Key Points
Pilot RCT shows feasibility of shorter LMWH + aspirin regimen vs 6-week LMWH for postpartum VTE prevention.
No major VTE or bleeding events; LMWH + aspirin may improve quality of life postpartum and further study needed.
Visual Abstract
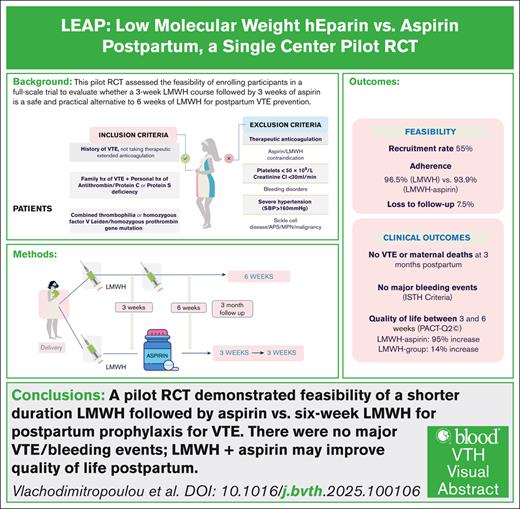
Low-molecular-weight heparin (LMWH) for 6 weeks postpartum for prophylaxis of venous thromboembolism (VTE) is suggested for patients with previous VTE or high-risk thrombophilia, yet evidence supporting this duration is limited. This study assesses the feasibility of a randomized controlled trial (RCT) comparing a shorter duration of LMWH postpartum. In a single-center pilot feasibility study, patients with previous VTE or high-risk thrombophilia were randomized to receive either 6 weeks of LMWH or a 3-week course of LMWH followed by 3 weeks of low-dose aspirin. We evaluated enrollment, adherence rates, and retention. Secondary outcomes included objectively confirmed VTE, bleeding events using the International Society on Thrombosis and Haemostasis criteria, and patient satisfaction via the Perception of Anti-Coagulant Treatment Questionnaire at 3 and 6 weeks postpartum. From October 2021 to July 2023, 67 individuals were screened, yielding a consent rate of 55%, with 30 participants enrolled. Fourteen patients were randomized to the LMWH group and 13 to the LMWH-aspirin group. Adherence was achieved in both groups (96.5% for LMWH, 93.9% for LMWH-aspirin, P = .027). Missing data were minimal. No VTE or maternal deaths occurred. One participant in the LMWH arm had delayed postpartum hemorrhage. Quality of life was improved in the combined treatment group at 6 weeks (P < .001). This single-center pilot study indicates that a larger RCT to evaluate a mixed regimen of LMWH and aspirin against traditional LMWH therapy is feasible. Enrollment and adherence rates were achieved, and quality of life may be improved. This trial was registered at www.ClinicalTrials.gov as #NCT05058924.
Introduction
For females not receiving extended-duration therapeutic anticoagulant who have previous unprovoked venous thromboembolism (VTE) or VTE associated with a hormonal risk factor (eg, pregnancy, combined oral contraception), the American Society for Hematology, the Royal College of Obstetricians and Gynecologists, and the Society of Obstetricians and Gynecologists of Canada recommend postpartum thromboprophylaxis with low-molecular-weight heparin (LMWH) for 6 weeks. These recommendations, however, are based primarily on expert consensus and observational data rather than robust evidence from randomized controlled trials (RCTs).
VTE occurring within 6 weeks postpartum affects 6.5% (95% confidence interval [CI], 4.3-9.7) of pregnancies in females with a previous VTE,1 compared with a risk of ∼0.6 per 1000 deliveries for the general population.2-5 The incidence of postpartum VTE peaks during the first week, at 9 per 10 000 deliveries, then declines sharply to 2.5 and 1.5 per 10 000 deliveries in the second and third weeks, respectively, with further reduction thereafter.2,5 Sixty-one percent of all postpartum VTEs have been described to occur within the first week, with the incidence sharply declining thereafter, reaching 2% by the sixth week.2 Additionally, a large retrospective study in the United States demonstrated that the median time for rehospitalization due to VTE was ∼2 weeks postpartum.6 These data suggest that a shorter duration of LMWH may be sufficient for many patients. A systematic review from the Cochrane Collaboration further highlighted the limited data supporting postpartum thromboprophylaxis, underscoring the need for high-quality RCTs to establish more definitive guidance.7
Aspirin (acetylsalicylic acid [ASA]) has emerged as a potential alternative for VTE prophylaxis in various settings, including postoperative care. ASA is an inexpensive, generic, and widely available oral antiplatelet medication with a well-established safety profile, antepartum8,9 and with breastfeeding.10 In contrast, LMWH is costly, requires daily subcutaneous injections, and is associated with a 1.98% (95% CI, 1.50-2.57) risk of significant bleeding, which includes antenatal bleeding, postpartum hemorrhage (>500 mL), and wound hematoma. Additionally, there is a 1.8% (95% CI, 1.34-2.37) risk of skin reactions and wound complications after cesarean delivery postpartum.11
Clinical trials and meta-analyses demonstrate that ASA may be noninferior to LMWH in preventing VTE, with at least comparable safety.12-14 ASA administered daily postoperatively has been shown to be noninferior to LMWH for VTE prophylaxis after total hip and knee arthroplasty (relative risk, 0.76; 95% CI, 0.37-1.56).15 Despite the particularly high risk of deep vein thrombosis (DVT) and pulmonary embolism (PE) within 90 days after total hip and knee replacement surgeries (with incidence rates of 5% and 2%, respectively, even with anticoagulation), ASA provides effective thromboprophylaxis.15 Moreover, ASA reduces VTE risk by at least one-third in patients with previous VTE without significantly increasing bleeding risk.13 In orthopedic settings, a 2-week administration of ASA after total knee replacement resulted in a gastrointestinal bleeding rate of 1.7 per 1000 patient-years, compared with 31.9 per 1000 patient-years with LMWH.16 A regimen of 5 days of rivaroxaban followed by 30 days of low-dose ASA (81 mg) was shown to be clinically safe and noninferior for thromboprophylaxis after total hip and knee arthroplasties,12 suggesting that a combination of limited duration thromboprophylaxis with LMWH followed by ASA could also be effective for postpartum prophylaxis.12
Given the significant burden associated with LMWH administration (costs, daily subcutaneous injections, etc) and the favorable evidence supporting ASA for VTE prophylaxis in other clinical contexts, the objective of this study was to explore the feasibility of a trial comparing 3 weeks of LMWH followed by 3 weeks of low-dose ASA to the standard 6 weeks of LMWH before embarking on a definitive study. A feasibility RCT is necessary to ensure acceptability of regimens and to identify potential barriers to enrollment and adherence before a large study.
Methods
Study design
This is a single-center, open-label feasibility RCT that evaluates the efficacy and safety of a postpartum thromboprophylaxis strategy of 3 weeks of LMWH followed by 3 weeks of low-dose ASA (henceforth referred to as the sequential antithrombotic group), compared with the standard care of 6 weeks of LMWH. Due to the nature of the interventions (subcutaneous LMWH vs oral ASA) blinding of participants and clinicians was not considered feasible because a previous study using LMWH during pregnancy1 had significant recruitment and feasibility issues with blinding and switched from a blinded trial to an open-label trial. Objective criteria were used to define clinical outcomes (eg, imaging-confirmed symptomatic VTE, International Society on Thrombosis and Haemostasis–defined bleeding events) to mitigate potential bias. The protocol was approved by the institutional ethics board and is registered at www.ClinicalTrials.gov as #NCT05058924. Written informed consent was obtained before randomization.
Participants
Participants were identified in the Hematology Clinic of the Medical Disorders Program at Mount Sinai Hospital in Toronto, Canada, a university-affiliated quaternary obstetrics center, from September 2021 to July 2023. Mount Sinai Hospital has ∼8000 births yearly. Eligible participants were pregnant patients aged >18 years with either (1) a previous objectively confirmed VTE (that is, diagnosed by compression ultrasound, venography, or computed tomography pulmonary angiogram perfusion scintigraphy) either unprovoked or provoked by hormonal or minor/major risk factors; or (2) a family history of VTE in addition to known high-risk thrombophilia (antithrombin deficiency, protein C or protein S deficiency); or (3) combined thrombophilia or homozygosity for the factor V Leiden mutation or prothrombin gene mutation, regardless of family history, as described in previous American Society of Hematology guidelines.17 Participants with high-risk inherited thrombophilia were preidentified through previous clinical testing or referral. Routine thrombophilia screening is not performed. Eligible participants were pregnant patients aged >18 years with either (1) a previous objectively confirmed VTE, or (2) a family history of VTE in addition to known high-risk inherited thrombophilia. Thrombophilias were defined using laboratory criteria consistent with established clinical standards. Protein S deficiency was defined as persistently low free protein S activity (<59%), measured outside of pregnancy, at least 6 weeks after an acute thrombotic event, and in the absence of vitamin K antagonist therapy. Protein C deficiency was defined as activity of <70%, using the same timing criteria. Antithrombin deficiency was defined as antithrombin activity of <79%. Genetic testing was used to confirm the factor V Leiden and prothrombin G20210A gene mutations, and homozygosity or compound heterozygosity for these mutations was considered high risk. These inclusion criteria reflect standard, consensus-based recommendations to identify patients at an elevated risk for VTE who may benefit from targeted prophylactic interventions postpartum. Exclusion criteria included an indication for therapeutic LMWH, a contraindication for LMWH, contraindication to ASA (such as known allergy, gastrointestinal ulcer, or platelet count below 50 × 109/L during current pregnancy or postpartum), active bleeding excluding physiological vaginal bleeding, known bleeding disorders, and/or severe hypertension (systolic blood pressure of >200 mm of Hg and/or diastolic blood pressure >120 mm Hg) during current pregnancy or postpartum.
We collected data on maternal age, body mass index (BMI), parity, and mode of delivery, as these are criteria recognized to be associated with VTE risk.18 BMI was recorded at the time of randomization in the third trimester. Females aged ≥35 years are recognized as being at increased risk of VTE. Obesity (BMI of ≥30 kg/m2) is a significant risk factor for VTE, with estimated odds ratios ranging between 2.0 and 3.0.19 Similarly, increased parity is associated with an elevated risk of VTE , as is cesarean delivery, with reported odds ratios ranging from 4.0 to 5.0.19 Antenatal ASA use was recorded at the time of randomization. It was prescribed primarily for preeclampsia prevention or placental dysfunction, consistent with routine clinical care.
Randomization
Participants eligible for this study were identified early in pregnancy; however, randomization was subsequently delayed until the third trimester after an early randomized participant experienced a miscarriage at 13 weeks of gestation. Participants were randomly assigned 1:1. An independent statistician generated the randomization list using computerized permuted blocks of varying sizes2,4,6 to ensure allocation concealment and balance. No stratification was applied in this pilot study due to the small sample size. The randomization process was facilitated through RedCap, allocating participants to either 6 weeks of prophylactic LMWH or sequential antithrombotics.
Intervention and procedures
The mode of delivery was determined by obstetricians. All participants were educated on subcutaneously self-administering prophylactic dose LMWH. The prophylactic dose was adjusted according to body weight at randomization (<100 kg: dalteparin 5000 U daily, or enoxaparin 40 mg daily, or tinzaparin 4500 U daily; ≥100 kg: dalteparin 10 000 U daily, or enoxaparin 80 mg daily, or tinzaparin 9000 U daily) as suggested by the Royal College of Obstetricians and Gynaecologists.18 Weight-adjusted dosing was based on the most recent body weight recorded at the time of randomization, typically measured during a third trimester clinic visit. LMWH postpartum was initiated for both groups within 24 hours of birth and at least 12 hours after spinal/epidural insertion or 4 hours after removal of epidural catheter if hemostasis was achieved consistent with the Society of Obstetric Anesthesia and Perinatology consensus statement.20 Data on the precise timing of LMWH initiation were not collected. For patients with a body weight of ≥100 kg, enoxaparin 40 mg was administered every 12 hours for the first 24 hours postpartum, followed by once-daily prophylactic dosing thereafter (eg, enoxaparin 40 mg twice daily followed by 80 mg daily). This dosing strategy reflects standardized postpartum thromboprophylaxis order sets at our institution. ASA was provided by the research team; LMWH was prescribed. ASA 81 mg was supplied for 3 weeks after 3 weeks of LMWH for the sequential antithrombotic group. All other medications were to be continued.
Participants were followed-up until 3 months postpartum and contacted by telephone 3 weeks, 6 weeks, and 12 weeks after birth. They were instructed to contact their physician and the study team with signs or symptoms of VTE or bleeding, upon which clinical assessment and diagnostic imaging would be performed.
Outcomes
The primary outcome is feasibility defined as enrollment rate (including consent) per month, adherence (including contamination), and retention.21,22 Adherence to study medication was assessed via participant self-reporting during scheduled follow-up telephone sessions at 3 and 6 weeks postpartum. Participants were asked to estimate the number of missed doses, and adherence was calculated as the proportion of prescribed doses taken during each time interval. Retention was measured as the proportion of participants completing all follow-up visits. Secondary clinical outcomes were assessed by telephone visits for symptomatic, objectively confirmed VTE (ie, diagnosed by compression ultrasound examination, venography, computed tomography pulmonary angiogram, or perfusion scintigraphy) at any time from delivery up to 3 months postpartum. VTE was defined as an occurrence of new DVT, PE, or venous thrombosis at an unusual site (eg, splanchnic vein or cerebral sinus thrombosis). After a diagnosis of thrombosis, patients were to be censored from the study. Other thrombotic events, such as distal thrombosis and superficial thrombophlebitis, were also recorded. Bleeding events were categorized as major or clinically relevant nonmajor bleeding as per the International Society on Thrombosis and Haemostasis criteria.23-25 Major bleeding events are defined as those that are fatal, involve critical areas or organs (eg, brain, spinal cord, eyes, joints), cause a reduction in hemoglobin concentration of ≥20 g/L, or require the transfusion of ≥2 units of red cells. Clinically relevant nonmajor bleeding events are those that are clinically significant but do not meet the criteria for major bleeding, such as prolonged epistaxis or gingival bleeding that require a face-to-face evaluation. A structured adverse event checklist was completed at 3 predefined contacts of 3 weeks, 6 weeks, and 3 months postpartum. At each visit participants were specifically asked about bleeding, thrombotic events, hospital readmissions, and any other serious adverse events. Because both ASA and LMWH have well-established obstetric safety profiles, additional regulatory pharmacovigilance reporting was not conducted for this pilot. Outcomes were not adjudicated because objective criteria were used to define clinical outcomes. The perception of anticoagulant treatment questionnaire (PACT- Q2) scale, a validated instrument to assess anticoagulant expectation (such as confidence in prevention of VTE, expectations of symptom relief) and satisfaction (such as feeling of reassurance, experience of side effects)26 was administered at 3 and 6 weeks postpartum.
Statistical analysis
Baseline characteristics are described using means, medians, standard deviations, and ranges for continuous variables; and frequencies and percentages for categorical variables. The primary outcome is feasibility, defined by the enrollment rate per month, adherence to medication at 3 and 6 weeks postpartum, and retention, measured by the proportion of participants completing follow-up visits. These metrics were assessed using frequency counts and percentages. Feasibility criteria were defined as (1) consent rate exceeding 60%, (2) adherence to the study medication of >80%, (3) compliance with study protocols of >80%, and (4) loss to follow-up of <25%.21,22 Secondary clinical outcomes, including VTE and bleeding event rates, are detailed using descriptive statistics, represented as the number of events and their corresponding percentages. Quality-of-life evaluations, assessed using the PACT-Q2, were analyzed as score variations between assessments at 3 and 6 weeks. The percentage change in PACT-Q2 scores was calculated as the difference between the 2 percentage scores, divided by the higher score, and multiplied by 100. Statistical analysis was conducted using the IBM SPSS statistical software (version 29). Items were reverse-scored (reversed item score = 6 − original item score), and the total score was subsequently summed and rescaled to a 0-to-100 scale, with higher scores indicating greater perceived convenience and satisfaction.26 With the anticipation to complete recruitment within 12 months, we targeted recruitment of 50 patients. This sample size was considered feasible for our single center.
Results
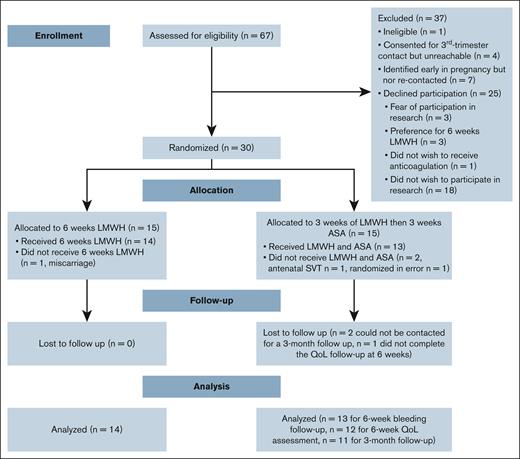
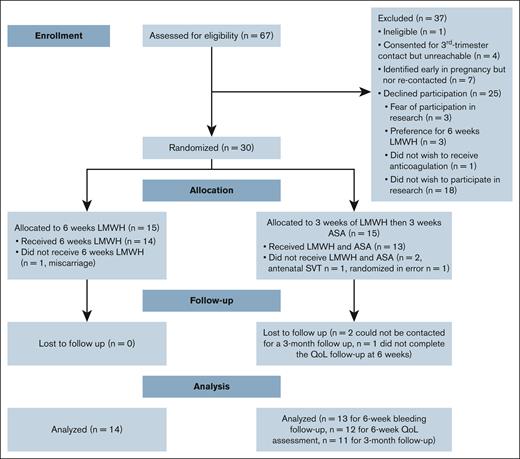
Between 1 October 2021 and 28 July 2023, 67 females were screened for participation in the study. Of these, 12 were excluded, including 1 who was deemed ineligible; 4 agreed to be contacted in the third trimester but were unreachable, and 7 were identified early in pregnancy and did not reach the third trimester by the end of the study (Figure 1). Among the remaining 55 eligible participants, 25 declined participation for reasons including fear of participating in research (n = 3), a preference for the 6-week LMWH group (n = 3), a preference to avoid anticoagulation altogether (n = 1), and unwillingness to participate in research (n = 18). Four individuals identified early in pregnancy consented to be recontacted in the third trimester but became unreachable; because they were not reassessed for eligibility or formally approached for consent at the time of randomization, they were not counted among those eligible for randomization. The pilot study was stopped as we achieved feasibility.
Participant flow. QoL, quality of life; SVT, superficial vein thrombosis.
The overall consent rate was 30 of 55 (55%; 95% CI, 41.4-67.6), with an average recruitment rate of 1.36 participants per month (95% CI, 0.91-1.82) over the 22-month study duration. No patient withdrew consent during the study. Participants were not excluded because of postpartum hemorrhage or hypertension. There were no deviations from the study protocol and there was no contamination with other medications. Adherence (± standard deviation) to study medication was 96.5% ± 8% in the LMWH group and 94% ± 8% in the sequential antithrombotic group during both the first and subsequent 3-week periods (Table 2). The total loss to follow-up was 3.7% (n = 1) at the 6-week quality-of-life follow-up, and 7.4% (n = 2) at the 3-month follow-up.
A total of 30 participants were randomly assigned to either the 6-week LMWH group (n = 15) or the sequential antithrombotic group (n = 15). After exclusions, 14 patients remained in the LMWH group and 13 in the sequential antithrombotic group (Figure 1). One participant had a subsequent pregnancy and participated in the study twice. Table 1 provides detailed characteristics at the time of randomization for each allocation group. The median age of participants was 34.3 ± 5.2 years, and the median BMI was 32.4 kg/m2 (interquartile range [IQR], 27.1-38.4). All pregnancies were singleton pregnancies. The median time from the previous VTE to the current pregnancy was 10.0 years (IQR, 4-11) in the LMWH group and 3.0 years (IQR, 2-7) in the sequential antithrombotic group (P = .003).
Twenty-nine patients (96.7%) had a previous VTE that was either unprovoked or related to the use of hormonal contraception, pregnancy, or postpartum. The provoking factors for previous VTE included hormonal contraception, assisted reproduction, VTE during a previous pregnancy or postpartum, unprovoked VTE, and air travel. Detailed distributions of these factors are described in Table 1. One participant with a previous unprovoked PE in the sequential antithrombotic group was previously found to be heterozygous for the factor V Leiden mutation, whereas another in the LMWH group was heterozygous for the prothrombin gene mutation.
The location of previous VTE varied: PE with or without DVT was more common in the LMWH group (64%) compared with the sequential antithrombotic group (29%; P = .14), whereas upper or lower extremity DVT was more frequent in the sequential antithrombotic group (71.4%) compared with the LMWH group (28.6%) (P = .03). One patient had a cerebral venous sinus thrombosis (LMWH group).
Participants were randomly assigned at a median gestational age of 33.5 weeks (IQR, 32.2-36.6) in the LMWH group and 34.3 weeks (IQR 31.3-36.0) in the sequential antithrombotic group. All pregnancies resulted in live births, with a mean gestational age at delivery of 37.8 ± 1.52 weeks in the LMWH group and 38.5 ± 1.22 weeks in the sequential antithrombotic group. The mode of delivery in the LMWH group comprised 4 vaginal deliveries (including instrumental births) and 10 cesarean sections and, in the sequential antithrombotic group, 7 vaginal deliveries (including instrumental deliveries) and 6 cesarean sections. Postpartum anticoagulation was initiated within 24 hours of delivery.
No serious adverse event or maternal deaths were reported during the study. Over the 3-month follow-up period, no hospital readmissions and no VTE events were observed. At 3 weeks postpartum, there were no incidences of major hemorrhage in either group. Clinically relevant delayed postpartum hemorrhage occurred in 1 patient (8% ± 0.05%) in sequential antithrombotic group 11 days postpartum. Gingival bleeding was observed in 3 patients (21% ± 0.12%) in the LMWH group and in 4 patients (30.8% ± 0.13%) in the sequential antithrombotic group (P = .678). Six weeks postpartum, there were no major or clinically relevant nonmajor bleeding events in either group.
The PACT-Q2 mean score was 27.1± 8.2 for the LMWH group and 29.3 ± 11.1 for the sequential antithrombotic group 3 weeks after birth (P = .479). Six weeks after birth, the mean score was 27.5 ± 6.7 for the LMWH group and 16.2 ± 2.9 for the sequential antithrombotic group (P < .001). The change in mean quality-of-life reversed scores from 3 to 6 weeks showed a slight increase of 14.1% in the LMWH group, whereas the sequential antithrombotic group experienced an increase of 95.0% (P < .001) (Table 2).
The PACT-Q2 mean reversed score at 3 weeks postpartum was 12.1% (mean score: 27.1 ± 8.2) for the LMWH group and 2.9% (mean score: 29.3 ± 11.1) for the sequential antithrombotic group (P = .479). By 6 weeks postpartum, the mean reversed scores were 10.4% (mean score: 27.5 ± 6.7) for the LMWH group and 57.5% (mean score: 16.2 ± 2.9) for the sequential antithrombotic group (P < .001) (Table 2).
Discussion
This pilot single-center RCT evaluated the feasibility of conducting a large multicenter RCT comparing shorter durations of postpartum prophylactic anticoagulation with the standard 6-week regimen. The study demonstrated the acceptability of the regimens as demonstrated by a high adherence rate of 94%, a low loss-to-follow-up rate that remained well below the 25% feasibility threshold, and minimal missing data, collectively confirming feasibility. The adherence rate to regimens in our pilot study was above the range reported in drug trials, with a median of 88% (range, 48-100).27 The overall consent rate was 55%, which we considered sufficient because the rate was potentially explained by approaching patients earlier in pregnancy. These findings compare favorably with other recent postpartum VTE pilot trials: the pilot PARTUM trial reported a 32% consent rate, 74% adherence, and 5% loss to follow-up28; and the PP-HEPP trial reported a 30% consent rate and adherence of >90%.29 Assessing enrollment and adherence rates is essential to determine the feasibility of a future large trial. Findings from this pilot study will help optimize recruitment strategies, improve adherence, and address barriers, ensuring the successful conduct of a full-scale RCT.
Secondary clinical outcomes, specifically bleeding and VTE recurrence, were not significantly different among groups; however, the sample size limits definitive conclusions. A significant improvement in quality-of-life scores in the sequential antithrombotic group compared with the LMWH group was observed. Patients in the sequential antithrombotic group experienced a 95% improvement in perceived convenience and satisfaction compared with a 14.1% improvement in the LMWH group (P < .001). This suggests that ASA reduced the physical and psychological burden of daily injections, leading to better patient satisfaction. Larger studies are needed to confirm these results.
A definitive RCT is needed because the risk of postpartum VTE is known to be significantly elevated in the context of pregnancy/postpartum (odd ratio, 33-54;30,31 absolute risk, 1%-12%)32-34; however, the risk 3 weeks after birth is considerably decreased, and the benefit of prophylaxis with LMWH has not been demonstrated in RCTs, particularly in those with previous VTE. The duration of the regimens in this study was selected based on extrapolation of the observed decline in VTE risk after 3 weeks postpartum. The HighLow study35 compared intermediate-dose and low-dose LMWH in pregnant and postpartum women with previous VTE. Although it did not find a statistically significant difference in VTE recurrence, post hoc analyses suggested a possible advantage of intermediate-dose LMWH in the postpartum period. However, this finding requires further confirmation in RCTs, specifically powered for the postpartum setting. Given these uncertainties, we adopted a pragmatic approach and conducted our study using prophylactic LMWH dosing.
The trial has limitations that are important to acknowledge. VTE recurrence and postpartum bleeding were assessed by the treating clinical team without independent, blinded adjudication; this may introduce misclassification bias in an open-label design. The trial was conducted in a single site, which may limit generalizability to other centers. However, a recent survey of practice reported that half of the expert clinicians were willing to support 3 weeks of LMWH followed by 3 weeks of ASA postpartum in individuals with a previous VTE >12 months before pregnancy, suggesting that a multicentered trial is feasible.36 The PACT-Q2 questionnaire was originally developed and validated in clinical trials involving warfarin and idraparinux for patients with DVT, PE, and atrial fibrillation.26 Hence, some items, such as the expectation of symptom relief, may have been less relevant to pregnant individuals receiving postpartum thromboprophylaxis compared with those using therapeutic anticoagulation. Nonetheless, PACT-Q2 has been validated in individuals with atrial fibrillation in addition to VTE, supporting its applicability across various patient populations. The median time from previous VTE to pregnancy was 10.0 years (IQR, 4-11) in the LMWH group and 3.0 years (IQR, 2-7) in the sequential antithrombotic group (P = .003). Although this difference is statistically significant, it should have resulted in a higher risk of recurrence in the sequential antithrombotic group (assuming a higher risk with a most recent history of VTE). We relied on patient-reported adherence rather than pill counts or drug diaries to confirm compliance with regimens. Although subject to recall bias, self-reported adherence is a widely accepted method in clinical studies, with validation studies demonstrating moderate correlation with objective measures, particularly in scenarios in which direct monitoring is impractical.37 One patient was randomized twice in the pilot trial. To maintain statistical independence, participants will only be able to participate once in the full-scale trial. The loss to follow-up criterion was initially set at a high proportion (25%) but should remain <15%.21,22 However, the actual loss to follow-up was 7.5%, well within the acceptable threshold. Finally, the Haematology Clinic at Mount Sinai Hospital is a focused, specialized clinic that assesses most obstetrical hematology patients, whereas many other centers may not have the volume and diversity of patients in 1 clinic.
We observed baseline imbalances between groups in the distribution of PE, hormonal risk factors, and unprovoked VTE. These differences likely reflect chance variation due to the small sample size and unstratified randomization. In the future planned LEAP (LMWH vs ASA postpartum) trial, we will incorporate stratification based on VTE phenotype to reduce potential bias. Additionally, antenatal ASA was prescribed in 37% of participants, mainly for preeclampsia prevention. This will be systematically recorded in the full trial and considered in planned sensitivity analyses to account for potential confounding effects on postpartum VTE risk. This single-center pilot RCT comparing 2 antithrombotic regimens for postpartum prophylaxis in individuals at high risk of recurrent VTE demonstrated feasibility and is, to our knowledge, the first to explore the duration of postpartum anticoagulation specifically for individuals with previous predominantly provoked or unprovoked VTE. Although some comparative statistics were reported for secondary outcomes, these analyses were strictly exploratory; the study was not powered for hypothesis testing, and findings should not be interpreted as inferential.
The results of this feasibility trial will directly inform the design and implementation of the planned LEAP trial, a multicenter, randomized, open-label, noninferiority study comparing 6 weeks of LMWH with 3 weeks of LMWH followed by 3 weeks of low-dose ASA. The primary outcome of LEAP will be a composite of VTE and all-cause mortality at 12 weeks postpartum. This pilot study was specifically designed to assess feasibility metrics (recruitment, adherence, and retention) that will guide the operational planning of the multicenter RCT.
Acknowledgments
The authors are grateful to the pregnant patients who participated in this study, as well as the research team, for their invaluable contributions.
This work was supported by an educational grant from the Division of Hematology at the University Health Network, Toronto, Canada.
Authorship
Contribution: E.V. and N.S. conceptualized and designed the study, supervised the research, collected and analyzed data, and wrote the manuscript; K.M. provided clinical expertise and manuscript review; M.C. contributed to study methodology and reviewed the manuscript; E.K. assisted with study design and manuscript review; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Evangelia Vlachodimitropoulou, Maternal and Fetal Medicine, Ontario Power Generation, Third Floor, 700 University Ave, Toronto, ON M7A 2S4, Canada; email: evangelia.vlachodimitropoulou@sinaihealth.ca.
References
Author notes
The data that support the findings of this study are available on request from the corresponding author, Evangelia Vlachodimitropoulou (evangelia.vlachodimitropoulou@sinaihealth.ca).