P-selectin is a membrane glycoprotein and a member of the selectin family of cell adhesion molecules. It is prestored in α-granules of platelets and Weibel-Palade bodies of endothelial cells and is rapidly expressed on their surfaces upon activation during the course of an inflammatory response. Although a critical component of the innate immune system, the interaction of P-selectin with its cognate ligand, P-selectin glycoprotein ligand 1 (PSGL-1) may mediate maladaptive events central to the pathophysiology of venous thromboembolism, cardiovascular disease, stroke, metabolic syndrome, and sickle cell disease, among other disorders. As a consequence, a growing understanding of the significance of P-selectin and PSGL-1 in human disease has motivated the design of inhibitors that target the P-selectin/PSGL-1 pathway. Herein, we review the development and evaluation of both biologic and small-molecule inhibitors, including preclinical studies and clinical trials that have evaluated therapeutic potential of these agents for a variety of diseases linked to dysregulated inflammatory and thrombotic responses.
Introduction
P-selectin (PADGEM, GMP-40, or CD62P) is a cell adhesion molecule found on the surface of activated endothelial cells and platelets. It was first identified in 1989, along with E- and L-selectin, as a member of the selectin family of cell surface glycoproteins.1 The expression of P-selectin is dynamically regulated, with rapid translocation from intracellular platelet α-granules or endothelial cell Weibel-Palade bodies to the cell surface in response to multiple inflammatory stimuli. P-selectin interacts with a high affinity ligand, P-selectin glycoprotein ligand 1 (PSGL-1), expressed on the surfaces of all leukocytes, facilitating the initial “capture and rolling” step in the leukocyte–endothelial cell adhesion cascade.2 Although P-selectin is an essential mediator of innate immune responses, sustained expression of P-selectin has been implicated in pathological processes linked to thromboinflammation and immunothrombosis.
Although conceptually similar, thromboinflammation emphasizes that both inflammatory and thrombotic pathways are interlinked, activated in concert, mutually amplifying, and together contribute to the progression of a variety of diseases, including atherosclerosis, thrombosis, metabolic syndrome, sickle cell disease (SCD), autoimmune diseases, and cancer.3-6 In contrast, immunothrombosis describes the role of the immune system in initiating and modulating thrombosis as a defense mechanism against pathogens, particularly through the actions of neutrophils and monocytes. If dysregulated, this response can lead to excessive thrombus formation and vascular complications.7,8 The P-selectin/PSGL-1 pathway plays a critical role in both thromboinflammation and immunothrombosis by mediating interactions between platelets, leukocytes, and the endothelium. Both processes share common mechanisms through the complex action of cytokines, chemokines, and tissue factor, among other mediators, on endothelial cells, platelets, and leukocytes. In the context of thromboinflammation, clot formation and ongoing inflammation can further stimulate the expression of P-selectin, enhancing the recruitment of leukocytes and generation of platelet-leukocyte aggregates that further enhance thrombin production and fibrin formation, perpetuating both inflammation and thrombosis. In immunothrombosis, P-selectin mediated interactions help localize leukocytes at sites of infection with the release of neutrophil extracellular traps and formation of microvascular thrombi that trap and clear pathogens. Excessive or inappropriate activation of this pathway during the course of a severe infection, sepsis, or a systemic inflammatory response can lead to pathological thrombosis, including venous thromboembolism or disseminated intravascular coagulation. The P-selectin/PSGL-1 axis lies at the nexus of the innate immune system, coagulation and complement cascades, platelet and endothelial activation, and the fibrinolytic system that potentiate both thromboinflammation and immunothrombosis. Recent detailed reviews of the role of thromboinflammation and immunothrombosis as common pathophysiologic mechanisms of diverse diseases can be found elsewhere.9-13
Significantly, multiple studies have corroborated the association of genetic variations in the SELP and SELPG genes, which encode P-selectin and PSGL-1, respectively, with cardiovascular disease, stroke, and venous thromboembolism. Specifically, SELP haplotypes have been linked to an increased risk of venous thromboembolism,14,15 and SELP polymorphisms have been correlated with cardiovascular risk as determined by increased carotid intima-medial thickness16 and elevated levels of soluble P-selectin.17SELP single-nucleotide polymorphisms have also been associated with thrombosis in male patients with severe COVID-19 infection, as well as with elevated levels of soluble P-selectin,18,19 indicative of an increased risk of cardiovascular disease, venous thromboembolism, and other chronic inflammatory disorders. Finally, specific SELP and SELPLG polymorphisms identified in the Atherosclerosis Risk in Communities study have also been associated with an increased or decreased risk of incident coronary artery disease and ischemic stroke20 and with cell surface levels of P-selectin and PSGL-1.21 These genetic population studies strongly support the significance of the PSGL-1/P-selectin pathway in human disease.
A structural foundation for the rational design of P-selectin inhibitors
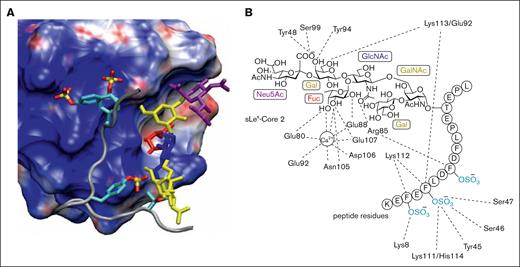
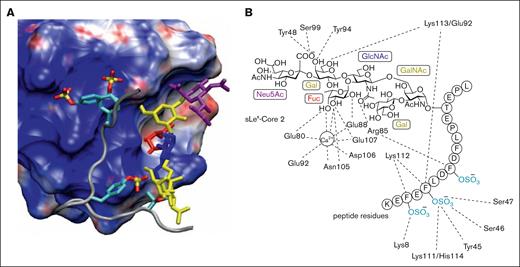
Detailed investigations, beginning in the 1990s, have significantly advanced our understanding of the structural features responsible for the interactions between selectins and multiple ligands.22 Early studies established that P-selectin bound strongly to a dimeric glycoprotein, referred to as PSGL-1, bearing an α-2,3-sialyl-Lewis x (sLeX) tetrasaccharide.23,24 Soon thereafter, the protein sequence of PSGL-1 was elucidated through expression cloning from a human myeloid cell complementary DNA library.25 Initial investigations demonstrated that the presence of a core 2 O-glycan expressing the unique tetrasaccharide sLeX promoted high affinity binding of PSGL-1 to P-selectin,26 with subsequent studies revealing that an anionic polypeptide domain, containing at least 1 tyrosine sulfate in addition to sLeX was critical for high affinity binding.27 The specific sugar and tyrosine sulfate residues responsible for high affinity binding were not well defined until detailed structure–function studies, enabled by the synthesis of a series of glycosulfopeptides of the N terminus of PSGL-1, revealed that the most important binding determinants were 3 tyrosine sulfates at positions 46, 48, and 51, along with fucose and sialic acid.28 The tyrosine sulfates were noted to increase binding affinity by 40-fold, with a nearly equivalent contribution by fucose. These findings were later supported by detailed crystal structures of P-selectin in complex with PSGL-1 along with nuclear magnetic resonance and molecular modeling studies.29-31 The 3 tyrosine sulfates were found to create an anionic pocket, greatly enhancing the affinity of PSGL-1 to P-selectin, with the adjacent glycan serving as an essential pharmacophore (Figure 1). Significantly, this was the first example of high affinity binding between a carbohydrate and a glycan-binding protein as a consequence of the tandem interactions of a glycan and an adjacent aglycone peptide domain. These experimental and computational studies established a foundation for targeting the P-selectin/PSGL-1 pathway through the rational design of orthosteric P-selectin antagonists, among other approaches.
Interactions of PSGL-1 with P-selectin. (A) Structure of the P-selectin complex with the PSGL-1 ligand containing tyrosine O-sulfates and the sLex hexasaccharide. The P-selectin surface is color coded according to electrostatic potential (acidic region, blue; basic region, red). In addition, individual sugars in the sLex are color coded (Neu5Ac, purple; Gal and GalNAc, yellow; GlcNAc, blue; and Fuc, red) and the sulfur and oxygen atoms within the sulfate groups are yellow and red, respectively. (B) Interaction map of specific carbohydrate and peptide functional groups with amino acids in the P-selectin binding domain (adapted with permission from Sladek et al,31 Copyright 2024, American Chemical Society).
Interactions of PSGL-1 with P-selectin. (A) Structure of the P-selectin complex with the PSGL-1 ligand containing tyrosine O-sulfates and the sLex hexasaccharide. The P-selectin surface is color coded according to electrostatic potential (acidic region, blue; basic region, red). In addition, individual sugars in the sLex are color coded (Neu5Ac, purple; Gal and GalNAc, yellow; GlcNAc, blue; and Fuc, red) and the sulfur and oxygen atoms within the sulfate groups are yellow and red, respectively. (B) Interaction map of specific carbohydrate and peptide functional groups with amino acids in the P-selectin binding domain (adapted with permission from Sladek et al,31 Copyright 2024, American Chemical Society).
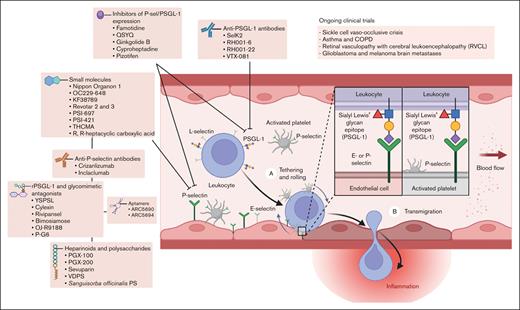
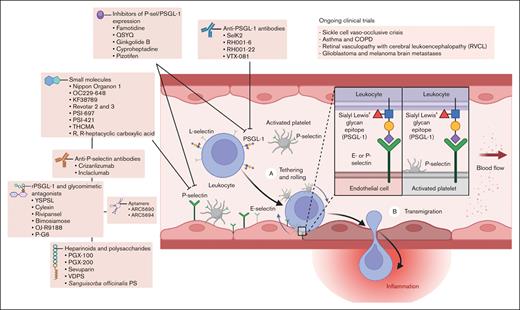
Strategies aimed at inhibiting P-selectin/PSGL-1 interactions have shown promise in preclinical studies and clinical trials. As a representative example of a systemic disorder of thromboinflammation, considerable evidence has substantiated that the PSGL-1/P-selectin pathway plays a pivotal role in the pathogenesis of acute sickle cell crises and other complications of SCD. Despite the inherent complexity of designing clinical trials to demonstrate a reduction in sickle cell vaso-occlusive crises in which the utilization of health care resources is used as a proxy for acute pain episodes, the clinical benefit of a P-selectin inhibitor has been observed in multiple patient populations with SCD.32-34 Herein, we review efforts toward the development of P-selectin inhibitors, including preclinical and clinical studies that support their potential role as a novel class of disease-modifying therapeutic for a broad range of human illnesses mediated by thromboinflammation and immunothrombosis that carry significant morbidity, compromising both quality of life and life expectancy (Figure 2).
P-selectin/PSGL-1–mediated leukocyte rolling, adhesion, and transmigration, including a summary of antagonists and current clinical applications.
P-selectin/PSGL-1–mediated leukocyte rolling, adhesion, and transmigration, including a summary of antagonists and current clinical applications.
Anti–P-selectin antibodies
Crizanlizumab (SelG1; Adakveo [Novartis]) is a humanized anti–P-selectin antibody that received US Food and Drug Administration approval in 2019 for the prevention of sickle cell vaso-occlusive crises based on the randomized, placebo controlled SUSTAIN trial.32,35 However, a subsequent European trial (STAND) failed to confirm these results, with withdrawal of approval for this therapeutic antibody within the European Union. The negative outcome of the STAND trial has been attributed, in part, to wide differences in cultural norms for pain treatment, access to care, and health care practices inherent to a multicountry study in which health care utilization is used as a surrogate for acute pain episodes.34,36-38 Nonetheless, additional studies have noted clinical benefit of crizanlizumab in a variety of patient populations with SCD with continued availability of this biologic in the United States.33
Crizanlizumab has also been evaluated for the treatment of COVID-19 vasculopathy (NCT04435184 and NCT04505774). Although an increase in D-dimer levels was observed, consistent with the induction of thrombolysis, along with a reduction in thrombin generation, a significant difference between crizanlizumab and placebo was not observed in primary clinical end points. Crizanlizumab is currently undergoing evaluation for the treatment of patients with glioblastoma and melanoma brain metastases in combination with nivolumab (NCT05909618), as a strategy to enhance immune cell infiltration. It is also being evaluated for the treatment of patients with retinal vasculopathy with cerebral leukoencephalopathy, a rare disease associated with vision loss, stroke, and cognitive decline as a consequence of a small-vessel vasculitis and thromboinflammation triggered by mutations in the repair exonuclease TREX1 (NCT04611880).39
Inclaclumab is a humanized anti–P-selectin antibody under evaluation by Pfizer.40 Inclaclumab was noted to reduce myocardial damage after percutaneous coronary intervention for the treatment of a non-ST–elevation myocardial infarction (non-STEMI) in a phase 2 clinical trial (NCT01327183).41 However, clinical benefit was not observed in a subsequent phase 2 trial of patients undergoing urgent coronary artery bypass surgery (NCT01245634).42 Currently, a multicenter phase 3 trial is in progress to determine the ability of inclaclumab to reduce the frequency of vaso-occlusive crises among individuals with SCD (Thrive-131; NCT04935879), as well as to evaluate the long-term safety of this agent in this patient population (Thrive-133 open-label extension; NCT05348915). A second phase 3 trial targeting SCD was terminated because of slow patient recruitment (Thrive-132; NCT04927247).
Anti–PSGL-1 antibodies and recombinant PSGL-1–immunoglobulin fusion protein
Tetherex Pharmaceuticals is developing SelK2, a humanized anti–PSGL-1 antibody that blocks interactions with P- and L-selectin, as well as chemokines.43 Interestingly, PSGL-1 can also function as a ligand for certain chemokines, such as CCL19 and CCL2144, as well as CCL27,45 independently of its recognition by P-selectin. In a randomized phase 2 clinical trial, administration of SelK2 as a single intravenous dose, either alone or in conjunction with enoxaparin, failed to reduce the incidence of venous thromboembolism after total knee replacement as compared with enoxaparin alone (NCT03812328). Further clinical studies are underway to assess the efficacy of SelK2 in preserving airway function and reducing pulmonary inflammation after allergen challenge in patients with asthma and chronic obstructive pulmonary disease (COPD) (NCT04540042). Li et al have also reported the generation of 2 anti–PSGL-1 antibodies, RH001-6 and RH001-22, with the former displaying potent reduction of leukocyte–endothelial interactions in vitro along with a commensurate decrease in inflammation and pancreatic injury in murine models of cerulein- and l-arginine–induced acute pancreatitis.46
In the late 1990s, Wyeth Pharmaceuticals developed a recombinant immunoglobulin chimera of PSGL-1 (YSPSL) that accelerated coronary thrombolysis,47 reduced myocardial ischemia reperfusion injury,48 and decreased postangioplasty restenosis in a variety of swine models.49 However, intravenous administration of YSPSL in conjunction with tissue plasminogen activator did not reduce infarct size or improve clinical outcomes among patients with acute myocardial infarction.50,51 Phase 2 clinical trials to assess the efficacy of this agent in the prevention of ischemia reperfusion injury in liver (NCT00876902) and kidney (NCT00298168) transplantation have also been conducted. YSPSL treatment demonstrated a trend toward improved early liver graft function with reduced postoperative liver enzymes and bilirubin levels, but primary study end points were not achieved.52 Likewise, intravenous administration of YSPSL reduced elevated creatinine levels after kidney transplantation, but the incidence of dialysis-delayed graft function was not reduced.53 Given the need to produce YSPSL through a biosynthetic manufacturing scheme, the possibility exists that the complex glycosylation structure of this recombinant glycoprotein was not consistently and fully recapitulated. In addition, ineffective dosing or a suboptimal pharmacokinetic profile may have contributed to the failure of these clinical trials.
Recent studies have determined that PSGL-1 serves as a key regulator of T-cell exhaustion. Engagement of PSGL-1 leads to increased expression of multiple inhibitory receptors (PD-1, LAG3, and TIM-3) and transcription factors (TOX and EOMES), which results in the loss of effector T-cell cytokine expression and inhibition of T-cell receptor activation.54-56 Although additional PSGL-1 binding partners, such as VISTA, may be responsible for this effect, targeting PSGL-1 in tumor-bearing mice by administration of a recombinant PSGL-1 as a “decoy molecule” or by antibody blockade, has been reported to enhance antitumor immunity and inhibit melanoma tumor growth.57 Verseau Therapeutics is developing an anti–PSGL-1-antibody, VTX-081, which has been noted to reduce tumor growth in several syngeneic murine tumor models, alone or in combination with PD-1 blockade.58 PSGL-1 is also a potential binding partner of Siglec-5, which is expressed on many leukocytes,59 but whether these interactions are involved in either T-cell functions or inflammatory responses are not yet known. In summary, although PSGL-1 represents the primary binding partner for P-selectin on activated platelets and endothelial cells, PSGL-1 displays additional binding interactions. As noted, PSGL-1 binds several chemokines and VISTA, which is expressed on myeloid cells, T cells, and antigen presenting cells, as well as a variety of cancer cells, including ovarian cancer, colorectal cancer, non–small cell lung cancer, and melanoma. As a consequence, preferentially targeting PSGL-1 rather than P-selectin may be desirable as a therapeutic intervention for autoimmune disorders and cancer.
Anti–P-selectin aptamers
Aptamers are short, synthetic single-stranded oligonucleotides and several have been generated that bind with high affinity to P-selectin.60 Archemix reported that ARC5690 and ARC5694 inhibited the adhesion of red blood cells and leukocytes to endothelial cells with increased red cell velocity in a murine model of SCD.61 However, ongoing studies of anti–P-selectin aptamers appear limited, potentially because of existing challenges associated with the use of aptamers as targeted therapeutics.62,63
Carbohydrate-based therapeutics
Glycomimetic P-selectin antagonists
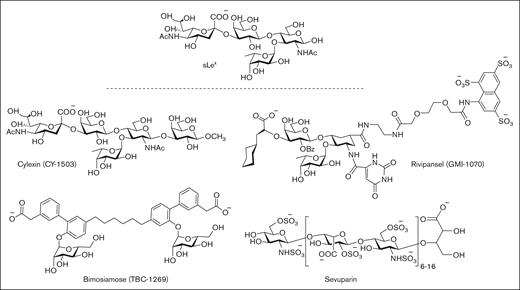
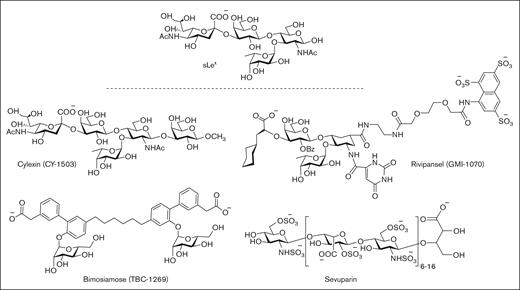
First-generation P-selectin inhibitors were designed to mimic the sLeX moiety of PSGL-1 but have displayed limited clinical efficacy, presumably because of low binding affinity to P-selectin (Figure 3).64 For example, cylexin (CY-1503; Epimmune) inhibited neutrophil–endothelial interactions65 and was observed to reduce reperfusion lung injury in an initial clinical study of patients undergoing pulmonary thromboendartectomy for chronic thromboembolic pulmonary hypertension.66 However, in a subsequent multicenter, double-blinded, placebo controlled trial of infants undergoing cardiac surgery, the ability to limit myocardial reperfusion injury was not demonstrated despite a trend toward reduced perioperative mortality (NCT00226369). GlycoMimetics has been at the forefront of the design of a series of pan-selectin inhibitors containing essential sLeX binding motifs, including fucose, galactose, and a carboxylate group as a sialic acid mimic, along with a sulfonated naphthalene ring.67 The most promising candidate, rivipansel (GMI-1070) demonstrated initial clinical benefit in a phase 2 trial of sickle cell pain crisis (NCT01119833), but its low activity (50% inhibitory concentration [IC50] of 423 μM) required infusion of ∼2 g of drug per day, and a later phase 3 trial failed to meet primary study end points (NCT02187003).68-70 Subsequent efforts have been directed at advancing uproleselan (GMI-1271) and GMI-1687 as E-selectin inhibitors for the treatment of SCD, venous thromboembolism, and acute myeloid leukemia. Promising results have been reported for GMI-1271 and GMI-1359 (an antagonist of both E-selectin and CXCR4) to treat patients with multiple myeloma.71
Pan-selectin carbohydrate–based inhibitors designed to mimic the sLeXmoiety of PSGL-1.
Pan-selectin carbohydrate–based inhibitors designed to mimic the sLeXmoiety of PSGL-1.
In a similar drug campaign, Encysive Pharmaceuticals designed a small-molecule glycomimetic through replacement of the essential binding motifs of sLeX with a biphenyl group and mannose. As a pan-selectin inhibitor, bimosiamose (TBC-1269) advanced to clinical trials directed at psoriasis (NCT00823693),72 COPD (NCT01108913),73 and asthma (NCT00962481).74 However, only a marginal benefit was observed, presumably as a result of modest inhibitory activity, exemplified by high micromolar inhibitory activity against P-, E-, and L-selectin.75
Anti–P-selectin heparinoids and plant-derived polysaccharides
Heparin derived drugs or heparinoids have been introduced through the chemical modification of native heparin to eliminate innate anticoagulant activity, while retaining pleiotropic anti-inflammatory properties, including the ability to inhibit P-selectin.76 Structure–function studies have identified 4 O- and N-linked sulfate groups in glucosamine that are essential for high affinity interactions between heparin and antithrombin, with a 3-O-sulfate group serving as a critical moiety for preserved antithrombin binding and anticoagulant activity.77,78 As a consequence, regioselective desulfation of heparin has been pursued to attenuate its anticoagulant activity while preserving other biological functions.79 Specifically, 2-O, 3-O-desulfation of glucosamine with retention of the 6-O-sulfate affords a heparinoid that displays reduced anticoagulant activity with the retained capacity to inhibit P-selectin.80,81 ParinGenix developed a nonanticoagulant 2-O, 3-O-desulfated heparin (PGX-100) that inhibited P-selectin with 60-fold less antithrombin activity82; a phase 2 trial, however, demonstrated lack of efficacy in the treatment of patients with an acute exacerbation of COPD (NCT00457951). PGX-200, an inhaled version of PGX-100, was introduced for the treatment of cystic fibrosis, asthma, and COPD, but further clinical assessment of this compound has not been reported. Sevuparin, a nonanticoagulant heparinoid developed by Modus Therapeutics prevented vaso-occlusion in murine models of SCD through blockade of P- and L-selectin.83 Nevertheless, a randomized phase 2 trial (NCT02515838) failed to demonstrate efficacy of continuous intravenous infusion of sevuparin in patients with SCD. Although heparin does exhibit anti-inflammatory properties, high micromolar concentrations of heparin are typically required to inhibit P-selectin, which may explain the limited efficacy of this class of compounds.84
Galactoxylan is a plant-derived hemicellulose polysaccharide composed of a backbone of xylose residues with galactose side chains. A galactoxylan polysaccharide, isolated from Viola diffusa, has been used as a traditional Chinese medicine for treating conjunctivitis, pleuritis, hepatitis, and other inflammatory diseases. In a recent report, V diffusa polysaccharide reduced endotoxin-induced acute lung injury by inhibiting P-selectin interactions with PSGL-1 with decreased leukocyte adhesion to endothelial cells, and diminished pulmonary inflammation.85 Polysaccharides derived from Sanguisorba officinalis, a plant in the Rosaceae family used as an herbal remedy, have also been reported to block interactions between P-selectin and PSGL-1.86
Synthetic glycolipids and glycopeptides
Nippon Organon introduced OJ-R9188, a glycolipid mimetic consisting of a fucose and furanose glycan linked to a dipeptide and diacyl group. As a pan-selectin antagonist, intravenous administration of OJ-R9188 displayed benefit in murine models of allergic dermatitis and reperfusion lung injury, but this compound has not been advanced to the clinic.87,88 Historically, the binding of a glycan to a glycan-binding protein has been viewed as an inherently low affinity interaction, in which high binding strength or avidity, if achieved, is primarily a function of multivalent interactions.
The high affinity interaction between P-selectin and PSGL-1 is unique, and a consequence of stereospecific interactions between P-selectin and both glycan and aglycone features present on PSGL-1, including a sLeX-containing hexasaccharide and clustered tyrosine sulfates on the adjacent peptide backbone. Although attempts to synthesize glycopeptide mimics of the N-terminal region of PSGL-1 have been pursued, both chemoenzymatically and chemically, these efforts have been limited by a variety of synthetic challenges; for enzymatic approaches these include difficulties in producing and handling enzymes and completeness of reactions, whereas for chemical synthesis difficulties include poor selectivity, incompatible protecting groups, and the instability of tyrosine sulfates. Overcoming many of these hurdles, we have been able to design and synthesize a broad range of glycopeptide mimics of PSGL-1, including highly stable analogues with low nanomolar binding affinity to P-selectin.89 These compounds can be administered by intravenous or subcutaneous routes and display promising pharmacokinetic and pharmacodynamic profiles. Notably, we have demonstrated that treatment of whole blood from individuals with SCD leads to a marked reduction of vaso-occlusion.90 Moreover, potent dose-dependent inhibition of venous thrombosis has been confirmed in both wild-type and tumor-bearing mice without an increase in bleeding risk. We believe that these promising results support the potential of these compounds for in-hospital or ambulatory thromboprophylaxis, particularly for patients at high risk of venous thromboembolism who may otherwise be vulnerable to major bleeding events or for whom direct oral anticoagulants are otherwise contraindicated.91
Small-molecule inhibitors of P-selectin
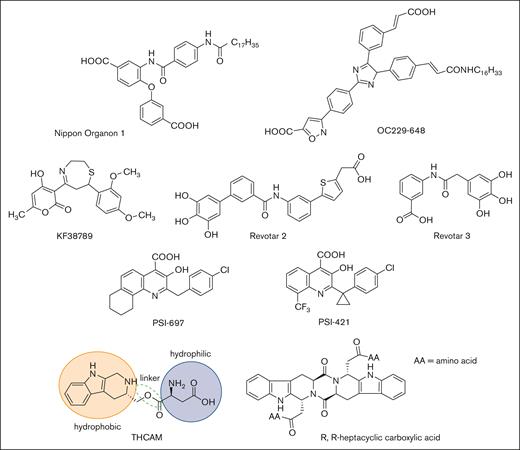
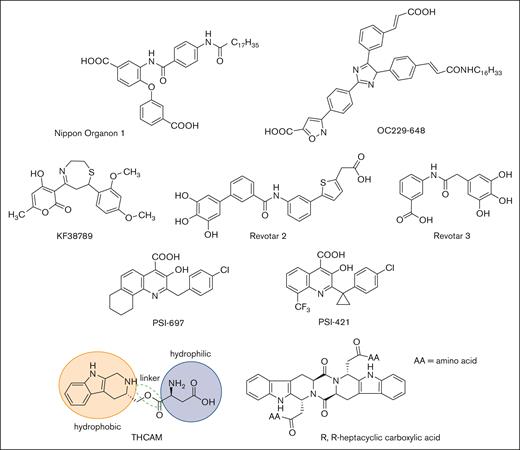
High-throughput screening and molecular modeling approaches have led to a number of nonsugar–based small-molecule inhibitors of P-selectin (Figure 4). Early studies led to the identification of polyanionic (phosphate or sulfate) derivatives of inositol as inhibitors of P-selectin, but their potency and specificity were not clear.92 Nippon Organon identified a pan-selectin inhibitor (Nippon Organon 1) with an IC50 of 6.1 μM,93 and Ontogen Corporation has disclosed an imidazole-based P-selectin antagonist (OC229-648).94 Molecular modeling of OC229-648 suggested that a single carboxylate group could act as a calcium chelator and in this manner serve as a substitute for the hydroxyl groups of fucose to facilitate P-selectin binding. Upon intravenous administration, OC229-648 reduced inflammation in a murine model of peritonitis. Kyowa Hakko Kogyo discovered a P-selectin inhibitor based on a 7-phenyl-1,4-thiazepine core and structure–activity studies led to the development of KF38789 with an IC50 of 1.97 μM. Intravenous administration of KF38789 reduced leukocyte recruitment in a murine model of peritonitis.95,96
Small-molecule P-selectin inhibitors in preclinical and early-stage clinical development.
Small-molecule P-selectin inhibitors in preclinical and early-stage clinical development.
Revotar Biopharmaceuticals has developed small-molecule pan-selectin antagonists derived from bimosiamose (Revotar 2 and Revotar 3), retaining 3 free hydroxyl and carboxyl binding motifs, which display anti–P-selectin activity.67,97 Other derivatives, including aromatic, cyclic, and nitrocatechol compounds have also been reported.67 Efforts at Wyeth have led to the discovery that quinoline salicylic acids inhibit P-selectin.98,99 Extensive high-throughput screening identified PSI-697 as the first orally available P-selectin inhibitor with activity in mouse, rat, and baboon models of atherogenesis,100 vascular injury,101 and venous thrombosis.102,103 PSI-697 also reduced thrombus formation in human blood perfused through an ex vivo chamber.104 However, PSI-697 did not display optimal pharmacokinetic properties nor was it effective in inhibiting circulating platelet-leukocyte aggregates among individuals who were active smokers (NCT03860506)105 or leukocyte rolling in scleral blood vessels of patients with autoimmune scleritis (NCT00367692). A second quinoline salicylic acid derivative, PSI-421, was designed with improved pharmacokinetic properties, exhibiting enhanced resolution of venous thrombosis in nonhuman primates, and reduced neointimal hyperplasia in a rat carotid angioplasty model.106 Nonetheless, both PSI-697 and PSI-421 have been associated with modest inhibitory activity (IC50, ∼200 μM), consistent with weak binding affinity and have not been further advanced to clinical trials.99,107 Recently, THCMA (3S-1,2,3,4-tetrahydro-β-carboline-3-methyl aspartyl ester) was designed as an orally available amphiphile containing pharmacophores of PSI-697. THCMA reportedly exhibits improved potency with enhanced anti-inflammatory and antithrombotic activity in vivo.108 Zhao et al have also reported P-selectin antagonists derived from an amino acid modified R,R-heptacyclic carboxylic acid compound, which have been evaluated for their ability to inhibit arterial thrombosis in rats.109 The inherent challenge of designing small-molecule orthosteric inhibitors of the P-selectin/PSGL-1 pathway is the presence of large and relatively flat surfaces that characterize the interaction between P-selectin and PSGL-1, along with the absence of a deep and well-defined binding pocket.
Inhibition of P-selectin and PSGL-1 expression
Serotonin antagonists, antihistamines, and a number of traditional Chinese medicines, have been noted to reduce the expression of P-selectin and PSGL-1 with potential beneficial effects. The 5-hydroxytryptamine 2A serotonin receptor antagonists, cyproheptadine and pizotifen, inhibit the expression of P-selectin and delay thrombotic occlusion in mice, albeit with an observed increased risk of bleeding.110 Famotidine, an H2 receptor blocker, has also been noted to reduce P-selectin expression in children with SCD, which has motivated the initiation of an ongoing pilot study (NCT05084521).111 Qishen yiqi dripping pills (QSYQ) are a Chinese herbal formulation used for the treatment of cardiovascular disease and has been reported to delay carotid artery occlusion and reduce stroke infarct area in several rodent models.112 QSYQ is composed of Astragalus membranaceus (huangqi), which, as a component of yiqi qubai, has been traditionally associated with anti-inflammatory effects, as well as Salvia miltiorrhiza (danshen), Panax notoginseng (sanqi), and Dalbergia odorifera (jiangxiang) that have been prescribed to promote circulation (huoxue). QSYQ and its yiqi qubai/huoxue components have been observed to inhibit platelet–leukocyte aggregation and decrease expression of P-selectin on platelets and PSGL-1 on leukocytes.112Ginkgo biloba has also been studied for potential cardiovascular benefits. In preclinical studies, ginkgolide B has displayed anti-atherogenic activity with attenuated platelet aggregation and reduced P-selectin expression within aortic plaques of ApoE−/− mice. The mechanism of action has been attributed to the inhibition of the PI3k/Akt pathway in activated platelets.113,114
Conclusion and outlook
Targeting the P-selectin/PSGL-1 pathway has unfolded with significant strides since initial studies were first reported in 1990s. The past 2 decades have witnessed intensive efforts toward the development of P-selectin inhibitors through wide ranging preclinical studies with significant promise of clinical benefit for a variety of diseases. The design and evaluation of glycomimetics, small molecules, and aptamers as P-selectin antagonists have all provided valuable insight and have set the stage for increasingly effective strategies in drug discovery campaigns. The failure to achieve clinical end points for many of these pharmacological agents likely reflect limitations in clinical trial design, inadequate dosing, poor pharmacokinetic profiles, and variable inhibitory potency (Table 1). Nonetheless, population genetic studies strongly support the significance of the PSGL-1/P-selectin pathway in human disease.14 Moreover, despite conflicting evidence, the results of the SUSTAIN trial and related clinical studies of crizanlizumab provides compelling evidence that P-selectin blockade is capable of mitigating the substantial morbidity attributable to diseases caused by dysregulated thrombotic and inflammatory responses.32,33,35
Acknowledgments
The authors acknowledge support from the US National Institutes of Health (National Heart, Lung, and Blood Institute grant RO1 HL128237, National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK107405, and National Institute of General Medical Sciences grant U01GM116196 [E.L.C.]) and the Blavatnik Therapeutic Challenge Award from Harvard Medical School (E.L.C.).
Authorship
Contribution: S.E. conceptualized the study, wrote the draft manuscript, and reviewed and edited the manuscript; and E.L.C. conceptualized the study, supervised the study, acquired funding, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elliot L. Chaikof, Department of Surgery, Beth Israel Deaconess Medical Center, 110 Francis St, Suite 9F, Boston, MA 02115; email: echaikof@bidmc.harvard.edu.