Key Points
IVCF was associated with reduced 30-day mortality in patients with cancer and VTE regardless of brain metastasis status.
IVCF placement in patients with brain metastasis and VTE was not assciated with decreased risk of intracranial hemorrhage at 180 days.
Visual Abstract
We investigated the association of inferior vena cava filter (IVCF) usage with early mortality and intracranial hemorrhage (ICH) in patients with cancer and venous thromboembolism (VTE) with and without brain metastasis. We used the California Cancer Registry data linked to hospitalization and emergency department databases to identify patients (all ages) with melanoma, kidney, breast, or lung cancers who had acute VTE between 2005 and 2017 at hospital admission. The primary outcomes were 30-day mortality and 180-day ICH post-index VTE hospitalization. Of the 16 847 patients with cancer and VTE, 19.1% had brain metastasis. Patients with brain metastasis were more likely to receive an IVCF (odds ratio, 2.24; 95% confidence interval [CI], 2.01-2.50). Among patients with active bleeding, IVCF placement was associated with ∼50% reduction in 30-day mortality (hazard ratio [HR], 0.53; 95% CI, 0.42-0.68), regardless of the presence or absence of brain metastasis. In patients without active bleeding, 30-day mortality decreased by nearly 30% among those with brain metastasis who received IVCF (HR, 0.72; 95% CI, 0.60-0.85), with no difference among those without brain metastasis who had an IVCF inserted. Patients with brain metastasis had an elevated hazard of 180-day mortality (HR, 5.14; 95% CI, 2.99-8.83), but no association was found between IVCF insertion and 180-day ICH. Our study suggests a potential mortality benefit of IVCF use among patients with selected cancers and VTE, particularly among patients with active bleeding and those with brain metastasis with no bleeding. IVCF use was not associated with 180-day ICH. Randomized clinical trials are warranted to confirm our results.
Introduction
Venous thromboembolism (VTE), which includes pulmonary embolism (PE) and/or deep venous thrombosis (DVT), is a major cause of morbidity and mortality among patients with cancer.1 Patients with brain metastasis are at a particularly high risk for cancer-associated thrombosis, with ∼20% of these patients developing VTE.2 Anticoagulation for patients with brain metastasis and acute VTE is controversial because of the perceived increased risk of intracranial hemorrhage (ICH), which may occur in up to 20% to 50% of patients.2 To decrease the risk of ICH, clinicians may recommend the insertion of an inferior vena cava filter (IVCF).3 However, IVCF does not treat the underlying cancer-associated hypercoagulable state and may increase the risk of subsequent DVT.4,5 Therefore, despite its widespread use in the United States over the past 50 years, the placement of IVCF in patients with cancer, particularly those with brain metastases, remains controversial.6,7 To date, there have been no high-quality prospective or randomized clinical studies that can help guide this treatment decision. Some studies have suggested that therapeutic anticoagulation does not increase the risk of ICH in patients with metastatic brain malignancies.2,8-10 Therefore, most professional medical societies advocate for IVCF placement only among those patients with absolute contraindication(s) for therapeutic anticoagulation (eg, active bleeding).6,11,12
With the lack of evidence in terms of efficacy4,13,14 and increasing evidence of harm,15 the American Society of Hematology has now placed appropriate patient selection for IVCF as an important component of their system-based hematology initiative.16 Population-based studies to evaluate IVCF use in patients with cancer have shown discordant results.17-19 A recent study suggested an improvement in PE-free survival among patients with cancer and VTE who had an IVCF inserted without an increase in the risk of new episodes of DVT.18 However, other reports have suggested an increased risk of DVT17 and higher odds of 30-day and 1-year mortality among patients with PE in whom an IVCF was inserted.19
The aim of this large retrospective population-based analysis was to evaluate the use of IVCF in a cohort of patients with common cancers with a high prevalence of brain metastasis (lung and breast cancers) and a high risk of ICH (melanoma and kidney cancers) who were hospitalized due to an acute VTE event. The primary outcomes were 30-day mortality and 180-day ICH from the discharge date of index VTE hospitalization. Our objective was to evaluate whether the use of IVCF was associated with decreased 30-day mortality and reduction of 180-day ICH among patients with brain metastasis hospitalized for acute VTE. We compared the outcomes between patients with and without brain metastasis.
Patients and methods
Data sources
We used data from the California Cancer Registry (CCR),20 which is linked to the California Department of Health Care Access and Information database.21 Health Care Access and Information includes the Patient Discharge Dataset (PDD) and Emergency Department Utilization (EDU) data. CCR was established as a cancer surveillance system in 1988. Given that cancer registration is mandatory in California, CCR captures >99% of cancer diagnoses in the state.20 The CCR provides tumor information (eg, primary and subsequent cancers, tumor stage based on the American Joint Committee on Cancer), patient demographics (eg, age at diagnosis, sex, race/ethnicity, neighborhood socioeconomic status [nSES], and health insurance at diagnosis), and clinical characteristics (eg, the first course of treatment [chemotherapy, radiation, surgery, and hematopoietic cell transplantation], as well as the date of cancer diagnosis and last known vital status [alive or dead]). The nSES is a combined measure of 7 indicators of education, poverty, and unemployment rates at the census block group level, and has been extensively used in studies using CCR data.22
The PDD, established in 1991, captures all hospital discharges from nonfederal hospitals in California.21 The EDU database was established in 2005 and also captures clinical encounters from nonfederal hospitals. We used the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM), and Current Procedure Terminology codes to identify up to 25 diagnoses and up to 21 procedures during each hospital encounter. The PPD and EDU data sets include the dates associated with all procedure codes. Since 1996, all medical diagnoses in the PDD have a present-on-admission (POA) indicator,23 which allowed us to ascertain whether a diagnosis was POA (POA = yes) or developed during hospitalization (POA = no/unknown) and was previously validated.24
Study population
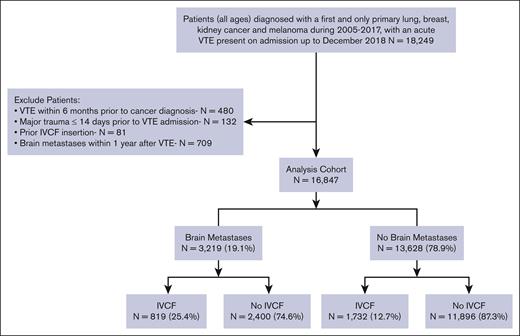
We identified all patients diagnosed with a first and only primary invasive melanoma, breast, lung, or kidney cancer, between 2005 and 2017, admitted to the hospital with an acute VTE event that was POA up to December 2018. We included the site and histology codes based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) (supplemental Table 1). Patients diagnosed with VTE within 6 months before their cancer diagnosis (n = 480), those with major trauma ≤14 days before their VTE admission (n = 132), patients who previously had an IVCF inserted (n = 81), and those with brain metastases ≤1 year after their VTE admission (n = 709) were excluded from the analysis. This last step was taken to ensure the brain metastases did not develop during our study period, which would have influenced the decision to place an IVCF (cohort diagram, Figure 1).
Identification of acute VTE hospitalization, IVCF, and brain metastases
Patients with the primary cancer type of interest who were hospitalized for acute PE and/or DVT POA (POA = yes) were identified using ICD-9-CM or ICD-10-CM codes (supplemental Table 2). We excluded patients with hospital-acquired VTE (POA = no/unknown), as it was not possible to ascertain the timing of IVCF insertion in relation to the VTE event. A prior history of VTE >6 months before cancer diagnosis included all ICD-9-CM or ICD-10-CM codes for acute VTE and additional other VTE not otherwise specified VTE, chronic VTE, and personal history VTE codes in any of the 25 diagnoses positions. Treating facilities’ characteristics for the VTE admission were recorded, such as facility type (hierarchical order: Kaiser Permanente, teaching, and private/others), facility location (urban vs rural), and facility size (0-99, 100-200, and ≥200 beds).
IVCF placement was determined using the ICD-9-CM, ICD-10-CM, or Current Procedure Terminology codes (supplemental Table 3). Patients with an IVCF placed after discharge from their index VTE event were classified as having no IVCF. Patients were classified as having brain metastases if they had an ICD-9-CM code 198.3, or ICD-10-CM code C79.31 present in the PDD or EDU either before or at the time of the index VTE admission.
Comorbidities and contraindications to anticoagulation
Comorbidities in the 2 years before cancer diagnosis were identified using the Elixhauser index, excluding the diagnosis of cancer.25 Comorbidities were classified as no preceding admission to the hospital (thus could not be ascertained), 0, 1 to 2, or ≥3 comorbidities. Similar to prior studies,15,17 we identified contraindications to anticoagulation using diagnoses or procedure codes noted either at or 14 days before the index VTE admission. These contraindications included coagulopathy, thrombocytopenia, aortic dissection, neurosurgical intervention in the brain or spine, orthopedic surgery, active bleeding, and/or inpatient transfusion (supplemental Table 4).
The primary outcomes for this study were 30-day mortality from the VTE admission date and ICH that occurred ≤180 days from the discharge date of the index VTE hospitalization. We identified hospitalizations for nontraumatic ICH using ICD-9-CM or ICD-10-CM codes present in the PDD or EDU (supplemental Table 5).
Statistical analysis
We used χ2 tests to compare the baseline characteristics of the patients with and without IVCF placement. To account for confounding by indication and immortal time bias, we used the propensity score methodology and time-dependent variables as follows.
Propensity score
We used logistic regression models to estimate each patient’s propensity for IVCF insertion with variables used in previous studies,15,17,26 which are likely associated with IVCF usage. These variables included sociodemographic characteristics (age at diagnosis, sex, race/ethnicity, health insurance, and nSES at diagnosis), hospital characteristics (type, location, and size), and clinical features (cancer site, stage at diagnosis, comorbidities, contraindications to anticoagulation, VTE location, and VTE year). Results are reported as odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Standardized mean difference was used to evaluate the effectiveness of the propensity models (supplemental Figure). Nearly all variables included in this study showed a standardized mean difference value of <10%, indicating a good balance of the study baseline covariates.27 The specific propensity methodology used varied according to the outcome of interest. For the analysis of 30-day mortality, we used Cox proportional hazards regression with inverse probability weighting (IPW) of the propensity score, whereas the propensity-matched conditional Cox proportional hazards regression model was used for the analysis of 180-day ICH.
Immortal time bias
Because the day of IVCF placement varies during hospitalization, patients are subject to immortal time bias. This means that patients who did not receive an IVCF may have died before receiving an IVCF, whereas all patients who had an IVCF inserted were alive at the time of the procedure. Therefore, they were “immortal” during the interval from the date of admission to the date of IVCF placement. To account for immortal time bias, IVCF insertion was used as a time-dependent variable in the IPW proportional hazard Cox model.28,29 In the propensity-matched analysis, patients who did not receive an IVCF had to be alive at the time the IVCF was placed in the matched case.
We tested for interactions to evaluate whether the association between IVCF placement and outcomes was modified by the presence or absence of brain metastases or active bleeding. Based on the results of previous studies15,17,26 and confirmation of a significant interaction between the use of IVCF and the presence of active bleeding, we stratified the 30-day mortality models by the presence or absence of active bleeding. For 180-day ICH, we used propensity matching at a ratio of 1:3 for patients who received and did not receive an IVCF using greedy matching with a 0.001 caliper. This approach allows the Fine-Gray methodology to adjust for the competing risk of death. The person time for all models was calculated from the index VTE admission until the outcome event (30-day mortality or 180-day ICH), date of death, last known alive, or end of the study (31 December 2020), whichever occurred first. There was no violation of the Cox proportional hazard assumption tested by the Schoenfeld residual test. All analyses were performed using SAS/STAT software Version 9.4 and 2-sided P values <.05 were considered statistically significant (including interaction terms). This study was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects and California Davis Institutional Review Boards.
Results
We identified 16 847 patients with invasive melanoma and breast, lung, or kidney cancer who were admitted to a nonfederal California hospital due to an acute VTE event between 2005 and 2017. Among these, 3219 patients (19.1%) had brain metastases. The median age at cancer diagnosis was 67 years (interquartile range [IQR], 58-76; Table 1). Most patients had lung cancer (57.1%), followed by breast cancer (26.7%), kidney cancer (10.8%), and melanoma (5.5%). The median follow-up time was 1.2 years (IQR, 0.4-2.8) for patients with brain metastases and 1.9 years (IQR, 0.4-6.2) for those without brain metastases. The median time from cancer diagnosis to acute VTE was 180 days for patients without brain metastases and 208 days for those with brain metastases.
Baseline characteristics of patients diagnosed with melanoma, kidney, breast, or lung cancer, and a VTE event on hospital admission, California, 2005-2017
| Variables . | All . | IVCF . | NO IVCF . | P value∗ . | |||
|---|---|---|---|---|---|---|---|
| N . | % . | n . | % . | n . | % . | ||
| All | 16 847 | 100.0% | 2551 | 100.0% | 14 296 | 100.0% | . |
| Sex | |||||||
| Male | 6 548 | 38.9% | 1140 | 44.7% | 5 408 | 37.8% | <.0001 |
| Female | 10 295 | 61.1% | 1411 | 55.3% | 8 884 | 62.1% | <.0001 |
| Race/Ethnicity | |||||||
| Non-Hispanic White | 11 100 | 65.9% | 1745 | 68.4% | 9 355 | 65.4% | .0036 |
| Non-Hispanic Black/African American | 1 720 | 10.2% | 227 | 8.9% | 1 493 | 10.4% | .0176 |
| Hispanic | 2 438 | 14.5% | 360 | 14.1% | 2 078 | 14.5% | .5755 |
| Asian/Pacific Islander | 1 450 | 8.6% | 208 | 8.2% | 1 242 | 8.7% | .3756 |
| Other/unknown | 139 | 0.8% | 11 | 0.4% | 128 | 0.9% | .017 |
| Age at cancer diagnosis, median (IQR) | 67 (58-76) | 67 (58-76) | 67 (58-76) | ||||
| Age at VTE diagnosis, median (IQR) | 68 (59-77) | 68 (59-77) | 68 (59-77) | ||||
| Insurance type at diagnosis | |||||||
| No insurance | 225 | 1.3% | 35 | 1.4% | 190 | 1.3% | .8617 |
| Private/military insurance | 7 010 | 41.6% | 1070 | 41.9% | 5 940 | 41.6% | .7098 |
| Medicaid/government | 1 863 | 11.1% | 244 | 9.6% | 1 619 | 11.3% | .009 |
| Medicare | 7 287 | 43.3% | 1132 | 44.4% | 6 155 | 43.1% | .2148 |
| Unknown insurance | 462 | 2.7% | 70 | 2.7% | 392 | 2.7% | .9955 |
| nSES | |||||||
| Low SES | 9 379 | 55.7% | 1335 | 52.3% | 8 044 | 56.3% | .0002 |
| High SES | 6 876 | 40.8% | 1132 | 44.4% | 5 744 | 40.2% | <.0001 |
| SES unknown | 592 | 3.5% | 84 | 3.3% | 508 | 3.6% | .5102 |
| Cancer site | |||||||
| Lung | 9 619 | 57.1% | 1584 | 62.1% | 8 035 | 56.2% | <.0001 |
| Breast | 4 491 | 26.7% | 512 | 20.1% | 3 979 | 27.8% | <.0001 |
| Melanoma | 921 | 5.5% | 187 | 7.3% | 734 | 5.1% | <.0001 |
| Kidney | 1 816 | 10.8% | 268 | 10.5% | 1 548 | 10.8% | .6285 |
| Stage at diagnosis | |||||||
| I | 3 210 | 19.1% | 398 | 15.6% | 2 812 | 19.7% | <.0001 |
| II | 2 129 | 12.6% | 258 | 10.1% | 1 871 | 13.1% | <.0001 |
| III | 3 350 | 19.9% | 455 | 17.8% | 2 895 | 20.3% | .0049 |
| IV | 7 229 | 42.9% | 1302 | 51.0% | 5 927 | 41.5% | <.0001 |
| Unknown | 929 | 5.5% | 138 | 5.4% | 791 | 5.5% | .8014 |
| Comorbidities† | |||||||
| No admission to the hospital | 8 529 | 50.6% | 1150 | 45.1% | 7 379 | 51.6% | <.0001 |
| 0 comorbidities | 841 | 5.0% | 148 | 5.8% | 693 | 4.8% | .0415 |
| 1-2 comorbidities | 2 846 | 16.9% | 489 | 19.2% | 2 357 | 16.5% | .0009 |
| ≥3 comorbidities | 4 631 | 27.5% | 764 | 29.9% | 3 867 | 27.0% | .0025 |
| Metastases to the brain | |||||||
| Yes | 3 219 | 19.1% | 819 | 32.1% | 2 400 | 16.8% | <.0001 |
| No | 13 628 | 80.9 | 1732 | 67.9% | 11 896 | 87.3% | <.0001 |
| Contraindication to anticoagulation‡ | |||||||
| Coagulopathy | 92 | 0.5% | 26 | 1.0% | 66 | 0.5% | .0004 |
| Thrombocytopenia | 466 | 2.8% | 101 | 4.0% | 365 | 2.6% | <.0001 |
| Aortic dissection | 72 | 0.4% | 10 | 0.4% | 62 | 0.4% | .7662 |
| Brain surgery | 109 | 0.6% | 61 | 2.4% | 48 | 0.3% | <.0001 |
| Spine surgery | 134 | 0.8% | 60 | 2.4% | 74 | 0.5% | <.0001 |
| Orthopedic surgery | 92 | 0.5% | 28 | 1.1% | 64 | 0.4% | <.0001 |
| Inpatient transfusion | 2 848 | 16.9% | 766 | 30.0% | 2 082 | 14.6% | <.0001 |
| Bleeding | 1 358 | 8.1% | 441 | 17.3% | 917 | 6.4% | <.0001 |
| VTE location | |||||||
| PE Only | 9 118 | 54.1% | 802 | 31.4% | 8 316 | 58.2% | <.0001 |
| PE + DVT | 2 754 | 16.3% | 761 | 29.8% | 1 993 | 13.9% | <.0001 |
| Proximal DVT | 2 811 | 16.7% | 639 | 25.0% | 2 172 | 15.2% | <.0001 |
| Distal DVT | 1 424 | 8.5% | 260 | 10.2% | 1 164 | 8.1% | .0006 |
| Lower extremity DVT, NOS | 740 | 4.4% | 89 | 3.5% | 651 | 4.6% | .0156 |
| Prior history of VTE | 805 | 4.8% | 117 | 4.6% | 688 | 4.8% | .6219 |
| Year of VTE discharge | |||||||
| 2005-2008 | 3 564 | 21.2% | 678 | 26.6% | 2 886 | 20.2% | <.0001 |
| 2009-2012 | 5 034 | 29.9% | 908 | 35.6% | 4 126 | 28.9% | <.0001 |
| 2013-2018 | 8 249 | 49.0% | 965 | 37.8% | 7 284 | 51.0% | <.0001 |
| VTE facility type | |||||||
| Kaiser | 2 265 | 13.4% | 228 | 8.9% | 2 037 | 14.2% | <.0001 |
| Teaching | 3 010 | 17.9% | 451 | 17.7% | 2 559 | 17.9% | .7886 |
| Private/other | 11 232 | 66.7% | 1816 | 71.2% | 9 416 | 65.9% | <.0001 |
| Unknown | 340 | 2.0% | 56 | 2.2% | 284 | 2.0% | .49 |
| VTE facility location | |||||||
| Rural | 588 | 3.5% | 29 | 1.1% | 559 | 3.9% | <.0001 |
| Urban | 15 843 | 94.0% | 2458 | 96.4% | 13 385 | 93.6% | <.0001 |
| Unknown | 416 | 2.5% | 64 | 2.5% | 352 | 2.5% | .8889 |
| VTE hospital size | |||||||
| 0-99 beds | 668 | 4.0% | 44 | 1.7% | 624 | 4.4% | <.0001 |
| 100-199 beds | 2 704 | 16.1% | 343 | 13.4% | 2 361 | 16.5% | .0001 |
| ≥200 beds | 12 981 | 77.1% | 2085 | 81.7% | 10 896 | 76.2% | <.0001 |
| Unknown | 494 | 2.9% | 79 | 3.1% | 415 | 2.9% | .5928 |
| Variables . | All . | IVCF . | NO IVCF . | P value∗ . | |||
|---|---|---|---|---|---|---|---|
| N . | % . | n . | % . | n . | % . | ||
| All | 16 847 | 100.0% | 2551 | 100.0% | 14 296 | 100.0% | . |
| Sex | |||||||
| Male | 6 548 | 38.9% | 1140 | 44.7% | 5 408 | 37.8% | <.0001 |
| Female | 10 295 | 61.1% | 1411 | 55.3% | 8 884 | 62.1% | <.0001 |
| Race/Ethnicity | |||||||
| Non-Hispanic White | 11 100 | 65.9% | 1745 | 68.4% | 9 355 | 65.4% | .0036 |
| Non-Hispanic Black/African American | 1 720 | 10.2% | 227 | 8.9% | 1 493 | 10.4% | .0176 |
| Hispanic | 2 438 | 14.5% | 360 | 14.1% | 2 078 | 14.5% | .5755 |
| Asian/Pacific Islander | 1 450 | 8.6% | 208 | 8.2% | 1 242 | 8.7% | .3756 |
| Other/unknown | 139 | 0.8% | 11 | 0.4% | 128 | 0.9% | .017 |
| Age at cancer diagnosis, median (IQR) | 67 (58-76) | 67 (58-76) | 67 (58-76) | ||||
| Age at VTE diagnosis, median (IQR) | 68 (59-77) | 68 (59-77) | 68 (59-77) | ||||
| Insurance type at diagnosis | |||||||
| No insurance | 225 | 1.3% | 35 | 1.4% | 190 | 1.3% | .8617 |
| Private/military insurance | 7 010 | 41.6% | 1070 | 41.9% | 5 940 | 41.6% | .7098 |
| Medicaid/government | 1 863 | 11.1% | 244 | 9.6% | 1 619 | 11.3% | .009 |
| Medicare | 7 287 | 43.3% | 1132 | 44.4% | 6 155 | 43.1% | .2148 |
| Unknown insurance | 462 | 2.7% | 70 | 2.7% | 392 | 2.7% | .9955 |
| nSES | |||||||
| Low SES | 9 379 | 55.7% | 1335 | 52.3% | 8 044 | 56.3% | .0002 |
| High SES | 6 876 | 40.8% | 1132 | 44.4% | 5 744 | 40.2% | <.0001 |
| SES unknown | 592 | 3.5% | 84 | 3.3% | 508 | 3.6% | .5102 |
| Cancer site | |||||||
| Lung | 9 619 | 57.1% | 1584 | 62.1% | 8 035 | 56.2% | <.0001 |
| Breast | 4 491 | 26.7% | 512 | 20.1% | 3 979 | 27.8% | <.0001 |
| Melanoma | 921 | 5.5% | 187 | 7.3% | 734 | 5.1% | <.0001 |
| Kidney | 1 816 | 10.8% | 268 | 10.5% | 1 548 | 10.8% | .6285 |
| Stage at diagnosis | |||||||
| I | 3 210 | 19.1% | 398 | 15.6% | 2 812 | 19.7% | <.0001 |
| II | 2 129 | 12.6% | 258 | 10.1% | 1 871 | 13.1% | <.0001 |
| III | 3 350 | 19.9% | 455 | 17.8% | 2 895 | 20.3% | .0049 |
| IV | 7 229 | 42.9% | 1302 | 51.0% | 5 927 | 41.5% | <.0001 |
| Unknown | 929 | 5.5% | 138 | 5.4% | 791 | 5.5% | .8014 |
| Comorbidities† | |||||||
| No admission to the hospital | 8 529 | 50.6% | 1150 | 45.1% | 7 379 | 51.6% | <.0001 |
| 0 comorbidities | 841 | 5.0% | 148 | 5.8% | 693 | 4.8% | .0415 |
| 1-2 comorbidities | 2 846 | 16.9% | 489 | 19.2% | 2 357 | 16.5% | .0009 |
| ≥3 comorbidities | 4 631 | 27.5% | 764 | 29.9% | 3 867 | 27.0% | .0025 |
| Metastases to the brain | |||||||
| Yes | 3 219 | 19.1% | 819 | 32.1% | 2 400 | 16.8% | <.0001 |
| No | 13 628 | 80.9 | 1732 | 67.9% | 11 896 | 87.3% | <.0001 |
| Contraindication to anticoagulation‡ | |||||||
| Coagulopathy | 92 | 0.5% | 26 | 1.0% | 66 | 0.5% | .0004 |
| Thrombocytopenia | 466 | 2.8% | 101 | 4.0% | 365 | 2.6% | <.0001 |
| Aortic dissection | 72 | 0.4% | 10 | 0.4% | 62 | 0.4% | .7662 |
| Brain surgery | 109 | 0.6% | 61 | 2.4% | 48 | 0.3% | <.0001 |
| Spine surgery | 134 | 0.8% | 60 | 2.4% | 74 | 0.5% | <.0001 |
| Orthopedic surgery | 92 | 0.5% | 28 | 1.1% | 64 | 0.4% | <.0001 |
| Inpatient transfusion | 2 848 | 16.9% | 766 | 30.0% | 2 082 | 14.6% | <.0001 |
| Bleeding | 1 358 | 8.1% | 441 | 17.3% | 917 | 6.4% | <.0001 |
| VTE location | |||||||
| PE Only | 9 118 | 54.1% | 802 | 31.4% | 8 316 | 58.2% | <.0001 |
| PE + DVT | 2 754 | 16.3% | 761 | 29.8% | 1 993 | 13.9% | <.0001 |
| Proximal DVT | 2 811 | 16.7% | 639 | 25.0% | 2 172 | 15.2% | <.0001 |
| Distal DVT | 1 424 | 8.5% | 260 | 10.2% | 1 164 | 8.1% | .0006 |
| Lower extremity DVT, NOS | 740 | 4.4% | 89 | 3.5% | 651 | 4.6% | .0156 |
| Prior history of VTE | 805 | 4.8% | 117 | 4.6% | 688 | 4.8% | .6219 |
| Year of VTE discharge | |||||||
| 2005-2008 | 3 564 | 21.2% | 678 | 26.6% | 2 886 | 20.2% | <.0001 |
| 2009-2012 | 5 034 | 29.9% | 908 | 35.6% | 4 126 | 28.9% | <.0001 |
| 2013-2018 | 8 249 | 49.0% | 965 | 37.8% | 7 284 | 51.0% | <.0001 |
| VTE facility type | |||||||
| Kaiser | 2 265 | 13.4% | 228 | 8.9% | 2 037 | 14.2% | <.0001 |
| Teaching | 3 010 | 17.9% | 451 | 17.7% | 2 559 | 17.9% | .7886 |
| Private/other | 11 232 | 66.7% | 1816 | 71.2% | 9 416 | 65.9% | <.0001 |
| Unknown | 340 | 2.0% | 56 | 2.2% | 284 | 2.0% | .49 |
| VTE facility location | |||||||
| Rural | 588 | 3.5% | 29 | 1.1% | 559 | 3.9% | <.0001 |
| Urban | 15 843 | 94.0% | 2458 | 96.4% | 13 385 | 93.6% | <.0001 |
| Unknown | 416 | 2.5% | 64 | 2.5% | 352 | 2.5% | .8889 |
| VTE hospital size | |||||||
| 0-99 beds | 668 | 4.0% | 44 | 1.7% | 624 | 4.4% | <.0001 |
| 100-199 beds | 2 704 | 16.1% | 343 | 13.4% | 2 361 | 16.5% | .0001 |
| ≥200 beds | 12 981 | 77.1% | 2085 | 81.7% | 10 896 | 76.2% | <.0001 |
| Unknown | 494 | 2.9% | 79 | 3.1% | 415 | 2.9% | .5928 |
NOS, not otherwise specified; SES, socioeconomic status.
P value: bivariate χ2 test
Elixhauser index assessed ≤2 years before cancer diagnosis
All contraindications included ≤14 days of VTE admission
Seventy-four percent of brain metastases were first coded within 3 months before the acute VTE event, with 39% being reported during the same hospital admission.
IVCF placement
The use of IVCF was more common among patients with vs without brain metastasis (25.4% vs 12.7%; Figure 1) and in patients with ≥1 contraindication(s) to anticoagulation at VTE admission than in those with no contraindications (26.4% vs 11.5%). Over time, there was a decreased use of IVCF, with the highest IVCF placement during 2005 to 2008 (19.0%) compared with 2013 to 2018 (11.7%). Among the patients with brain metastasis, the use of IVCF decreased from 32.3% in 2005 to 2008 to 19.9% in 2013 to 2018.
On multivariable logistic analysis used to create the propensity score, the presence of brain metastases was an independent predictor of IVCF placement (OR, 2.24; 95% CI, 2.01-2.50; Figure 2). Compared with patients with kidney cancer, those with melanoma were more likely to receive IVCF (OR, 1.40; 95% CI, 1.11-1.77), with no difference among patients with lung or breast cancer. Higher IVCF use was observed among males, patients who underwent major surgeries, who had PE plus DVT or DVT only (vs PE only), who received treatment at teaching or private/other hospitals (vs Kaiser Permanente) and large hospitals (≥200 beds), who had a major trauma, bleeding, and inpatient transfusion at baseline. Patients diagnosed with a VTE in the more recent period (2013-2018) were less likely to have an IVCF inserted than those diagnosed from 2005 to 2008 (OR, 0.53; 95% CI, 0.47-0.60).
Factors associated with IVCF use among patients with melanoma, kidney, breast, or lung cancers and a VTE event at hospital admission, California, 2005 to 2017. ^Contraindication to anticoagulation. The reference category corresponds to patients with no contraindications. The model was also adjusted for VTE age, prior VTE, sex, race/ethnicity, insurance, stage at diagnosis, and comorbidities at cancer diagnosis. LE, lower extremity; NOS, not otherwise specified.
Factors associated with IVCF use among patients with melanoma, kidney, breast, or lung cancers and a VTE event at hospital admission, California, 2005 to 2017. ^Contraindication to anticoagulation. The reference category corresponds to patients with no contraindications. The model was also adjusted for VTE age, prior VTE, sex, race/ethnicity, insurance, stage at diagnosis, and comorbidities at cancer diagnosis. LE, lower extremity; NOS, not otherwise specified.
Outcomes
Thirty-day mortality
Death within 30 days from the index VTE admission occurred in 1198 (37.2%) patients with metastatic brain tumors and 3376 (24.8%) patients without brain metastases. In the IPW multivariable analysis, after adjusting for sociodemographic and clinical factors, we found an interaction between IVCF placement and active bleeding (P = .0006), therefore we stratified the analysis according to the presence or absence of bleeding. Among patients with active bleeding, IVCF use was associated with reduced 30-day mortality (hazard ratio [HR], 0.53; 95% CI, 0.42-0.68; Table 2). The hazard of 30-day mortality was not different among patients with and without brain metastases (HR, 1.27; 95% CI, 0.98-1.65). In patients with no active bleeding, there was an interaction between IVCF and brain metastases (P = .0318), thus we stratified the model according to the presence or absence of brain metastasis. We found a nearly 30% reduction in 30-day mortality among patients with brain metastasis and no active bleeding who received an IVCF (HR, 0.72; 95% CI, 0.60-0.85), whereas there was no difference in 30-day mortality among patients without brain metastasis who received an IVCF (HR, 0.91; 95% CI, 0.80-1.05).
Association of IVCF usage and brain metastases with 30-day mortality in patients diagnosed with melanoma, kidney, breast, or lung cancers and a VTE by the presence or absence of active bleeding
| Variable∗ . | HR (95% CI) . |
|---|---|
| Active bleeding†(no interaction between IVCF and brain metastasis) | |
| Brain metastases | |
| Yes | 1.27 (0.98-1.65) |
| No | Reference |
| IVCF | |
| Yes | 0.53 (0.42-0.68) |
| No | Reference |
| No active bleeding‡(interaction between IVCF & brain metastasis) | |
| Brain metastases | |
| IVCF | |
| Yes | 0.72 (0.60-0.85) |
| No | Reference |
| No brain metastases | |
| IVCF | |
| Yes | 0.91 (0.80-1.05) |
| No | Reference |
| Variable∗ . | HR (95% CI) . |
|---|---|
| Active bleeding†(no interaction between IVCF and brain metastasis) | |
| Brain metastases | |
| Yes | 1.27 (0.98-1.65) |
| No | Reference |
| IVCF | |
| Yes | 0.53 (0.42-0.68) |
| No | Reference |
| No active bleeding‡(interaction between IVCF & brain metastasis) | |
| Brain metastases | |
| IVCF | |
| Yes | 0.72 (0.60-0.85) |
| No | Reference |
| No brain metastases | |
| IVCF | |
| Yes | 0.91 (0.80-1.05) |
| No | Reference |
Inverse propensity-weighted Cox proportional hazard regression models were used. IVCF was included as time-dependent covariate. All models were also adjusted for age, year, and location of VTE; prior history of VTE; sex; race/ethnicity; health insurance; neighborhood socioeconomic status; cancer site; stage at diagnosis; comorbidities; and each contraindication for anticoagulation (in the active bleeding model, aortic dissection and brain, orthopedic, and spine surgeries were consolidated into 1 variable).
There was an interaction between IVCF and active bleeding (P = .0006) warranting stratified models.
Active bleeding: there was no interaction between IVCF and brain metastases (P = .7046).
No active bleeding: there was an interaction between IVCF and brain metastases (P = .0318).
One hundred eighty–day intracranial hemorrhage
During follow-up, 145 (0.9%) patients experienced ICH within 180 days of the discharge date of VTE hospitalization. Among those, 86 patients (59.3%) had brain metastases. In the propensity-matched conditional Cox proportional hazard regression model, the presence of brain metastases was associated with an increased hazard of 180-day ICH (overall model, HR, 5.14; 95% CI, 2.99-8.83; Table 3). There was no association between IVCF placement and risk of ICH (HR, 1.20; 95% CI, 0.77-1.88). There was no interaction between IVCF and brain metastasis. (P = .9535).
Association of IVCF and brain metastases with 180-day ICH among patients diagnosed with melanoma, kidney, breast, or lung cancers and a VTE event by the cancer bleeding risk
| Variable . | HR (95% CI) . |
|---|---|
| Overall cohort∗ | |
| Brain metastases | |
| Yes | 5.14 (2.99-8.83) |
| No | Reference |
| IVCF | |
| Yes | 1.20 (0.77-1.88) |
| No | Reference |
| Variable . | HR (95% CI) . |
|---|---|
| Overall cohort∗ | |
| Brain metastases | |
| Yes | 5.14 (2.99-8.83) |
| No | Reference |
| IVCF | |
| Yes | 1.20 (0.77-1.88) |
| No | Reference |
There was no interaction between IVCF and brain metastases (P = .9535). A propensity-matched conditional Cox proportional hazard regression model was used. The model was adjusted for the year of VTE diagnosis, sex, race/ethnicity, and active bleeding ≤14 days before the index VTE admission date.
Discussion
The management of VTE in patients with brain metastases poses significant challenges for clinicians, who must decide whether to administer anticoagulation or place an IVCF in these patients. Individuals with brain metastases are either entirely excluded30 or underrepresented31,32 in randomized controlled trials of anticoagulation for cancer-associated VTE. Owing to the paucity of prospective or randomized data, clinicians are limited to observational data to help guide treatment decisions.9 In this study, we confirmed that brain metastasis is a strong predictor of ICH and that patients with brain metastasis and acute VTE are more likely to receive IVCF. Unlike our previous study,17 we found that in patients with cancer and VTE, the use of IVCF was associated with a notable decrease in 30-day mortality. This finding was more pronounced among patients with active bleeding, regardless of the presence or absence of brain metastasis, but also relevant among those without active bleeding with brain metastasis. Although our study confirmed an association of the presence of brain metastasis with increased hazard of ICH, we did not find any association between IVCF and ICH at 180 days.
The variation in clinical practice is best reflected by the nearly 25-fold increased use of IVCF in the United States compared with a similar cohort of patients in Europe.33 In our large population-based analysis, there was a higher use of IVCF in patients with cancer and brain metastases who were hospitalized for acute VTE than in those without brain metastases. The likely reason for this is clinician discomfort in giving therapeutic anticoagulation in patients with brain metastases. However, despite the lack of prospective randomized studies addressing this question, observational studies and systematic reviews suggest therapeutic anticoagulation is not associated with clinically relevant increased ICH in patients with brain metastases and VTE.2,8-10
The potential harms of IVCF use, including device migration, fracture, embolization, and poor retrieval rates, have been previously described.34,35 These adverse events were the driving force behind the 2010 US Food and Drug Administration safety advisory,36 which was updated in 2014.37 Our study is consistent with prior findings, which have demonstrated a trend toward reduced IVCF usage since the 2010 US Food and Drug Administration advisory38 and suggest some success in educational efforts to decrease inappropriate use of IVCF.
Notably, we found that IVCF placement was associated with decreased 30-day mortality. The reduced mortality was more pronounced among patients with active bleeding but was also observed among patients with no active bleeding or brain metastasis. In our prior analysis of patients diagnosed with cancer and VTE between 2005 and 2009 in California, we showed no improvement in short-term mortality with IVCF insertion, with an increased risk of recurrent DVT.17 This previous study included nearly all cancer types, and the significant heterogeneity of treatment effects of IVCF across different cancer populations may explain why our prior findings differ from the current study. We chose lung and breast cancers because they are common; therefore, clinicians will often see these patients with VTE and brain metastases. We selected kidney cancer and melanoma because of their predisposition to bleeding. This study illustrates the importance of examining the interactions between relevant variables and stratifying the analysis in regression models when interactions are found.
Identifying patients with cancer who might benefit from the placement of an IVCF is crucial.7 A prior study, which used inpatient and emergency department data from California, Florida, and New York, showed increased short-term mortality risk associated with IVCF placement in patients with a contraindication to anticoagulation.15 Conversely, a population-based study using data from California and Florida showed improved PE-free survival associated with IVCF placement in patients with cancer and DVT and suggested that the use of IVCF in carefully selected patients with cancer may improve outcomes.18 These diverse findings and conclusions can be attributed, in part, to the substantial heterogeneity in study populations, outcomes, and the analytic methods used across various studies. For instance, some studies have failed to account for immortal time bias.39 This underscores the need for careful consideration of these factors when interpreting literature.
Our results confirmed that the presence of brain metastases is a strong predictor of ICH, with the risk of ICH being more than fivefold higher than that in patients without brain metastasis. However, the use of IVCF was not associated with ICH among patients with selected cancers or VTE, regardless of the presence or absence of brain metastasis. Therefore, if the purpose of placing an IVCF is to reduce the risk of ICH, our findings do not support its use. A retrospective single-center matched cohort analysis in the United States demonstrated a 19% 1-year cumulative incidence of ICH in patients with brain metastases and VTE.2 Interestingly, the use of enoxaparin did not seem to further increase the risk of ICH in these patients. This study used a blinded review of all neuroimaging of the patients included in the analysis, which ensured uniform ascertainment of ICH. Conversely, a recent US nationwide study examined patterns of ICH among patients hospitalized with brain metastasis and observed that patients diagnosed with primary melanoma or kidney cancer and those with long-term anticoagulation had a higher risk of ICH.40 This highlights the need for carefully weighing the risks and benefits of anticoagulation in these high-risk patients.
Our study had several limitations. First, there is a potential risk of misclassification of brain metastasis in the administrative health claims data.41 Second, the absence of data on anticoagulation is a limitation of the PDD and EDU databases and may confound the interpretation of our results. However, as in our previous studies,17,26,42 we accounted for the presence of active bleeding and other contraindications for anticoagulation (eg, thrombocytopenia, aortic dissection, brain, major orthopedic, or spine surgeries). We propose that these factors serve as proxies for refraining from anticoagulation treatment in high-risk patients. Additionally, for 30-day mortality, we are not aware of strong evidence suggesting the influence of anticoagulation on short-term mortality in patients with cancer. Thus, the lack of anticoagulation data should not significantly confound the interpretation of our findings on mortality. Third, we did not have data on patients treated exclusively in the outpatient setting, which could have underestimated the incidence of VTE in our cohort. Studies, including clinical trials and population-based data, suggest that 70% to 98% of patients diagnosed with PE are admitted to the hospital for treatment.43-46 However, because all cases in our study were admitted to a hospital, they likely represent a sicker cohort of patients than the overall population of those with cancer-associated thrombosis. Lastly, although our propensity model for the placement of IVCF effectively balanced baseline covariates, there is likely residual confounding due to unmeasured covariates. The strengths of our study include a very large cohort of patients from a high-quality cancer registry linked to hospitalization and emergency department data sources as well as the application of robust statistical methods that accounted for immortal time bias and confounding by indication.
Conclusion
With ongoing improvements in the management of patients with cancer, the prevalence of patients surviving with brain metastases is expected to rise. A more evidence-based management strategy for VTE in these patients is greatly needed. Our study suggests a potential role for IVCF in the acute management of VTE in some patients with cancer, especially those with active bleeding, regardless of the presence of brain metastases, and in patients without active bleeding with brain metastases. However, there was no evidence of an association between IVCF use and a reduction in the 180-day risk of ICH. Future randomized controlled studies should focus on assessing the role of IVCF in patients with brain metastases and VTE.
Acknowledgments
This study was supported by the National Cancer Institute (NCI) (award number National Institutes of Health 5T32HL007149 [V.K.]). The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885, Centers for Disease Control and Prevention’s National Program of Cancer Registries under cooperative agreement 5NU58DP006344, the NCI’s Surveillance, Epidemiology and End Results (SEER) Program under contract HHSN261201800032I (awarded to the University of California, San Francisco), contract HHSN261201800015I (awarded to the University of Southern California), and contract HHSN261201800009I (awarded to the Public Health Institute).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, State of California, Department of Public Health, the NCI, the CDC, or their contractors and subcontractors.
Authorship
Contribution: V.K., A.B., and T.W. contributed to the concept and design of the study; A.B., T.W., and R.A. contributed to the acquisition and analysis of the data; all authors contributed to the drafting of the manuscript and provided final approval.
Conflict-of-interest disclosure: V.K. received honoraria from STAGO. N.S.K. reports consultancy from uniQure, BioMarin, and Pfizer; received research funding from the National Heart, Lung, and Blood Institute; and served as the chair of the grants committee member at Novo Nordisk. T.W. was a study steering committee member for Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Renata Abrahão, Division of Hematology and Oncology, University of California Davis Comprehensive Cancer Center, 4501 X St, Suite 3016, Sacramento, CA 95817; email: rabrahao@ucdavis.edu.
References
Author notes
Presented orally at the International Society on Thrombosis and Haemostasis, Montreal, Canada, 24 to 28 June 2023.
For deidentified data requests, please contact author Ted Wun (twun@ucdavis.edu).
The full-text version of this article contains a data supplement.