically significant.
Ethics approval was granted by our institution's human research ethics committee (approval 460/22) in accordance with the Declaration of Helsinki. This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology checklist.
Results and discussion
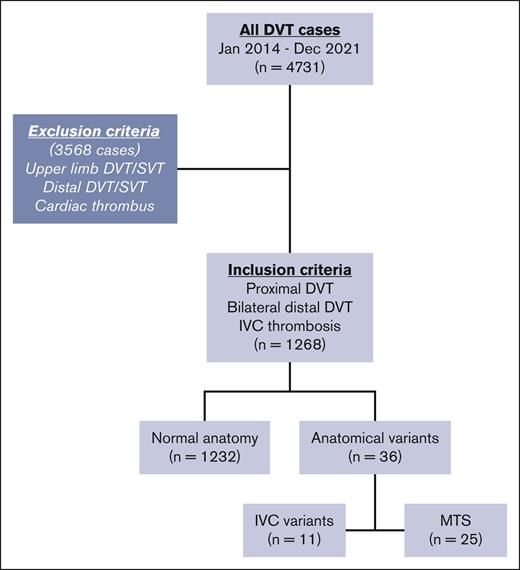
During the study period, 4731 DVTs were identified, with 1268 meeting inclusion criteria (IVC/iliac/femoral/popliteal/bilateral; Figure 1). There were 36 anatomical variants (2.84%), including 25 MTS (1.97%) and 11 hypoplastic IVC (0.87%) variants. In females <50 years of age, the rate was 14.39%. The median age of all included patients was 63 years (interquartile range, 47-76), with 784 males (62%). Anatomical variant cases were more likely younger (median age, 37 years [interquartile range, 25-48]; P < .0001) and female (67% vs 37%; P = .0007). Baseline characteristics are shown in Table 1.
Flowchart of study population. IVC, inferior vena cava; SVT, superficial venous thrombosis.
Flowchart of study population. IVC, inferior vena cava; SVT, superficial venous thrombosis.
Baseline characteristics of study population
| . | All patients with DVT (n = 1268) (%) . | Anatomical variants (n = 36) (%) . |
|---|---|---|
| Male | 784 (62) | 12 (33) |
| Female | 484 (38) | 24 (67) |
| Median age (IQR), y | 63 (47-76) | 37 (25-48) |
| . | All patients with DVT (n = 1268) (%) . | Anatomical variants (n = 36) (%) . |
|---|---|---|
| Male | 784 (62) | 12 (33) |
| Female | 484 (38) | 24 (67) |
| Median age (IQR), y | 63 (47-76) | 37 (25-48) |
| . | Nonanatomical variants (n = 1232) (%) . | Anatomical variants (n = 36) (%) . |
|---|---|---|
| Presenting symptoms | ||
| DVT symptoms: leg swelling/pain | 811 (66) | 31 (86) |
| PE symptoms: dyspnea/tachycardia | 98 (8) | |
| Screening | 231 (19) | |
| Scan results | 28 (2) | 4 (11) |
| Other | 64 (5) | 1 (3) |
| Provoking factors | ||
| Unprovoked | 289 (23) | 29 (81) |
| Provoked | 943 (77) | 7 (19) |
| Trauma | 289 (23) | |
| Lower limb fractures | 216 (18) | |
| Surgery (major) | 303 (25) | 1 (3) |
| Cellulitis/leg wounds | 73 (6) | |
| Immobility | 568 (46) | 5 (14) |
| Line-related | 120 (10) | |
| Malignancy | 208 (17) | |
| Risk factors | ||
| Risk factors present (exclude family history and previous VTE) | 218 (18) | 36 (100) |
| Inflammatory | 18 (1) | 1 (3) |
| COCP | 20 (2) | 7 (19) |
| Heparin-induced thrombocytopenia | 6 (0) | |
| Anatomical variants | ||
| MTS | 0 | 25 (69) |
| IVC variants | 0 | 11 (31) |
| Thrombophilia | 71 (6) | 2 (6) |
| Factor V Leiden mutation | 38 (3) | |
| Prothrombin gene mutation | 2 (0) | |
| Protein S deficiency | 3 (0) | |
| Protein C deficiency | 3 (0) | 1 |
| Antithrombin deficiency | 3 (0) | |
| Antiphospholipid syndrome | 17 (1) | 1 |
| Myeloproliferative neoplasm | 5 (0) | |
| Smoking | 55 (4) | 6 (17) |
| Obesity | 51 (4) | 3 (8) |
| Family history | 58 (5) | 4 (11) |
| Previous VTE | 225 (20) | 12 (33) |
| Dominant side of clot | ||
| Left | 497 (40) | 26 (72) |
| Right | 524 (43) | 4 (11) |
| Bilateral | 211 (17) | 6 (17) |
| Extent of DVT | ||
| Proximal | 1050 (85) | 34 (94) |
| Distal bilateral | 117 (9) | 0 |
| IVC involvement | 65 (5) | 2 (6) |
| Diagnosis | ||
| Initial imaging | ||
| Vascular Doppler ultrasound | 1177 (96) | 34 (94) |
| Angiography | 6 (0) | |
| CT | 33 (3) | 2 (6) |
| Other | 16 (2) | |
| Further imaging | ||
| Vascular Doppler ultrasound | 887 (72) | 4 (11) |
| Angiography | 14 (1) | 14 (39) |
| CT | 78 (6) | 16 (44) |
| Other | 9 (1) |
| . | Nonanatomical variants (n = 1232) (%) . | Anatomical variants (n = 36) (%) . |
|---|---|---|
| Presenting symptoms | ||
| DVT symptoms: leg swelling/pain | 811 (66) | 31 (86) |
| PE symptoms: dyspnea/tachycardia | 98 (8) | |
| Screening | 231 (19) | |
| Scan results | 28 (2) | 4 (11) |
| Other | 64 (5) | 1 (3) |
| Provoking factors | ||
| Unprovoked | 289 (23) | 29 (81) |
| Provoked | 943 (77) | 7 (19) |
| Trauma | 289 (23) | |
| Lower limb fractures | 216 (18) | |
| Surgery (major) | 303 (25) | 1 (3) |
| Cellulitis/leg wounds | 73 (6) | |
| Immobility | 568 (46) | 5 (14) |
| Line-related | 120 (10) | |
| Malignancy | 208 (17) | |
| Risk factors | ||
| Risk factors present (exclude family history and previous VTE) | 218 (18) | 36 (100) |
| Inflammatory | 18 (1) | 1 (3) |
| COCP | 20 (2) | 7 (19) |
| Heparin-induced thrombocytopenia | 6 (0) | |
| Anatomical variants | ||
| MTS | 0 | 25 (69) |
| IVC variants | 0 | 11 (31) |
| Thrombophilia | 71 (6) | 2 (6) |
| Factor V Leiden mutation | 38 (3) | |
| Prothrombin gene mutation | 2 (0) | |
| Protein S deficiency | 3 (0) | |
| Protein C deficiency | 3 (0) | 1 |
| Antithrombin deficiency | 3 (0) | |
| Antiphospholipid syndrome | 17 (1) | 1 |
| Myeloproliferative neoplasm | 5 (0) | |
| Smoking | 55 (4) | 6 (17) |
| Obesity | 51 (4) | 3 (8) |
| Family history | 58 (5) | 4 (11) |
| Previous VTE | 225 (20) | 12 (33) |
| Dominant side of clot | ||
| Left | 497 (40) | 26 (72) |
| Right | 524 (43) | 4 (11) |
| Bilateral | 211 (17) | 6 (17) |
| Extent of DVT | ||
| Proximal | 1050 (85) | 34 (94) |
| Distal bilateral | 117 (9) | 0 |
| IVC involvement | 65 (5) | 2 (6) |
| Diagnosis | ||
| Initial imaging | ||
| Vascular Doppler ultrasound | 1177 (96) | 34 (94) |
| Angiography | 6 (0) | |
| CT | 33 (3) | 2 (6) |
| Other | 16 (2) | |
| Further imaging | ||
| Vascular Doppler ultrasound | 887 (72) | 4 (11) |
| Angiography | 14 (1) | 14 (39) |
| CT | 78 (6) | 16 (44) |
| Other | 9 (1) |
COCP, combined oral contraceptive pill; IQR, interquartile range; PE, pulmonary embolism.
We identified a rate of 3.10% for anatomical variants and 2.15% for MTS. The estimated incidence of MTS is 2% to 5% of all DVTs, but this may be an underestimation.4 Waheed et al reported a high prevalence of 21 MTS cases of 51 DVTs over a 7-year cohort study.13 A metanalysis identified that 46% of over 1000 patients who underwent thrombolysis had underlying iliac vein compression.14 Furthermore, fibrous spurs on the left common iliac vein were identified in 22% to 33% of 430 autopsies, suggesting MTS anatomy is often undetected until a small proportion manifests as an acute DVT often provoked by factors such as a combined oral contraceptive pill.15 Underdiagnosis of MTS may be contributed to by the lack of a standard definition of the degree of left common iliac compression on imaging.6 Our study identified 0.87% of DVT cases had IVC anomalies. The large-scale Registro Informatizado de Enfermedad TromboEmbólica study found an incidence of 0.06%9; however, another prospective study depicted a higher incidence of 5.15%.16 Comparison is difficult given that each study has different inclusion criteria, population differences, and thresholds for further investigation. The incidence is likely underestimated because of lack of awareness and appropriate diagnostic imaging.4
Nonanatomical DVTs were mostly provoked (943, 74%) by ≥1 provoking factors, including immobility (568, 46%), trauma (289, 23%), major surgery (303, 25%), lower limb fractures (216, 18%), and malignancy (208, 17%; P = .0023). Only 18% (218) had risk factors, most commonly being thrombophilia (71, 6%). In contrast, most anatomical variant DVTs were unprovoked (81% vs 23%; P < .0001). If provoked, it was mostly due to immobility (5, 14%) or major surgery (1, 3%). Anatomical variants were more likely female (67% vs 37%; P = .0007), on the combined oral contraceptive pill (19% vs 2%; P < .0001), active smokers (17% vs 4%; P = .0061) and left-sided (72% vs 40%; P = .0002). Left-sided predominance is entirely attributed to MTS (P < .0001), whereas IVC variants were evenly distributed (P > .9999). MTS is believed to be more common in females because of an accentuation of lumbar lordosis, which pushes the iliocaval venous system anteriorly.4 No significant association with anatomical variants was identified for thrombophilia (6% vs 6%; P > .9999), obesity (8% vs 4%; P = .1949), family history (11%, vs 5%; P = .0946), and previous VTE (33% vs 20%; P = .0577; Table 1).
After the initial diagnosis of DVT by Doppler ultrasound, only 7% of patients overall had further imaging: 6% computed tomography (CT) and 1% angiography. Unprovoked DVTs more likely underwent further imaging (29.6%), and of these, 24.5% had an anatomical variant. Anatomical variant DVTs more frequently underwent subsequent angiography (39% vs 1%; P < .0001) or CT (44% vs 6%; P < .0001). CT and angiography are the modalities of choice for the diagnosis of IVC variants, and there is >95% sensitivity and specificity for CT or magnetic resonance imaging for MTS.5,17,18 The widespread use of noninvasive ultrasound is likely to miss anatomical variants because of limited views of the proximal iliac vein (and lack of views of IVC) and limitations with obesity, bowel gas, and significant operator variation.19 Therefore, in our nonanatomical variant group, MTS or IVC variants were likely missed. This is unlike other variants, such as Klippel-Trenaunay syndrome, which is a clinical diagnosis and less likely to be underdiagnosed because of lack of imaging.20 Our study results support the targeted screening with CT venography in at-risk cohorts such as young females with unprovoked DVT.
Generally, nonanatomical variants were treated with anticoagulation (1113, 90%) unless there were contraindications such as bleeding, with 740 (60%) for a time-limited duration consistent with the burden of provocation of thromboses. Only 53 (4%) received interventional treatment, most commonly thrombolysis (18, 1%). In comparison, all anatomical variants were treated with anticoagulation, and most were treated indefinitely (83% vs 40%; P < .0001). Furthermore, most underwent interventional treatment (69% vs 3%; P < .0001) involving thrombolysis (58% vs 1%; P < .0001), angioplasty (47% vs 0%; P < .0001) or thrombectomy (3% vs 1%; P = .251; Figure 2). After a median follow-up of 2992 days, there was more PTS in anatomical variants (22% vs 9%; P = .0192), but no difference in VTE recurrence (6% vs 7%; P > .9999). There was no difference in PTS rate within the anatomical variant group regardless of treatment, anticoagulation alone vs interventional management (37.5% vs 20%; P = .6781).
Comparison of interventional management for anatomical variants and nonanatomical variants. In contrast to nonanatomical variants, anatomical variant associated DVTs were more likely to receive interventional treatment involving thrombolysis (58% vs 1%; P < .0001), angioplasty (47% vs 0%; P < .0001), or thrombectomy (3% vs 1%; P = .251).
Comparison of interventional management for anatomical variants and nonanatomical variants. In contrast to nonanatomical variants, anatomical variant associated DVTs were more likely to receive interventional treatment involving thrombolysis (58% vs 1%; P < .0001), angioplasty (47% vs 0%; P < .0001), or thrombectomy (3% vs 1%; P = .251).
Endovascular treatment in MTS may reduce VTE recurrence and PTS; however, these benefits are not well established.10 Mickley et al and Kim et al reported a reduction in VTE recurrence in MTS treated with stenting in addition to thrombectomy (73%-13%, and 37%-73% to 17%-39%).10,21 Not specific to anatomical variants, the Catheter-Directed Thrombolysis for Deep Vein Thrombosis trial found a reduced PTS rate in thrombolysis cases, whereas the ATTRACT trial suggested no difference in PTS and an increased risk of bleeding, though a post hoc analysis demonstrated a reduced PTS rate if there was early thrombus removal.22-24 A pediatric meta-analysis for MTS outcomes observed a PTS incidence of 61% and DVT recurrence of 38%, with no significant reduction in PTS rates in endovascular treatment (P = .57).25 Unlike MTS, IVC variants have a significantly higher likelihood of PTS but no difference in VTE recurrence.9 Our results suggest that PTS rates are higher in anatomical variants but no clear benefit of endovascular treatment in reducing PTS or VTE recurrence. However, this is likely confounded by the fact that those receiving interventional treatment have a more extensive clot burden contributing to PTS and would benefit from further studies.
Our study strengths are its large sample size and level of care as a major metropolitan tertiary center. However, as a statewide trauma center without obstetrics and gynecology, some provoked DVTs are secondary to trauma, which may not be representative of other cohorts and affect prevalence. Furthermore, it is limited in its single center and retrospective study design.
Overall, this study identified anatomical variants in 2.84% of lower limb DVT and 14.39% in females aged <50 years. However, they are likely underdiagnosed, as only 7% of individuals underwent further diagnostic imaging. At-risk cohorts, such as young females, should be considered for further workup of anatomical variants, as such variants can affect management.
Acknowledgment
J.M. is supported by a National Heart Foundation Future Leader Fellowship.
Authorship
Contribution: J.S. and C.D. collected data; J.S., C.D., and J.D.M. interpreted the data; J.S., C.D., and J.D.M. wrote the manuscript with input from all authors; W.C., H.G., and H.T. reviewed the manuscript; and all authors conceived the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joanne So, Alfred Health, 55 Commercial Rd, Melbourne, VIC 3004, Australia; email: joanne.so.yc@gmail.com.
References
Author notes
Data are available on request from the corresponding author, Joanne So (joanne.so.yc@gmail.com).
The full-text version of this article contains a data supplement.