Key Points
SVEP-1 emerges as a potential mTOR activator in iMCD-TAFRO.
iMCD-TAFRO is characterized by coagulopathy, hypofibrinolysis, complement activation, endotheliopathy and immunothrombosis.
Visual Abstract
Idiopathic multicentric Castleman disease (iMCD) is an inflammatory disease associated with a cytokine storm, activation of the PI3K/AKT/mTOR pathway, coagulopathy, and increased risk of thrombosis. The mechanisms underlying these pathologic processes remain elusive. We studied novel markers of mTOR activation and thrombosis in 1 patient with typical features of iMCD with TAFRO (thrombocytopenia, anasarca, fevers, reticulin myelofibrosis, and organomegaly) syndrome (iMCD-TAFRO). Plasma levels of SVEP1 (Sushi, von Willebrand factor type A, epidermal growth factor, and pentraxin domain–containing 1 protein), a newly identified mTOR activator associated with cardiovascular diseases and dementia, in addition to cytokines, chemokines and components of the coagulation cascade and complement system were evaluated by enzyme-linked immunosorbent assay (ELISA) and arrays. Compared with healthy controls, a 15-fold increase in SVEP1 was observed. High levels of factor VIIa/antithrombin and microparticles expressing functional tissue factor (TF) were detected. The anticoagulants thrombomodulin and soluble endothelial protein C receptor were elevated, indicating shedding from endothelial cells. Plasminogen activator inhibitor 1 was increased, consistent with hypofibrinolysis, whereas high levels of C3b and C5a are in keeping with complement activation. Furthermore, markers of endothelial cell activation (e.g. von Willebrand factor, angiopoietin-2), cell adhesion molecules, and angiogenesis mediators were upregulated. SVEP1 emerges as a potential mechanism of mTOR activation in iMCD-TAFRO, while multiple pathways influence coagulopathy. Immunothrombosis emerges as a potential therapeutic target for iMCD.
Introduction
Castleman disease (CD) is a heterogeneous group of disorders that share morphological features. It is classified into unicentric CD and multicentric CD (MCD). Unicentric CD involves a solitary enlarged lymph node, and patients typically have mild symptoms; excision surgery is often curative. MCD involves multiple regions of enlarged lymph nodes, constitutional symptoms, and organ dysfunction due to a cytokine storm including elevated interleukin 6 (IL-6).1-3 MCD is further divided into human herpesvirus 8 (HHV-8)–associated MCD, which occurs in individuals who are immunocompromised, and HHV-8-negative/idiopathic MCD (iMCD). A subtype of iMCD with TAFRO (thrombocytopenia, anasarca, fevers, reticulin myelofibrosis, and organomegaly) syndrome (iMCD-TAFRO) affects patients who present with normal or only slightly elevated immunoglobulin levels. iMCD can also present in association with POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes) syndrome.1-3 Patients with iMCD frequently undergo multiple biopsies, several admissions for life-threatening conditions, and often receive inadequate treatment because of the lack of a clear diagnosis.1 iMCD is also accompanied by coagulopathy and increased incidence of thrombosis.4 More recently, studies identified phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR), Janus kinase/signal transducer and activator of transcription (JAK/STAT), and type I interferon (IFN) pathways as possible drivers in iMCD.1-7 However, the mechanisms of mTOR activation and thrombosis in iMCD remain incompletely understood. Understanding these mechanisms is particularly important given the high morbidity and mortality of these patients.1-7
In this report, we have studied novel markers of mTOR activation and coagulopathy/thrombosis in 1 patient who met criteria for iMCD-TAFRO.8,9 We investigated the plasma level of SVEP1 (Sushi, von Willebrand factor type A, epidermal growth factor [EGF], and pentraxin domain–containing 1 protein), a large extracellular protein found in plasma10 and recently identified as a ligand for the orphan receptor platelet and endothelial aggregation receptor 1 (PEAR1).11 This interaction promotes PEAR1 phosphorylation and disease associated PI3K/AKT/mTOR signaling in vascular cells and platelets. Additionally, SVEP1 binds to Tie-1, which modulates the function of endothelial cells and lymphangiogenesis.12 Furthermore, SVEP1 regulates monocyte recruitment and differentiation phenotypes through an integrin α4β1/α9β1 dependent mechanism.13 SVEP1 has other functions, and recent studies have shown that elevated plasma levels are associated with vascular disease and dementia.14,15
Given the patient’s coagulopathy and thrombotic vasculopathy, we studied markers of immunothrombosis, which is the bidirectional interaction between innate immunity and coagulation. This process involves multiple cell types and proteolytic reactions that mediate numerous aspects of thromboinflammatory processes.16-20 Accordingly, we used arrays and enzyme-linked immunosorbent assay (ELISA) to evaluate cytokine levels, endotheliopathy, coagulation and complement activation, and fibrinolysis. The findings suggest that immunothrombosis plays a role in iMCD-TAFRO pathogenesis, and it emerges as a potential therapeutic target in addition to currently available immunomodulators.
Study design
Blood samples were collected into Vacutainer tubes (BD, Franklin Lakes, NJ) containing sodium citrate (3.2%), as previously described.21 Plasma was collected from controls (n = 20), during the patient’s flare (coinciding with a peak in C-reactive protein [CRP], ferritin, and creatinine), after 4 days of treatment (1 dose rituximab and steroids), and during remission 6 months later when clinical symptoms subsided and inflammatory markers normalized. Tests for all hematologic parameters, prothrombin time (PT), activated partial thromboplastin time (aPTT), D-dimer, and fibrinogen were performed with a Siemens CS-5100 (Siemens, Malvern, PA) at the Hematology Core Laboratory at Johns Hopkins Hospital (JHH). Routine chemistry analytes were performed at the Clinical Chemistry Laboratory at JHH. Routine tests for autoantibodies and infectious agents were performed at the Clinical Immunology Laboratory and Microbiology Laboratory at JHH, respectively. Routine serum IL-6, sIL-2Rα (CD25), and vascular endothelial growth factor (VEGF) were sent to Quest Diagnostics (Chantilly, VA).
DuoSet ELISA kits for human soluble endothelial protein C receptor (DY2245), plasminogen activation inhibitor 1 (PAI-1, DY1786), and thrombomodulin (DY3947) and the human sIL-2Rα/CD25 Quantikine ELISA Kit were from R&D (Minneapolis, MN). Asserachrom factor VIIa/antithrombin (FVIIa/AT) complex was from Stago (Asnières sur Seine, France). Zymogen microparticle tissue factor (MP-TF) was from Hyphen Biomed/Aniara Diagnostica (West Chester, OH). ELISA for citrullinated histone H3 was from Cayman Chemical (Ann Arbor, MI). ELISA for SVEP1 (MBS015992) was from MyBioSource (San Diego, CA). ELISA was performed according to the manufacturers’ instructions and with a Synergy HTX multimode microplate reader, interfaced with Gen5 2.09 Software (BioTek Instruments, Winooski, VT), as previously reported.21 Ixolaris was purified as described.22
Array 1 is a clinical-grade electrochemiluminescence assay (V-PLEX Human Biomarkers), performed as described.23 Briefly, precoated electrochemiluminescence assay plates were incubated with a blocking solution. Plates were then washed and incubated with diluted plasma samples and/or calibrators. Bound analytes were detected using sulfo-TAG detection antibodies and plates were analyzed on a SECTOR S 120 plate reader (MSD). Data were analyzed using MSD Discovery Workbench version 4.0. Array 2 is a clinical-grade fluorescence assay (EVE Technologies, Calgary, Canada) that compared the fold change in plasma markers of the complement cascade, coagulation cascade, and angiogenesis during the flare and in remission. The following arrays were used: human coagulation panel 3 4-plex assay, human complement 13-plex discovery assay, human angiogenesis 17-plex discovery assay, human cardiovascular disease panel 3 9-plex discovery assay, and the human supplemental biomarker 10-plex discovery assay.
Immunostaining for phospho-S6 ribosomal protein (Ser235/236, pS6) was performed on formalin-fixed paraffin-embedded sections on a Ventana Discovery Ultra autostainer (Roche Diagnostics) at the Oncology Tissue Services Core Laboratory at JHH. Briefly, after dewaxing and rehydration on board, epitope retrieval was carried out using Ventana Ultra CC1 buffer (catalog no. 6414575001, Roche Diagnostics) at 96°C for 64 minutes. Primary antibody, anti-pS6 (1:400 dilution; catalog no. CST 2211, Cell Signaling Technology) was applied at 36°C for 60 minutes and detected using an anti-rabbit HQ detection system (catalog no. 7017936001 and 7017812001, Roche Diagnostics). This step was followed by detection with Chromomap DAB IHC kit (catalog no. 5266645001, Roche Diagnostics), counterstaining with Mayer hematoxylin, dehydration, and mounting. Other routine immunostains (CD138, κ, λ, and TdT) were performed by the Histopathology laboratory of the Surgical Pathology Division at JHH. Flow cytometry was performed at the Flow Cytometry laboratory of the Hematopathology division at JHH.
Health Insurance Portability and Accountability Act (HIPAA) identifiers were removed. All procedures were in accordance with the ethical standards of the respective local research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Results and discussion
An adult patient presented to a hospital with low-grade fevers, myalgias/arthralgias, shortness of breath, fatigue, and weight gain. Laboratory results showed an increase in nonspecific inflammatory markers. A computerized tomography (CT) scan of the chest showed mild hilar and mediastinal adenopathy, and a CT scan of the abdomen and pelvis showed mesenteric and retroperitoneal adenopathy. Two lymph node core biopsies were performed, which were inconclusive but concerning for sarcoidosis, and the patient was started on prednisone. On follow-up, the patient continued to exhibit fever, weight loss, night sweats, and joint pain, and was found to have adenopathy on imaging studies. Another excisional lymph node biopsy demonstrated reactive features. The patient proceeded to develop tenderness in both wrists, knees, and ankles, raising concern for an inflammatory arthropathy for which methotrexate was started. The patient was admitted with worsening dyspnea on exertion with diagnostic consideration of secondary hemophagocytic lymphohistiocytosis (HLH) and treated with dexamethasone, anakinra, and etoposide.
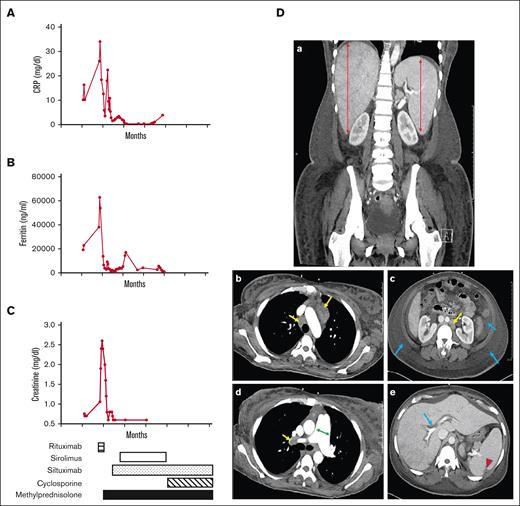
Months later, the patient presented with shortness of breath, anasarca, abdominal distension, and severe pulmonary hypertension requiring hospitalization. Routine and send-out laboratory results showed an increase in CRP (Figure 1A), ferritin (Figure 1B), and creatinine (Figure 1C). Additional results are presented in Table 1. The complete blood count showed anemia, thrombocytopenia, and mild leukocytosis. The coagulogram revealed elevated D-dimers and reduced fibrinogen with prolonged PT and aPTT, consistent with some degree of disseminated intravascular coagulation. Liver and kidney functions were notable for low albumin and increased creatinine, respectively. Liver alkaline phosphatase was borderline elevated whereas lactate dehydrogenase was significantly increased. Other inflammatory markers (e.g. IL-6, sIL-2Rα/CD25, VEGF, ferritin, CRP, and erythrocyte sedimentation rate [ESR]) were also elevated. Mild hypergammaglobulinemia was present. An infectious disease workup was negative for Epstein-Barr virus, cytomegalovirus, HHV-8, HIV, hepatitis A, hepatitis B, hepatitis C, severe acute respiratory syndrome coronavirus 2, and several other respiratory viruses. Most autoimmune markers associated with rheumatologic diseases were negative, except for low titers of antinuclear antibodies (ANA), cyclic citrullinated peptide (CCP) and rheumatoid factor (Table 1). Figure 1D shows the CT scan of the chest and abdomen, revealing hepatosplenomegaly, hepatic vascular congestion, and arterial dilation. It also shows volume overload, including anasarca, ascites, bilateral pleural effusion, and splenic hypodensities concerning for infarcts. An incisional biopsy of a lymph node showed a reactive process (see below). Given the clinical and pathologic findings, the patient was diagnosed with iMCD-TAFRO, fulfilling all 4 required clinical criteria, the required morphologic pathologic criteria, and 1 additional criterion (elevated creatinine).9 A bone marrow biopsy did not show a definitive increase in fibrosis or megakaryocytic hyperplasia (not shown), which are supportive features but not essential for the diagnosis. The patient was treated with rituximab, sirolimus, cyclosporine, siltuximab, and methylprednisolone, in additional to anticoagulants and aspirin. The timeline for administration of these medications is presented in Figure 1C (bottom).
Inflammatory markers, impaired renal function, and imaging studies in iMCD-TAFRO. Plasma levels of (A) CRP (reference, <0.5 mg/dL), (B) ferritin (reference, 13-150 ng/mL), and (C) creatinine (reference, 0.5-1.2 mg/dL) are associated with a flare. Timeline for the administration of medications (rituximab, sirolimus, siltuximab, cyclosporine, and methylprednisolone) is indicated. (D) Coronal CT image showing (a) hepatosplenomegaly (red double arrow). Other panels (b-e) are axial images of the chest, abdomen, and pelvis. (b) Multiple enlarged mediastinal lymph nodes (yellow arrows). (c) Volume overload with diffuse anasarca and moderate volume ascites (blue arrows) with enlarged para-aortic lymph node (yellow arrow). (d) Mild dilatation of the main pulmonary artery (green double arrow), and adenopathy (yellow arrows). (e) Periportal edema (blue arrow) and wedge-shaped hypodensity in the posterior aspect of the spleen, concerning for small infarcts (red arrowhead).
Inflammatory markers, impaired renal function, and imaging studies in iMCD-TAFRO. Plasma levels of (A) CRP (reference, <0.5 mg/dL), (B) ferritin (reference, 13-150 ng/mL), and (C) creatinine (reference, 0.5-1.2 mg/dL) are associated with a flare. Timeline for the administration of medications (rituximab, sirolimus, siltuximab, cyclosporine, and methylprednisolone) is indicated. (D) Coronal CT image showing (a) hepatosplenomegaly (red double arrow). Other panels (b-e) are axial images of the chest, abdomen, and pelvis. (b) Multiple enlarged mediastinal lymph nodes (yellow arrows). (c) Volume overload with diffuse anasarca and moderate volume ascites (blue arrows) with enlarged para-aortic lymph node (yellow arrow). (d) Mild dilatation of the main pulmonary artery (green double arrow), and adenopathy (yellow arrows). (e) Periportal edema (blue arrow) and wedge-shaped hypodensity in the posterior aspect of the spleen, concerning for small infarcts (red arrowhead).
Relevant laboratory values during a flare
| . | Values (flare) . | Reference range . |
|---|---|---|
| Hematology | ||
| Hemoglobin | 10.3 g/dL | 12-15 g/dL |
| Platelets | 23 × 103/μL | 150 × 103/μL to 350 × 103/μL |
| White blood cells | 11.9 × 103/μL | 4.5 × 103/μL to 11 × 103/μL |
| Coagulation | ||
| PT | 13.9 s | 9.3-11.7 s |
| INR | 1.32 | 0.9-1.1 |
| aPTT | 31.8 s | 23.1-30.9 s |
| D-dimer | >30 mg/dL | 0-0.49 mg/dL |
| Fibrinogen | 97 mg/dL | 170-422 mg/dL |
| Liver and other enzymes | ||
| Albumin | 2.8 g/dL | 3.5-5.3 g/dL |
| Alanine transaminase | 22 U/L | <31 U/L |
| Alkaline phosphatase | 197 U/L | 30-120 U/L |
| Aspartate aminotransferase | 38 U/L | <31 U/L |
| Lactate dehydrogenase | 1800 U/L | 122-220 U/L |
| Kidney | ||
| Creatinine | 2 mg/dL | 0.5-1.2 mg/dL |
| Inflammatory markers | ||
| VEGF | 491 pg/mL | 31-86 pg/mL |
| IL-6 | 123 pg/mL | <10 pg/mL |
| sIL2Rα (CD25) | 54 275 pg/mL | 532-1891 pg/mL |
| Ferritin | 18 188 ng/mL | 13-150 ng/mL |
| CRP | 18.4 mg/dL | <0.5 mg/dL |
| ESR | 103 mm/h | 4-25 mm/h |
| Immunoglobulins | ||
| IgG, serum | 2 818 mg/dL | 610-1616 mg/dL |
| IgM, serum | 248 mg/dL | 35-242 mg/dL |
| IgA, serum | 439 mg/dL | 61-348 mg/dL |
| Autoantibodies | ||
| Nuclear antibody (ANA) screen, serum | Present | Negative |
| Nuclear antibody (ANA) pattern, serum | Nuclear, speckled | Negative |
| ANA titer | 1:80 | Negative |
| Cyclic citrullinated peptide 3 IgG, serum | 36.3 AU | <20 AU |
| dsDNA antibody screen, serum | Antibodies not present | Antibodies not present |
| Rheumatoid factor, serum | 16 IU/mL | ≤12 IU/mL |
| Infectious agents (serology and NAT) | ||
| SARS-CoV-2 NAT | Negative | Negative |
| EBV Quant NAT (viral load), plasma | Target not detected | Detection, ≥20 IU/mL |
| CMV Quant NAT (viral load), plasma | Target not detected | Detection, ≥34.5 IU/mL |
| HHV-8, Quant PCR | <1000 copies per mL | <1000 copies per mL |
| EBV AB VCA, IgM | <36 U/mL | <36 U/mL |
| EBV AB VCA, IgG | >600 U/mL | <18 U/mL |
| HIV-1/2 Ag/Ab screen | Nonreactive | Nonreactive |
| . | Values (flare) . | Reference range . |
|---|---|---|
| Hematology | ||
| Hemoglobin | 10.3 g/dL | 12-15 g/dL |
| Platelets | 23 × 103/μL | 150 × 103/μL to 350 × 103/μL |
| White blood cells | 11.9 × 103/μL | 4.5 × 103/μL to 11 × 103/μL |
| Coagulation | ||
| PT | 13.9 s | 9.3-11.7 s |
| INR | 1.32 | 0.9-1.1 |
| aPTT | 31.8 s | 23.1-30.9 s |
| D-dimer | >30 mg/dL | 0-0.49 mg/dL |
| Fibrinogen | 97 mg/dL | 170-422 mg/dL |
| Liver and other enzymes | ||
| Albumin | 2.8 g/dL | 3.5-5.3 g/dL |
| Alanine transaminase | 22 U/L | <31 U/L |
| Alkaline phosphatase | 197 U/L | 30-120 U/L |
| Aspartate aminotransferase | 38 U/L | <31 U/L |
| Lactate dehydrogenase | 1800 U/L | 122-220 U/L |
| Kidney | ||
| Creatinine | 2 mg/dL | 0.5-1.2 mg/dL |
| Inflammatory markers | ||
| VEGF | 491 pg/mL | 31-86 pg/mL |
| IL-6 | 123 pg/mL | <10 pg/mL |
| sIL2Rα (CD25) | 54 275 pg/mL | 532-1891 pg/mL |
| Ferritin | 18 188 ng/mL | 13-150 ng/mL |
| CRP | 18.4 mg/dL | <0.5 mg/dL |
| ESR | 103 mm/h | 4-25 mm/h |
| Immunoglobulins | ||
| IgG, serum | 2 818 mg/dL | 610-1616 mg/dL |
| IgM, serum | 248 mg/dL | 35-242 mg/dL |
| IgA, serum | 439 mg/dL | 61-348 mg/dL |
| Autoantibodies | ||
| Nuclear antibody (ANA) screen, serum | Present | Negative |
| Nuclear antibody (ANA) pattern, serum | Nuclear, speckled | Negative |
| ANA titer | 1:80 | Negative |
| Cyclic citrullinated peptide 3 IgG, serum | 36.3 AU | <20 AU |
| dsDNA antibody screen, serum | Antibodies not present | Antibodies not present |
| Rheumatoid factor, serum | 16 IU/mL | ≤12 IU/mL |
| Infectious agents (serology and NAT) | ||
| SARS-CoV-2 NAT | Negative | Negative |
| EBV Quant NAT (viral load), plasma | Target not detected | Detection, ≥20 IU/mL |
| CMV Quant NAT (viral load), plasma | Target not detected | Detection, ≥34.5 IU/mL |
| HHV-8, Quant PCR | <1000 copies per mL | <1000 copies per mL |
| EBV AB VCA, IgM | <36 U/mL | <36 U/mL |
| EBV AB VCA, IgG | >600 U/mL | <18 U/mL |
| HIV-1/2 Ag/Ab screen | Nonreactive | Nonreactive |
Ag/Ab, antigen/antibody; ANA, antinuclear antibody; aPTT, activated partial thromboplastin time; CMV, cytomegalovirus; dsDNA, double-stranded DNA; EBV, Epstein-Barr virus; ESR, erythrocyte sedimentation rate; Ig, mmunoglobulin; INR, international normalized ratio; NAT, nucleic acid test; Quant NAT, quantitative nucleic acid test; PT, prothrombin time; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VCA, viral capsid antigen.
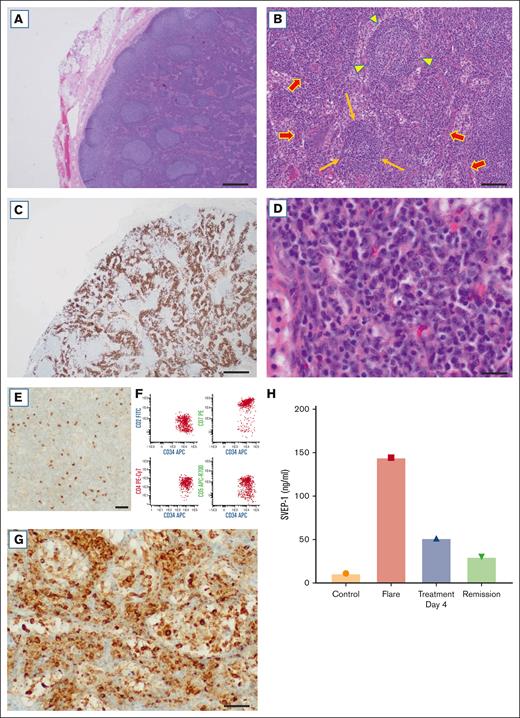
This case highlights the difficulty in the diagnosis and management of a patient with iMCD-TAFRO. Such patients frequently require multiple biopsies to reach a diagnosis, are hospitalized for life-threatening conditions, and often receive inadequate treatment because of the lack of a clear diagnosis, as described by Fajgenbaum et al.1 iMCD exhibits a histological spectrum, namely, hypervascular, plasmacytic, or “mixed” subtypes.2,8 The biopsy from the patient revealed reactive follicular and paracortical hyperplasia on hematoxylin and eosin staining (Figure 2A). Some follicles showed mantle zone lymphocytes arranged in concentric rings (resembling an “onion skin” pattern), whereas others had regressed germinal centers in a background with increased vascularity (Figure 2B). There were also abundant plasma cells present in the interfollicular areas, highlighted by CD138 (Figure 2C). κ and λ immunostains revealed a polyclonal population (not shown). At high power the plasma cells exhibited mature morphology (Figure 2D). In addition, scattered mononuclear cells were positive for TdT by immunohistochemistry (Figure 2E). This immature population was also detected by flow cytometry as TdT+ T-lymphoblasts expressing CD34, CD7 (bright) and dim-to-negative CD2, CD4, and CD5 (Figure 2F), as described.24
Histopathology, mTOR activation, and SVEP1 in iMCD-TAFRO. (A) Reactive follicular and paracortical hyperplasia (original magnification, ×20); scale bar, 500 μm. (B) Concentric mantle zones (arrowhead), regressed follicle (long arrows), and increased vascularity (arrows; original magnification, ×100); scale bar, 100 μm. (C) Areas with abundant plasma cells are positive for CD138 (original magnification, ×20); scale bar, 500 μm. (D) Plasma cells have mature morphologic features (original magnification, ×400); scale bar, 30 μm. (E) Scattered cells are positive for TdT (original magnification, ×400); scale bar, 30 μm. (F) Flow cytometry shows a population of T lymphoblasts positive for CD34 and CD7 (bright) and dim-to-negative CD2, CD4, and CD5. (G) Expression of pS6, a marker of mTOR activation, in areas with abundant plasma cells (original magnification, ×400); scale bar, 30 μm. (H) ELISA for SVEP1 shows results for controls (Controls), flare (Flare), 4 days after treatment (Treatment Day 4), and during remission (Remission) 6 months later. The biopsy was obtained during a flare.
Histopathology, mTOR activation, and SVEP1 in iMCD-TAFRO. (A) Reactive follicular and paracortical hyperplasia (original magnification, ×20); scale bar, 500 μm. (B) Concentric mantle zones (arrowhead), regressed follicle (long arrows), and increased vascularity (arrows; original magnification, ×100); scale bar, 100 μm. (C) Areas with abundant plasma cells are positive for CD138 (original magnification, ×20); scale bar, 500 μm. (D) Plasma cells have mature morphologic features (original magnification, ×400); scale bar, 30 μm. (E) Scattered cells are positive for TdT (original magnification, ×400); scale bar, 30 μm. (F) Flow cytometry shows a population of T lymphoblasts positive for CD34 and CD7 (bright) and dim-to-negative CD2, CD4, and CD5. (G) Expression of pS6, a marker of mTOR activation, in areas with abundant plasma cells (original magnification, ×400); scale bar, 30 μm. (H) ELISA for SVEP1 shows results for controls (Controls), flare (Flare), 4 days after treatment (Treatment Day 4), and during remission (Remission) 6 months later. The biopsy was obtained during a flare.
mTOR activation has been reported in iMCD with expression of pS6, p4EBP1, and p70S6K in the lymph nodes of these patients.7 Of note, Arenas et al. quantified pS6 expression by immunofluorescence and showed that plasma cells represent a large proportion of pS6+ cells, among other cell types.7 Our immunohistochemical studies also revealed nodal expression of pS6 (Figure 2G) in areas with abundant plasma cells identified by morphology and positive staining for CD138, although we cannot exclude expression by interspersed cells. More recently, SVEP1 was identified as a novel activator of PI3K/AKT/mTOR signaling in vascular cells and platelets.11 Notably, there was a 15-fold increase in plasma SVEP1 during a flare compared to normal controls (Figure 2H). Accordingly, our results suggest that SVEP1 may contribute to mTOR signaling in iMCD, among other proinflammatory effects. However, the relative contribution of SVEP1 to this process and the cell types involved in this response remains to be determined. It is also unclear by which mechanisms SVEP1 plasma levels are elevated, although it may be related to cellular and tissue expression of this protein, which include adipocytes, fibroblasts, vascular smooth muscle cells, arterial tissue, and bone marrow.10-15 Further studies are required to determine whether SVEP1 represents a potential disease marker and/or therapeutic target.
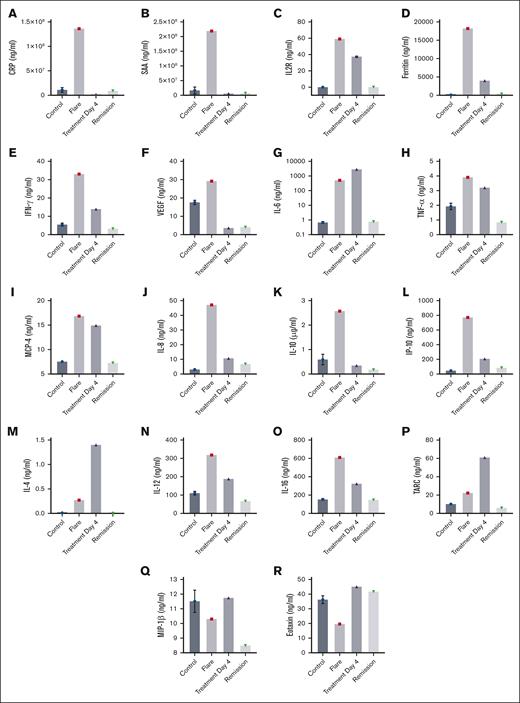
In addition to high serum levels of IL-6, other cytokines (IL-1β, tumor necrosis factor α, IL-10, and IL-23), chemokines (CXCL13, CXCL-10, CCL23, CCL21, and CCL-14), growth factors (vascular endothelial growth factor A [VEGF-A] and platelet derived growth factor subunit A [PDGF-A]), and acute phase reactants (serum amyloid A, haptoglobin, and CRP) are known to be upregulated in iMCD during flares.6,25 Transcriptome and proteome analyses of iMCD also showed cytokine upregulation.6,26 To further characterize the cytokine profile in iMCD-TAFRO, we used two complementary cytokine/chemokine arrays and ELISAs. The first array (designated array 1) quantified markers in healthy controls, during the patient’s flare, after 4 days of treatment, and in remission 6 months later. Array 1 demonstrates an increase in acute phase proteins, including CRP (Figure 3A) and serum amyloid A during the patient’s flare (Figure 3B). High levels of soluble interleukin-2 receptor (sIL2Rα/CD25) (Figure 3C) and ferritin (Figure 3D) were also observed. Both markers are associated with HLH and macrophage activation syndrome.27 These entities are associated with a cytokine storm,28 share clinical and laboratory overlap with iMCD and are important differential diagnoses, as recently reviewed.8 In this respect, the patient’s clinical course, laboratory results, and histology findings that define TAFRO by criteria, exclusion of all other causes associated with HLH, and response to treatment are consistent with iMCD-TAFRO.8,9 Array 1 also revealed an increase in IFN-γ, critical to both innate and adaptive immunity (Figure 3E), and high levels of VEGF, as previously reportedly in iMCD (Figure 3F). IL-6 was markedly increased in our patient during a flare (510 μg/mL) compared with controls (average, 0.6 μg/mL; Figure 3G). IL-6 level was determined before siltuximab was administered and we did not detect a spurious increase that has been reported after most patients take this medication.29 Of note, IL-6 is produced by various cells upon several stimuli and has pleiotropic functions. When IL-6 production increases, fatigue, low appetite, weight loss, high fever, and lymph node swelling occur. Abnormal laboratory findings associated with elevated IL-6 include renal dysfunction, anemia, thrombocytosis, hypoalbuminemia, and increases of acute phase proteins and hypergammaglobulinemia.30
Cytokine and chemokines in iMCD-TAFRO. Array 1 shows plasma levels for CRP (A), serum amyloid A (SAA) (B), ferritin (D), IFN-γ (E), VEGF (F), IL-6 (G), TNF-α (H), MCP-4 (I), IL-8 (J), IL-10 (K), IP-10 (L), IL-4 (M), IL-12 (N), IL-16 (O), thymus- and activation-regulated chemokine (TARC) (P), macrophage inflammatory protein 1β (MIP-1β) (Q), and eotaxin (R). (C) sIL2Rα (CD25) was determined by ELISA. Array 1 and ELISA results are for controls (Controls), flare (Flare), 4 days after treatment (Treatment Day 4), and during remission (Remission) 6 months later.
Cytokine and chemokines in iMCD-TAFRO. Array 1 shows plasma levels for CRP (A), serum amyloid A (SAA) (B), ferritin (D), IFN-γ (E), VEGF (F), IL-6 (G), TNF-α (H), MCP-4 (I), IL-8 (J), IL-10 (K), IP-10 (L), IL-4 (M), IL-12 (N), IL-16 (O), thymus- and activation-regulated chemokine (TARC) (P), macrophage inflammatory protein 1β (MIP-1β) (Q), and eotaxin (R). (C) sIL2Rα (CD25) was determined by ELISA. Array 1 and ELISA results are for controls (Controls), flare (Flare), 4 days after treatment (Treatment Day 4), and during remission (Remission) 6 months later.
Tumor necrosis factor α, a mediator of inflammation, was also elevated during the flare and may contribute to endothelial activation and coagulation (Figure 3H).31 We also identified high levels of MCP-4 (CCL13), which attracts monocytes and macrophages to sites of inflammation, contributing to adhesion molecule expression and secretion of proinflammatory cytokines (Figure 3I). IL-8, a chemoattractant of neutrophils and endothelial cells, showed a marked increase during the flare (Figure 3J). IL-10 was also increased compared with controls, conceivably acting as a negative regulator of the excessive inflammatory responses (Figure 3K). Furthermore, IP-10/CXCL10, which is usually associated with type 1 T-helper cells, showed an ∼10-fold increase during the flare (Figure 3L). Other cytokines and chemokines such as IL-4 (Figure 3M), IL-12 (Figure 3N), IL-16 (Figure 3O), and thymus- and activation-regulated chemokine (TARC) (Figure 3P) were also elevated, whereas macrophage inflammatory protein 1β (Figure 3Q) and eotaxin (Figure 3R) showed reduced plasma levels. Four days into treatment, the levels of most markers downtrended and ultimately reached baseline levels during remission.
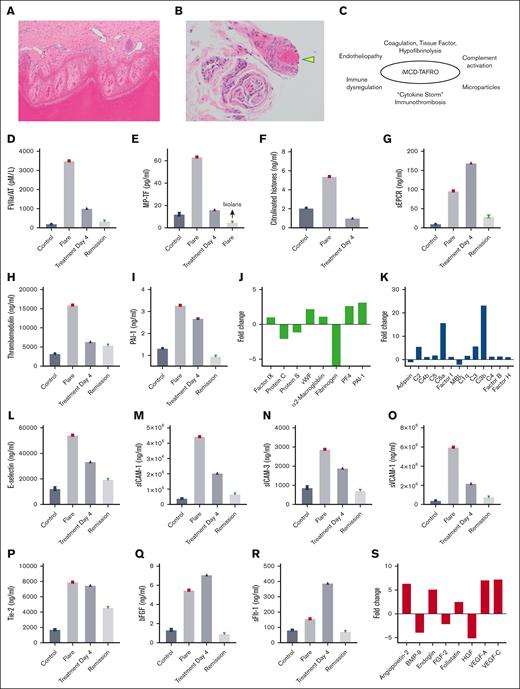
During the patient’s hospitalization, there was some degree of a consumption coagulopathy, based on prolonged PT and aPTT, thrombocytopenia, high D-dimers, and reduced fibrinogen levels. A thrombotic event was also present, based on biopsy proven epidermal digital necrosis (Figure 4A) and thrombotic vasculopathy (Figure 4B). It was hypothesized that iMCD-TAFRO results in coagulation and complement activation, immune dysregulation, and heightened immunothrombosis, as depicted in Figure 4C. To investigate the mechanisms of coagulopathy and thrombosis in the disease, ELISA was performed for coagulation factors, anticoagulants, and fibrinolytic factors.14Figure 4D reveals an elevation in the FVIIa/AT complex. This marker suggests in vivo upregulation of TF, the clotting initiator which forms FVIIa/TF complex, resulting in FXa generation.32 The TF/FVIIa/FXa complex also promotes inflammation through activation of protease activated receptors, with endothelial cell activation and release of inflammatory cytokines.19Figure 4E depicts an increase in microparticles containing functional TF (MP-TF), with procoagulant activity blocked by the specific TF inhibitor Ixolaris.22 Of note, MP-TF contributes to the amplification of the coagulation cascade, thereby potentiating thrombotic events.20 The source of MP-TF in iMCD is not known, but monocytes play a major role in coagulation dysregulation by expressing TF and releasing MP-containing TF in sepsis and other conditions.16-20 Furthermore, an increase in the ratio of classical (CD14+CD16−) to nonclassical (CD14−CD16+) monocytes has been observed in iMCD-TAFRO and associated with a type I IFN response.33 Notably, CD14+CD16− monocytes selectively express TF and produce multiple proinflammatory cytokines in patients with chronic HIV infection,34 suggesting a similar role for these cells in iMCD-TAFRO related coagulopathy. Accordingly, inhibitors of TF may have therapeutic potential in these patients, as reported for monkeys chronically infected with Simian immunodeficiency virus.34 Finally, we detected an increase in citrullinated histones (Figure 4F), a marker of neutrophil extracellular traps (NETs), which may contribute to thrombosis.35
Thrombotic vasculopathy, immunothrombosis, and endotheliopathy in iMCD-TAFRO. (A) Acral skin with full thickness epidermal necrosis (original magnification, ×100). (B) Small vessels in the dermis and subcutaneous fat show thrombi (arrowhead; original magnification, ×200). (C) iMCD-TAFRO, inflammation, and immunothrombosis. (D) ELISA for FVIIa/AT, FVIIa/antithrombin complex. (E) MP-TF functional assay (TF activity was blocked by the inhibitor Ixolaris22). ELISA for citrullinated histones, a marker for neutrophil extracellular traps (NETs) (F), soluble endothelial protein C receptor (sEPCR) (G), thrombomodulin (H), and PAI-1 (I). Array 2 shows the fold change in coagulation factors (J) and components of the complement cascade (K). Array 1 shows plasma levels for E-selectin (L), sICAM-1 (M), sICAM-3 (N), sVCAM-1 (O), Tie-2 (P), bFGF (Q), and sFlt1 (R). (S) Array 2 shows the fold change in markers associated with endothelial cell activation and angiogenesis. Array 1 and ELISA results are for controls (Controls), flare (Flare), 4 days after treatment (Treatment Day 4), and in remission (Remission) 6 months later. Array 2 results are expressed as fold change during the flare vs remission.
Thrombotic vasculopathy, immunothrombosis, and endotheliopathy in iMCD-TAFRO. (A) Acral skin with full thickness epidermal necrosis (original magnification, ×100). (B) Small vessels in the dermis and subcutaneous fat show thrombi (arrowhead; original magnification, ×200). (C) iMCD-TAFRO, inflammation, and immunothrombosis. (D) ELISA for FVIIa/AT, FVIIa/antithrombin complex. (E) MP-TF functional assay (TF activity was blocked by the inhibitor Ixolaris22). ELISA for citrullinated histones, a marker for neutrophil extracellular traps (NETs) (F), soluble endothelial protein C receptor (sEPCR) (G), thrombomodulin (H), and PAI-1 (I). Array 2 shows the fold change in coagulation factors (J) and components of the complement cascade (K). Array 1 shows plasma levels for E-selectin (L), sICAM-1 (M), sICAM-3 (N), sVCAM-1 (O), Tie-2 (P), bFGF (Q), and sFlt1 (R). (S) Array 2 shows the fold change in markers associated with endothelial cell activation and angiogenesis. Array 1 and ELISA results are for controls (Controls), flare (Flare), 4 days after treatment (Treatment Day 4), and in remission (Remission) 6 months later. Array 2 results are expressed as fold change during the flare vs remission.
With respect to anticoagulants associated with the endothelium, both soluble endothelial protein C receptor (Figure 4G) and thrombomodulin (Figure 4H) were augmented in the plasma during the flare, indicating shedding from activated endothelial cells. This results in decreased activated protein C (APC) with impaired inactivation of cofactors FVa and FVIIIa by APC, as well as reduced APC anti-inflammatory effects toward the endothelium.19 Additionally, there was an increase in PAI-1, the main inhibitor of tissue-type plasminogen activator that is released by endothelial cells and platelets, contributing to hypofibrinolysis (Figure 4I). A second array (designated array 2) compared the fold change in markers of coagulation and complement activation and endothelial cells/angiogenesis during the flare vs during remission. Figure 4J shows some reduction of protein C, protein S, and fibrinogen but mostly preserved levels of α2-macroglobulin and FIX, suggesting a somewhat compensated state of coagulation activation. There was an increase in von Willebrand factor and platelet factor 4, reflecting endothelium and platelet activation, respectively (Figure 4J). PAI-1 was also elevated in agreement with ELISA results. Array 2 revealed more than a 10-fold elevation in C3b and C5a, consistent with complement activation (Figure 4K).
Array 1 shows an increase in markers associated with endothelial cell activation including E-selectin (Figure 4L), soluble intercellular adhesion molecule 1 (sICAM-1) (Figure 4M), sICAM-3 (Figure 4N), and vascular cell adhesion molecule 1 (VCAM-1) (Figure 4O) during the flare, compared with controls. These soluble markers are endothelial receptors, which mediate leukocyte adhesion to the endothelium and trafficking upon inflammatory stimuli. Our results also revealed a marked elevation of soluble receptor tyrosine kinase (Tie-2) (Figure 4P), indicating shedding from endothelial cells. When Tie-2 becomes inactivated, important molecular brakes are released in the endothelium that, in turn, potentiate inflammation and vascular leakage.36Figure 4Q-R show upregulation of basic fibroblast growth factor (bFGF) and soluble fms-like tyrosine kinase 1 (sFLT-1), respectively, supporting the view of angiogenesis dysregulation. Array 2 confirmed endothelial activation based on high levels of angiopoietin (Tie-2 ligand) and molecules affecting angiogenesis such as endoglin, VEGF-A, VEGF-C and follistatin, among others (Figure 4S). These results imply that endotheliopathy may be a hallmark of iMCD contributing to impaired microcirculation and a prothrombotic state. Overall, it is conceivable that a cytokine storm, TF-driven coagulopathy, complement activation, and immunothrombosis shift hemostasis toward a prothrombotic and antifibrinolytic state in iMCD.29 This notion also suggests that anticoagulants targeting TF 22,34 and complement inhibitors (eg, eculizumab)37 may be beneficial for patients with iMCD-TAFRO.
Although 1 of the limitations of this paper is that it reports on a single patient with iMCD-TAFRO, given the rarity of the disease, several novel aspects of iMCD pathogenesis have been described. First, SVEP1 possibly contributes to mTOR activation–dependent inflammatory processes in vivo. Second, iMCD is accompanied by endothelial activation and endotheliopathy, likely triggered by a cytokine storm, immune and hemostasis dysregulation, with loss of endothelium surface anticoagulants. Third, TF, likely expressed by monocytes and NETs released by neutrophils, may play a role in coagulopathy. Fourth, high levels of PAI-1 released by platelets and endothelial cells may contribute to hypofibrinolysis. Fifth, complement dysregulation may contribute to thrombotic events through cross talk with coagulation activation. Together, these events result in heightened immunothrombosis,16,17 which emerges as a possible therapeutic target in combination with the immunomodulatory therapy currently available for iMCD. Confirmation of our findings in similar patients may provide additional support to our conclusions regarding the pathophysiology of iMCD-TAFRO.
Acknowledgments
The authors are thankful to Samantha Olszewski for administrative support.
This study was supported by Johns Hopkins University School of Medicine grant 80053630 (I.M.B.F.), and National Institutes of Health grants K99HL150594 (S.C.) and U01HL143402 (K.R.M.).
Authorship
Contribution: All authors participated in study design, data analysis, and writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ivo M. B. Francischetti, Pathology Department, Johns Hopkins University School of Medicine, Sheik Zayed Building, Room B 1020 J, 1800 Orleans St, Baltimore, MD 21287; email: ivofrancischetti@gmail.com.
References
Author notes
Data are available upon reasonable request from the corresponding author, Ivo M. B. Francischetti (ivofrancischetti@gmail.com).