Key Points
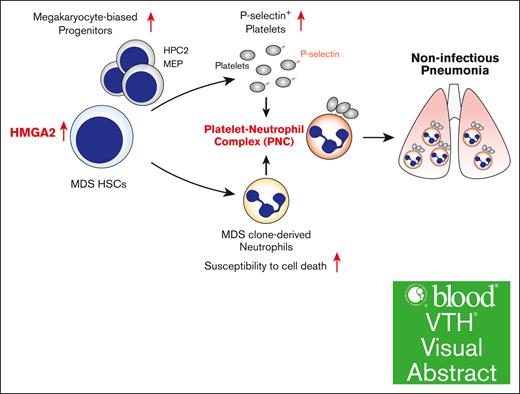
HMGA2 expression in HSC/Ps is significantly associated with the incidence of noninfectious pneumonia in patients with MDS.
HMGA2-mediated PNC formation is critical for the development of pulmonary tissue damage in MDS.
Visual Abstract
High mobility group AT-hook 2 (HMGA2) is an architectural transcription factor that functions as an oncogene in various cancers. Although overexpression of HMGA2 has been reported in several myeloid malignancies, its role varies considerably in different disease contexts. Here, we identified a distinct role of HMGA2 as a mediator of noninfectious pneumonia in myelodysplastic syndrome (MDS). The expression level of HMGA2 in CD34+ hematopoietic stem cells and progenitors (HSC/Ps) was significantly associated with the incidence of noninfectious pneumonia, a common systemic complication in patients with MDS. Consistent with this clinical investigation, HMGA2 overexpression in a mouse model of an MDS-associated mutation led to the development of lethal noninfectious pneumonia. Mechanistically, HMGA2 overexpression conferred a megakaryocytic lineage bias to HSC/Ps and contributed to platelet activation in MDS mice. P-selectin–positive activated platelets interacted with MDS clone–derived neutrophils that exhibit increased susceptibility to cell death and formed platelet-neutrophil complexes (PNCs). Both the frequency of PNCs and neutrophil cell death within the lung microenvironment increased in MDS mice overexpressing HMGA2. Genetic inhibition of P-selectin attenuated pulmonary tissue damage in MDS mice. These findings indicate that PNCs could be a new therapeutic target for noninfectious pneumonia in patients with MDS and provide new insights into the mechanistic basis of the systemic complications of MDS.
Introduction
High mobility group AT-hook 2 (HMGA2) is an architectural transcription factor that lacks intrinsic transcriptional activity but modulates gene expression by altering the 3-dimensional structure of chromatin, facilitating the binding of other transcription factors.1,2 HMGA2 is ubiquitously expressed in early embryonic stages and regulates embryonic development, cell differentiation, and cellular proliferation.3,4 Its expression becomes more restricted to specific tissues and cell lineages during fetal development and remains at a low level during adulthood.1 In contrast, elevated HMGA2 expression has been identified in many cancers.5-8 In the context of cancer pathogenesis, HMGA2 functions as an oncogene that facilitates oncogenic transformation, promotes epithelial-to-mesenchymal transition, and enhances metastatic potential, highlighting its importance as a potential therapeutic target.5-8
In addition to its well-established role in solid cancers, emerging evidence indicates the significance of HMGA2 in the hematopoietic system. In normal hematopoiesis, HMGA2 is predominantly expressed in hematopoietic stem cells and progenitors (HSC/Ps) and plays a crucial role in maintaining the fitness of HSC/Ps.9 Similar to what has been observed in solid cancers, elevated HMGA2 expression has been reported in several myeloid malignancies including myelodysplastic syndromes (MDSs),10 myeloproliferative neoplasms (MPNs),11,12 and acute myeloid leukemia (AML)13 that arise from HSC/Ps with genetic alterations. Among the intricate molecular processes underlying the development of these myeloid malignancies, dysregulation of HMGA2 has emerged as a compelling factor in the oncogenic landscape. Indeed, the role of HMGA2 as a fibrosis mediator in MPN has been well documented.14-16 Additionally, high HMGA2 expression is associated with immature transcriptional signature, lower frequency of complete remission, and higher frequency of relapse in AML.13 However, its specific role in the pathogenesis of MDS is not well studied.
Therefore, in this study, we investigated the role of HMGA2 and the effect of its expression on MDS in humans and mice.
Methods
Patients
Patients with MDS were examined as approved by the institutional review board. All patients provided written informed consent in accordance with the Declaration of Helsinki. Patients were selected according to the following criteria: (1) diagnosis of MDS, (2) age of ≥20 years, and (3) patients who visited hospitals between January 2011 and December 2017. The current study included inpatients (n = 17) for whom data of HMGA2 expression were available and who were followed-up from admission to discharge (including discharge by death) between May 2013 and May 2015. Mononuclear cells were isolated from bone marrow (BM) aspirates or peripheral blood (PB). From these mononuclear cells, CD34+ cells were purified using an EasySep Human CD34 Selection Kit (Stemcell Technologies). Total RNA was isolated using an RNeasy Micro Kit (Qiagen) and was reverse transcribed to complementary DNA using ReverTra Ace quantitative polymerase chain (PCR) reaction reverse transcription Master Mix with genomic DNA Remover (Toyobo). Expression of HMGA2 in purified CD34+ cells was quantified via real-time PCR using TaqMan Gene Expression Assays (HMGA2: Hs00171569_m1 and glyceraldehyde-3-phosphate dehydrogenase: Hs03929097_g1; Life Technologies) and TaqMan Universal PCR Master Mix (Life Technologies). Patients with MDS with noninfectious pneumonia in this analysis were patients who had a history of lung damage on chest computed tomography during the course of the disease and for whom the source of infection could not be identified by various culture tests.
Mice
C57BL/6 mice (7 weeks old, female) were purchased from Tokyo Laboratory Animal Science. All animal experiments were approved by and conducted according to the institutional animal care protocol and guidelines of the Tokyo University of Pharmacy and Life Sciences.
Flow cytometry
Flow cytometric analysis was performed using fluorescence-activated cell sorting (FACS): FACSCanto, FACSCelesta, and FACSAriaIII (BD Biosciences). Phosphate-buffered saline (PBS) containing 2% fetal bovine serum and 2 mM ethylenediaminetetraacetic acid was used as a FACS buffer. Data were analyzed using the FlowJo software (BD Biosciences). PB was collected using heparin-coated capillaries with an inner diameter of 0.93 mm and was promptly transferred to an EDTA-containing tube to prevent platelet aggregation. In addition, a smear specimen was prepared from a portion of the collected PB. Diff-Quik staining was performed to confirm the absence of platelet aggregation by microscopic observation. BM cells were isolated from euthanized mice by flushing their bones. Lung tissue specimens were isolated from euthanized mice and incubated with a solution containing DNase (100 μg/mL; Roche) and collagenase IV (120 U/mL; Gibco) for 30 minutes at 37°C. Subsequently, the lung tissue specimens were mechanically homogenized on a 100-μm mesh filter, followed by rinsing with FACS buffer to collect lung-infiltrated hematopoietic cells. Murine PB, BM cells, and lung-infiltrated cells were incubated with the following anti-mouse antibodies: CD11b (M1/70), c-Kit (2B8), Sca1 (D7), CD48 (HM48-1), CD150 (TC15-12F12.2), CD115 (AFS98), Gr-1 (RB6-8C5), CD45 (30-F11), CD41 (MWReg30), CD62P(RMP-1), and Ly6G (1A8) (BioLegend). For HSC/P and lung-infiltrated cell analyses, biotin-conjugated anti-mouse CD3e (145-2C11), CD4 (RM4-5), CD8a (53-6.7), CD19 (6D5), Ter119 (Ter-119), CD11b (M1/70), Gr-1 (RB6-8C5), NK1.1 (PK136), and B220 (RA3-6B2) antibodies (BioLegend) were used, followed by staining with streptavidin-PerCP/Cy5.5 for lineage exclusion. The nerve growth factor receptor protein was detected using an anti-human CD271 (ME20.4) antibody (BioLegend). An annexin V apoptosis detection kit (Biolegend) was used for phosphatidylserine detection.
H&E and reticulin staining
The collected lung and tibia tissues were fixed using a 10% formalin neutral buffer solution (Fujifilm Wako Pure Chemical Corporation). Lung specimens were embedded in paraffin for hematoxylin and eosin (H&E) staining. Tibial specimens were decalcified and embedded in paraffin for H&E and reticulin staining. Staining was performed by GenoStaff (Tokyo, Japan). Lung injury scoring was performed according to the system reported by the official American Thoracic Society Workshop.17 More than 10 random high-power fields were scored.
Immunofluorescence staining and confocal microscopy
FAC sorted (FACSAriaIII, BD Biosciences) CD45+ CD11b+ lung-infiltrated cells from 4 groups of mice were mixed with Smear Gell (GenoStaff) and spread on slides. Cells were then fixed with 4% paraformaldehyde in PBS, after which 15 mmol/L glycine in PBS was added. After PBS washing, cells were stained with the following antibodies: Alexa Fluor 647–conjugated anti-mouse Ly-6G (127609; Biolegend), anti-CD41 (N2C1; GeneTex), and Alexa Fluor 555–conjugated anti-rabbit immunoglobulin G (H+L; a21428, Invitrogen). Cells were then washed 3 times with PBS, mounted with Hoechst 33342, and examined using an Olympus FV3000-IX83 confocal fluorescence microscope (Olympus).
LPS administration
Mice were anesthetized via intraperitoneal injection of a mixture of 3 agents: medetomidine hydrochloride (0.75 mg/kg), midazolam (4 mg/kg), and butorphanol tartrate (5 mg/kg). After sufficient anesthesia, low-dose lipopolysaccharide (LPS) (5 μg/body; Sigma-Aldrich) was administered intranasally and delivered into the trachea. The mice were analyzed 16 hours after LPS administration.
Statistical analysis
GraphPad Prism (version 8; GraphPad) was used for statistical analyses. Survival of mice was analyzed using the log-rank test. Comparison of 2 independent, normally distributed samples was performed using the 2-tailed Student t test. For multiple pairwise comparisons, the data were analyzed using 1-way analysis of variance followed by Tukey multiple comparison test. The area under the receiver operating characteristic curve (AUC) was calculated using GraphPad Prism. Statistical significance was set at P < .05.
Detailed methods for quantitative reverse transcription PCR, immunoblot analysis, retrovirus and lentivirus generation, mouse BM transplantation (BMT) experiment, RNA-sequencing (RNA-seq) and data analysis, gene set enrichment analysis (GSEA), pathway enrichment analysis, and complete blood counts are described in supplemental Materials.
Results
HMGA2 expression level in HSC/Ps correlates with the incidence of noninfectious pneumonia in patients with MDS
To investigate the significance of a high expression level of HMGA2 in MDS, we examined the association between its expression levels in CD34+ BM cells and the clinical findings in patients with MDS (n = 17; supplemental Table 1). Based on the AUC, we first evaluated the effect of HMGA2 expression levels on blood counts, which are important biomarkers for prognostic prediction that are used the revised International Prognostic Scoring System. We found a significant association between the occurrence of neutropenia (<800 neutrophils per μL) and HMGA2 expression (AUC, 0.8286; P = .0248; Figure 1A-B). In contrast, no association was found between HMGA2 expression and anemia (Hb < 10 g/dL; AUC, 0.5833) or thrombocytopenia (<10 × 104 platelets per μL; AUC, 0.5857; Figure 1A,C-D). Because HMGA2 has been shown to be involved in disease progression in many cancers, we evaluated several factors related to leukemic transformation. However, neither the incidence of leukemic transformation (AUC, 0.6032), excessive blasts (AUC, 0.700), nor the existence of a poor karyotype (AUC, 0.6429) exhibited a significant association with HMGA2 expression levels (Figure 1E-H). Patients with MDS often develop various noninfectious complications and organ disorders due to systemic inflammation,18,19 and ∼20% of patients with MDS exhibit BM fibrosis.20 Thus, we also evaluated the incidence of several major systemic complications in MDS, including organizing pneumonia, kidney injury, hemorrhage, and BM fibrosis. Among these, the incidence of noninfectious pneumonia was significantly associated with HMGA2 expression (AUC, 0.9524; P = .0066; Figure 1I-M). In contrast, although HMGA2 has been reported as a potent mediator of BM fibrosis in JAK2-mutated MPN,10,14-16 we did not observe a significant correlation between the expression level of HMGA2 and the grade of BM fibrosis in patients with MDS. These findings indicate a disease context-dependent role for HMGA2 in MDS.
High expression level of HMGA2 is associated with the development of noninfectious pneumonia in patients with MDS. (A) Receiver operating characteristic (ROC) curves showing the association between HMGA2 messenger RNA (mRNA) level in CD34+ BM cells and cytopenias in patients with MDS (n = 17). Corresponding AUC values are shown. (B-D) mRNA expression levels of HMGA2 in CD34+ BM cells from the indicated groups. The horizontal bar shows the median value. (E) ROC curves showing the association between HMGA2 mRNA level in CD34+ BM cells and parameters for disease progression in patients with MDS (n = 17). Corresponding AUC values are shown. (F-H) mRNA expression levels of HMGA2 in CD34+ BM cells from the indicated groups. The horizontal bar shows the median value. (I) ROC curves showing the association between HMGA2 mRNA level in CD34+ BM cells and major systemic complications in patients with MDS (n = 17). Corresponding AUC values are shown. (J-M) mRNA expression levels of HMGA2 in CD34+ BM cells from the indicated groups. The horizontal bar shows the median value.
High expression level of HMGA2 is associated with the development of noninfectious pneumonia in patients with MDS. (A) Receiver operating characteristic (ROC) curves showing the association between HMGA2 messenger RNA (mRNA) level in CD34+ BM cells and cytopenias in patients with MDS (n = 17). Corresponding AUC values are shown. (B-D) mRNA expression levels of HMGA2 in CD34+ BM cells from the indicated groups. The horizontal bar shows the median value. (E) ROC curves showing the association between HMGA2 mRNA level in CD34+ BM cells and parameters for disease progression in patients with MDS (n = 17). Corresponding AUC values are shown. (F-H) mRNA expression levels of HMGA2 in CD34+ BM cells from the indicated groups. The horizontal bar shows the median value. (I) ROC curves showing the association between HMGA2 mRNA level in CD34+ BM cells and major systemic complications in patients with MDS (n = 17). Corresponding AUC values are shown. (J-M) mRNA expression levels of HMGA2 in CD34+ BM cells from the indicated groups. The horizontal bar shows the median value.
HMGA2 overexpression in HSC/Ps causes lethal pneumonia in MDS mice
To determine the role of HMGA2 in the pathogenesis of MDS, we generated a mouse model of MDS using patient-derived RUNX1 S291 frameshift mutation (RUNX1S291fs) via in vivo BMT21,22 and integrated HMGA2 overexpression into this model (RUNX1S291fs/HMGA2-OE mice; Figure 2A; supplemental Figure 1). Mice were transplanted with HMGA2-OE cells (HMGA2-OE mice) or empty vector–transduced cells (Empty mice). Consistent with previous reports,22,23RUNX1S291fs mice did not become moribund within 200 days after BMT (Figure 2B). In contrast, RUNX1S291fs/HMGA2-OE and HMGA2-OE mice developed a lethal phenotype within 200 days after BMT (Figure 2B). Notably, the median survival of RUNX1S291fs/HMGA2-OE mice (158 days) was significantly shorter than that of HMGA2-OE mice (185 days; Figure 2B). These results suggest that high expression of HMGA2 in MDS clones is associated with progression of severe adverse events. Leukocytopenia and neutropenia were observed in the PB of the debilitated RUNX1S291fs/HMGA2-OE mice (Figure 2C; supplemental Figure 2A-B). These results were consistent with the findings observed in patients with MDS with high HMGA2 expression. In the BM, c-Kit+ fraction increased and mature granulocyte fraction decreased in RUNX1S291fs/HMGA2-OE mice compared with Empty mice, suggesting the occurrence of impaired BM granulopoiesis in this model (Figure 2D-G). The double-transduced RUNX1S291fs/HMGA2-OE clones were dominant within population of lineage marker–negative c-Kit+ HSC/Ps in RUNX1S291fs/HMGA2-OE mice (supplemental Figure 2C). Further analysis of the HSC/P population revealed an increase in several progenitor fractions, particularly fractions of hematopoietic progenitor cell 2 (HPC2), lineage marker–negative c-Kit+, and myeloid progenitors compared with the Empty mice (Figure 2H-J; supplemental Figure 2D-E). In contrast, RUNX1S291fs/HMGA2-OE mice did not develop significant anemia or thrombocytopenia (Figure 2B). Additionally, although the role of HMGA2 as a fibrosis mediator in MPN has been reported,14-16 BM fibrosis was not observed in RUNX1S291fs/HMGA2-OE mice (supplemental Figure 2F). Thus, abnormalities in blood count parameters, including leukocytopenia, are unlikely to be the cause of the lethal conditions observed in RUNX1S291fs/HMGA2-OE mice. In contrast, consistent with observations in patients, RUNX1S291fs/HMGA2-OE mice developed severe pneumonia (Figure 3A-B). Moribund HMGA2-OE mice also exhibited lung injury with long latency (Figure 3A-B). Flow cytometric analysis revealed a prominent infiltration of CD11b+ myeloid cells, particularly neutrophils, into the lung tissue (Figure 3C-D). We confirmed that the double-transduced RUNX1S291fs/HMGA2-OE clones were dominant in these lung-infiltrated cells from RUNX1S291fs/HMGA2-OE mice (supplemental Figure 2G). Taken together and concordant with clinical observations, these results suggest that high expression levels of HMGA2 in MDS HSC/Ps could cause the development of pulmonary tissue damage in mice.
HMGA2 overexpression in HSC/Ps results in impaired granulopoiesis and neutropenia in MDS mice. (A) Schematic of experimental design. (B) Survival of Empty (n = 5), RUNX1S291fs (n = 5), HMGA2 (n = 6), and RUNX1S291fs/HMGA2 mice (n = 6). (C) White blood cell (WBC) counts, hemoglobin (Hb) concentration, and platelet counts in the PB of mice. Data are represented by the mean ± standard deviation (SD). (D-E) Flow cytometric analysis of BM cells of indicated mice (n = 5 per each group). Frequency of c-Kit+ fraction is shown in panel E. Data are presented as the mean ± SD. (F-G) Flow cytometric analysis of BM cells of indicated mice (n = 5 per each group). Frequency of granulocyte fraction is shown in panel G. Data are presented as the mean ± SD. (H-J) Flow cytometric analysis of BM cells of indicated mice (n = 5 per each group). Frequency of CD150+ CD48− HSC, CD150− CD48− MPP, CD150− CD48+ HPC1, and CD150+ CD48+ HPC2 in BM cells is shown in panel I. Data are presented as the mean ± SD. Frequency of LSK, lineage marker–negative c-Kit+ (LK), CMP, GMP, and in BM cells is shown in panel J. Data are presented as the mean ± SD. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. CMP, common myeloid progenitors; GMP, granulocyte-macrophage progenitors; LSK, lineage marker− Sca1+ c-Kit+ cells; MEP, megakaryocyte-erythroid progenitors; MPP, multipotent progenitors.
HMGA2 overexpression in HSC/Ps results in impaired granulopoiesis and neutropenia in MDS mice. (A) Schematic of experimental design. (B) Survival of Empty (n = 5), RUNX1S291fs (n = 5), HMGA2 (n = 6), and RUNX1S291fs/HMGA2 mice (n = 6). (C) White blood cell (WBC) counts, hemoglobin (Hb) concentration, and platelet counts in the PB of mice. Data are represented by the mean ± standard deviation (SD). (D-E) Flow cytometric analysis of BM cells of indicated mice (n = 5 per each group). Frequency of c-Kit+ fraction is shown in panel E. Data are presented as the mean ± SD. (F-G) Flow cytometric analysis of BM cells of indicated mice (n = 5 per each group). Frequency of granulocyte fraction is shown in panel G. Data are presented as the mean ± SD. (H-J) Flow cytometric analysis of BM cells of indicated mice (n = 5 per each group). Frequency of CD150+ CD48− HSC, CD150− CD48− MPP, CD150− CD48+ HPC1, and CD150+ CD48+ HPC2 in BM cells is shown in panel I. Data are presented as the mean ± SD. Frequency of LSK, lineage marker–negative c-Kit+ (LK), CMP, GMP, and in BM cells is shown in panel J. Data are presented as the mean ± SD. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. CMP, common myeloid progenitors; GMP, granulocyte-macrophage progenitors; LSK, lineage marker− Sca1+ c-Kit+ cells; MEP, megakaryocyte-erythroid progenitors; MPP, multipotent progenitors.
HMGA2 overexpression in HSC/Ps causes lethal noninfectious pneumonia in MDS mice. (A-B) H&E staining of lung section from indicated mice. Scale bars: top panels, 250 μm (original magnification ×40); middle panels, 50 μm (original magnification ×100); and bottom panels, 25 μm (original magnification ×400). Lung injury score of individual samples is shown in panel B (n = 3 per each group). More than 10 random high-power fields were scored. Data are presented as the mean ± SD. (C-D) Flow cytometric analysis of lung-infiltrated cells of indicated mice (n = 3 per each group). Frequencies of CD11b+ myeloid cells and Ly6G+ neutrophils within lung-infiltrated cells are shown in panel D. Data are presented as the mean ± SD. ∗∗P < .01.
HMGA2 overexpression in HSC/Ps causes lethal noninfectious pneumonia in MDS mice. (A-B) H&E staining of lung section from indicated mice. Scale bars: top panels, 250 μm (original magnification ×40); middle panels, 50 μm (original magnification ×100); and bottom panels, 25 μm (original magnification ×400). Lung injury score of individual samples is shown in panel B (n = 3 per each group). More than 10 random high-power fields were scored. Data are presented as the mean ± SD. (C-D) Flow cytometric analysis of lung-infiltrated cells of indicated mice (n = 3 per each group). Frequencies of CD11b+ myeloid cells and Ly6G+ neutrophils within lung-infiltrated cells are shown in panel D. Data are presented as the mean ± SD. ∗∗P < .01.
HMGA2 overexpression leads to a distinct gene signature in MDS clones
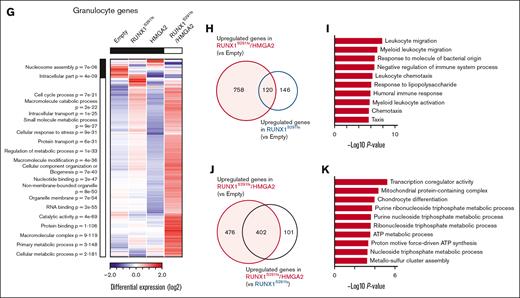
To determine the effect of HMGA2 overexpression on MDS HSC/Ps, we performed RNA-seq analysis of HSC/Ps obtained from RUNX1S291fs/HMGA2-OE mice and other groups of mice. Principal component analysis revealed that high expression levels of HMGA2 in HSC/Ps altered gene expression patterns in both MDS and wild-type (WT) clones (Figure 4A). Among the differentially expressed genes within the 4 groups, those related to neutrophil development, such as S100a9, Lcn2, Mmp9, and Cebpe, were downregulated in RUNX1S291fs/HMGA2-OE and HMGA2-OE mice (Figure 4B). This was consistent with the reduction of granulocyte population within the BM in these mice. In contrast, genes related to the expansion and fitness of HSC/Ps, such as Esam, Meis1, Hlf, and Gata2, were upregulated in RUNX1S291fs/HMGA2-OE mice, suggesting that HMGA2 overexpression in combination with RUNX1 mutant upregulates the expression of an additional subset of genes involved in expansion and fitness of HSC/Ps (Figure 4B). GSEA confirmed these gene expression signatures in the comparison of RUNX1S291fs/HMGA2-OE and Empty mice (Figure 4C-D; supplemental Table 2). Genes related to megakaryocyte development and platelet activation, such as Selp, Mpl, and Pf4, were expressed at higher levels in RUNX1S291fs and HMGA2-OE mice than in Empty mice. Notably, these genes were further upregulated in RUNX1S291fs/HMGA2-OE mice (Figure 4B,E; supplemental Table 2). Consistent with this gene signature, RUNX1S291fs/HMGA2-OE mice exhibited an expansion of the HPC2 population, which has been suggested to have megakaryocytic potential24,25 (Figure 2H-J; supplemental Figure 2D-E).
We also performed RNA-seq analysis of neutrophils obtained from individual mouse groups. Unlike HSC/Ps, the gene expression pattern of RUNX1S291fs/HMGA2-OE mice was distinct from that of HMGA2-OE mice and the other groups of mice (Figure 4F). Analysis of differentially expressed genes among the 4 groups revealed that MDS mutant-mediated alterations in gene expression primarily occurred and that HMGA2 overexpression synergistically affected them (Figure 4G). Genes upregulated in both RUNX1S291fs/HMGA2-OE and RUNX1S291fs mice compared with Empty mice were significantly enriched in leukocyte migration–related signaling pathways, indicating that MDS clone–derived neutrophils exhibited a migration advantage to tissues (Figure 4H-I; supplemental Table 3). Given that RUNX1S291fs mice do not develop lethal pneumonia, we hypothesized that HMGA2-mediated synergistic effects on downstream gene expression may confer a tissue-damaging ability to MDS clone–derived neutrophils. Concordantly, genes upregulated in RUNX1S291fs/HMGA2-OE mice compared with RUNX1S291fs and Empty mice were significantly enriched in pathways related to mitochondrial oxidative phosphorylation and metabolism, which have been suggested to contribute to neutrophil cytotoxic potential and cell death26 (Figure 4J-K; supplemental Table 3). Taken together, these data suggest that HMGA2 overexpression–mediated alterations in gene expression in MDS clones are responsible for the lethal pathological conditions observed in RUNX1S291fs/HMGA2-OE mice.
Platelets form PNCs in MDS mice with pneumonia
Increasing evidence demonstrates that the interplay between activated platelet and innate immune cells, particularly neutrophils, is a critical mediator of pulmonary tissue damage.27 Transcriptomic analysis showed that genes related to platelet activation signaling are significantly upregulated in RUNX1S291fs/HMGA2-OE mice HSC/Ps in which megakaryocyte lineage–committed HPC2 as well as megakaryocytes-erythrocyte progenitors were expanded (Figures 2H-J and 4B,E; supplemental Figure 2D-E); thus, we hypothesized that platelet activation occurs and contributes to development of pneumonia in this model. Among the genes encoding the major platelet activation molecules, Selp, which encodes P-selectin (also known as CD62P), was the most highly expressed (supplemental Figure 3A). We confirmed protein expression using flow cytometry and found that the frequency of Selp protein–expressing platelets significantly increased in RUNX1S291fs/HMGA2-OE mice (Figure 5A-C). Many experimental and clinical studies have demonstrated that P-selectin accelerates platelet-neutrophil aggregation and activates neutrophils. Thus, to evaluate whether platelets formed platelet-neutrophil complexes (PNCs) in RUNX1S291fs/HMGA2-OE mice, we isolated lung-infiltrated hematopoietic cells and performed flow cytometric analysis (Figure 5D). As expected, the frequency of CD41+ CD11b+ population increased in the lung tissue of RUNX1S291fs/HMGA2-OE mice (Figure 5E-F; supplemental Figure 3B). To further confirm the existence of PNCs, we performed immunofluorescence staining of Ly6G and CD41 using CD11b+ myeloid cells obtained from the lungs of the 4 groups of mice at 4 months after BMT. Consistent with the results of flow cytometric analysis, we found a higher frequency of PNCs in the lung of the RUNX1S291fs/HMGA2-OE mice than in those in other groups (Figure 5G-H; supplemental Figure 3C). GSEA also revealed that genes related to platelet activation signaling and inflammatory pathways were significantly upregulated, even in neutrophils from RUNX1S291fs/HMGA2-OE mice (supplemental Figure 3D). These findings indicate the occurrence of PNCs in the HMGA2-OE MDS model. To determine the effect of PNC formation on neutrophils, we evaluated the susceptibility to cell death in lung-infiltrated neutrophils obtained from RUNX1S291fs/HMGA2-OE and Empty mice. The frequency of annexin V–positive cells significantly increased in lung-infiltrated neutrophils from RUNX1S291fs/HMGA2-OE mice (Figure 5I-J), indicating that these neutrophils, which contain a high frequency of PNCs, were more prone to cell death than those in control mice. These data suggest that platelets and neutrophils derived from HMGA2-OE MDS HSC/Ps interact with each other and that the interplay between them could alter the features of neutrophils in MDS.
Platelets interact with neutrophils in HMGA2-overexpressing MDS mice. (A) Schematic of sample collection and FACS gating strategy. (B-C) Flow cytometric analysis of PB cells from indicated mice. Frequency of CD62P+ (P-selectin positive) platelets is shown in panel C. Data are presented as the mean ± SD. (D) Schematic of sample collection and FACS gating strategy. (E-F) Flow cytometric analysis of lung-infiltrated cells of Empty (n = 5) and RUNX1S291fs/HMGA2 mice (n = 6). Frequency of CD41+ cells within CD11b+ lung-infiltrated cells is shown in panel F. Data are presented as the mean ± SD. The difference between the 2 groups is approximately twofold (1.95 times). (G-H) Representative immunofluorescence images of CD41+ Ly6G+ PNCs within CD11b+ cells obtained from the lung of RUNX1S291fs/HMGA2 mice at 4 months after BMT. Scale bar, 5 μm. (E) Frequency of PNCs (≥100 cells per sample) from Empty (n = 5) and RUNX1S291fs/HMGA2 mice (n = 3). Data are represented by the mean ± SD. (I-J) Annexin V flow cytometric analysis of lung-infiltrated cells of Empty (n = 5) and RUNX1S291fs/HMGA2 mice (n = 6). Frequency of annexin V+ cells within lung-infiltrated neutrophils is shown in panel J. Data are presented as the mean ± SD. ∗P < .05 and ∗∗P < .01.
Platelets interact with neutrophils in HMGA2-overexpressing MDS mice. (A) Schematic of sample collection and FACS gating strategy. (B-C) Flow cytometric analysis of PB cells from indicated mice. Frequency of CD62P+ (P-selectin positive) platelets is shown in panel C. Data are presented as the mean ± SD. (D) Schematic of sample collection and FACS gating strategy. (E-F) Flow cytometric analysis of lung-infiltrated cells of Empty (n = 5) and RUNX1S291fs/HMGA2 mice (n = 6). Frequency of CD41+ cells within CD11b+ lung-infiltrated cells is shown in panel F. Data are presented as the mean ± SD. The difference between the 2 groups is approximately twofold (1.95 times). (G-H) Representative immunofluorescence images of CD41+ Ly6G+ PNCs within CD11b+ cells obtained from the lung of RUNX1S291fs/HMGA2 mice at 4 months after BMT. Scale bar, 5 μm. (E) Frequency of PNCs (≥100 cells per sample) from Empty (n = 5) and RUNX1S291fs/HMGA2 mice (n = 3). Data are represented by the mean ± SD. (I-J) Annexin V flow cytometric analysis of lung-infiltrated cells of Empty (n = 5) and RUNX1S291fs/HMGA2 mice (n = 6). Frequency of annexin V+ cells within lung-infiltrated neutrophils is shown in panel J. Data are presented as the mean ± SD. ∗P < .05 and ∗∗P < .01.
Genetic inhibition of P-selectin attenuates development of lung injury in MDS mice
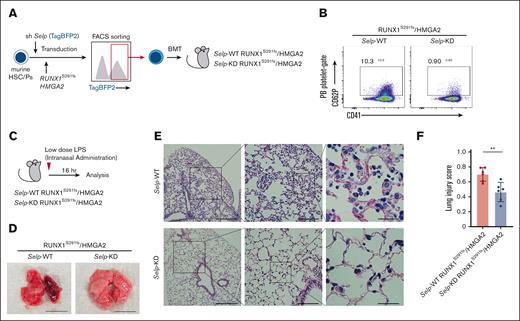
To investigate in vivo therapeutic efficacy of targeting PNCs in the pathogenesis of MDS, we integrated a lentiviral knockdown (KD) system into our BMT mouse model and generated RUNX1S291fs/HMGA2-OE mice with or without Selp KD (Figure 6A). We tested 3 short hairpin RNAs targeting different sequences within cording region of Selp messenger RNA. We then used short hairpin RNA number 1 for in vivo experiments (supplemental Figure 4A). Knock down of Selp was confirmed at the protein level via flow cytometric analysis of PB platelets obtained from RUNX1S291fs/HMGA2-OE mice with Selp KD (Figure 6B). We also determined Selp expression in other lineages. However, we found little Selp protein expression in PB leukocytes and BM myeloid cells and no significant differences between Selp-WT and Selp-KD RUNX1S291fs/HMGA2-OE mice (supplemental Figure 4B). When we analyzed Selp-WT and Selp-KD RUNX1S291fs/HMGA2-OE mice at 1.5 to 2 months after transplantation, there was no difference in the frequency of c-Kit+ BM cells between the 2 groups (supplemental Figure 4C). No obvious difference in gross lung findings was observed between them (supplemental Figure 4D). These findings indicate that Selp protein expression is highly specific to platelet population. Considering that the RUNX1S291fs/HMGA2-OE mice develop lethal lung injury several months after BMT, not only intrinsic but also some extrinsic stimuli likely contribute to the onset of lung injury. To evaluate the effect of Selp KD on the development of lung injury in MDS, we performed low-dose administration of LPS, which is widely used in animal models to experimentally recapitulate chronic inflammation mediated by both pathogen-associated molecular patterns and damage-associated molecular patterns (DAMPs).28,29 After intranasal administration of mice with low-dose LPS (5 μg per body), lung injury in RUNX1S291fs/HMGA2-OE mice was more severe than that in control mice (supplemental Figure 5A-B). Despite the difference in pulmonary tissue damage, the frequencies of lung-infiltrated myeloid cells and neutrophils were comparable between the control and RUNX1S291fs/HMGA2-OE mice (supplemental Figure 5C-D). These results suggest that alterations in neutrophil features are critical for the development of lung injury. Next, we intranasally administered low-dose LPS to RUNX1S291fs/HMGA2-OE mice with or without Selp KD (Figure 6C). Genetic inhibition of P-selectin significantly attenuated the development of lung injury in RUNX1S291fs/HMGA2-OE mice (Figure 6D-F). These results suggest that P-selectin–mediated PNC formation is a critical mediator of pulmonary tissue damage through the induction of neutrophil death in the pathogenesis of MDS.
Genetic inhibition of P-selectin attenuates lung injury in MDS mice. (A) Schematic of the experimental procedure. (B) Confirmation of P-selectin knockdown in PB platelets from RUNX1S291fs/HMGA2 mice. (C) Schematic of the experimental procedure for intranasal LPS administration. (D) Gross morphology of lung from indicated mice after low-dose LPS administration. Representative gross morphology is shown. Scale bar indicates 10 mm. (E-F) H&E staining of a lung section from RUNX1S291fs/HMGA2 mice with or without Selp knocked down after low-dose LPS administration (n = 6 per each group). Scale bars: left panels, 250 μm (original magnification ×40); middle panels, 50 μm (original magnification ×100); and right panels, 25 μm (original magnification ×400). Lung injury score of individual samples is shown in panel F. More than 10 random high-power fields were scored. Data are presented as the mean ± SD. ∗∗P < .01.
Genetic inhibition of P-selectin attenuates lung injury in MDS mice. (A) Schematic of the experimental procedure. (B) Confirmation of P-selectin knockdown in PB platelets from RUNX1S291fs/HMGA2 mice. (C) Schematic of the experimental procedure for intranasal LPS administration. (D) Gross morphology of lung from indicated mice after low-dose LPS administration. Representative gross morphology is shown. Scale bar indicates 10 mm. (E-F) H&E staining of a lung section from RUNX1S291fs/HMGA2 mice with or without Selp knocked down after low-dose LPS administration (n = 6 per each group). Scale bars: left panels, 250 μm (original magnification ×40); middle panels, 50 μm (original magnification ×100); and right panels, 25 μm (original magnification ×400). Lung injury score of individual samples is shown in panel F. More than 10 random high-power fields were scored. Data are presented as the mean ± SD. ∗∗P < .01.
Discussion
MDS comprises a group of refractory clonal HSC disorders characterized by ineffective hematopoiesis and dysplasia. Patients with MDS frequently exhibit concomitant autoimmune/inflammatory diseases and systemic organ damage. Additionally, some patients with MDS exhibit BM fibrosis, which is an independent adverse prognostic factor regardless of disease risk classification.20 Recent studies have revealed the dysregulation of innate immune signaling pathways in MDS, providing molecular insights into the clinical evidence linking inflammation and MDS pathogenesis. In this study, we show that HMGA2 expression in the MDS clone is significantly associated with the incidence of noninfectious pneumonia, one of the systemic complications in patients with MDS, and that P-selectin–mediated formation of PNCs is critical for the development of pulmonary tissue damage in MDS pathogenesis and is therefore a potential therapeutic target for the disease.
Although high expression levels of HMGA2 in WT HSC/Ps also eventually resulted in the development of pneumonia in BMT mice with long latency, we found that the progression of the HMGA2-OE–mediated phenotype was markedly accelerated in a mouse model of MDS-associated mutation. This indicates that the interplay between MDS clone–derived factors and HMGA2 overexpression–mediated activation of downstream genes is critical for the development of pneumonia. Despite the presence of genetic alterations, MDS HSC/Ps retain differentiation capacity and continue producing aberrant mature cell populations in all lineages including innate immune cells.23,30 Moreover, unlike MPN and AML, the proliferation ability of MDS clones is not prominent but rather defective because of increased cell death.23,29 Increasing evidence also demonstrated that chronic inflammation due to dysregulated innate immune signaling pathways resulting from various genetic alterations plays a pivotal role in initiating MDS pathogenesis.23,31,32 Importantly, activation of innate immune signaling pathways could promote cell death mechanisms, such as pyroptosis and necroptosis, that contribute to the release of abundant DAMPs.31,32 Such DAMPs further accelerate the activation of innate immune signaling in MDS clones.31,32 This phenomenon underscores the significance of cell death in the development of inflammatory MDS pathogenesis. Consistent with this, we found that gene signatures related to neutrophil cell death and leukocyte migration were induced in both RUNX1S291fs and RUNX1S291fs/HMGA2-OE mice (but not in HMGA2-OE mice). Upregulated genes in RUNX1S291fs/HMGA2-OE neutrophils compared with neutrophils of Empty mice were significantly enriched in interferon signaling pathways, which are among the most significantly dysregulated innate immune pathways in MDS. These findings indicate that the MDS clone exhibits increased susceptibility to the induction of cell death compared with the WT clone. It has been well demonstrated that inflammatory cell deaths mediated by pathogen-associated molecular patterns or DAMPs could trigger the release of large amounts of DAMPs and cytokines into the surrounding environment, leading to tissue damage.33-35
Our data demonstrated that a high expression level of HMGA2 in MDS clones was specifically associated with pulmonary tissue damage rather than damage to other organs in both humans and mice. Lung megakaryocytes were first described in 1893,36 but platelet production from megakaryocytes has long been believed to occur primarily in BM. However, recent findings from multiple studies have provided clear evidence that megakaryocytes not only exist in the BM but also in pulmonary vasculature and interstitium, in which circulation of a large number of megakaryocytes and efficient platelet production have been demonstrated.37-39 Emerging evidence further demonstrates that the number of lung megakaryocytes increase in various pathological conditions including inflammatory diseases and cancers.40 More recently, marked increase in the number of lung megakaryocytes has been reported in patients with severe COVID-19.41,42 We note that high HMGA2 expression not only conferred megakaryocytic lineage bias to HSC/Ps but also contributed to upregulation of genes related to platelet activation, such as Selp. Various recent studies demonstrated that activated platelets could form PNCs that promote neutrophil activation and cell death in various diseases including severe complications during COVID-19.43-45 Our data revealed the significance of complex formation between platelets and neutrophils in the development of pulmonary tissue damage in MDS mice. Furthermore, increased susceptibility to the induction of cell death was observed in neutrophils derived from the MDS clone. In MDS, aberrant mature cells, including neutrophils, are consistently generated from MDS HSC/Ps and circulate throughout highly vascularized organs. Considering the abundance of megakaryocytes and platelet production in the lungs, the pulmonary microenvironment in MDS pathogenesis likely provides a favorable setting for the formation of PNCs, leading to pulmonary tissue damage due to excessive neutrophil cell death.
In summary, our data from clinical investigations and experiments using murine models reveal a novel disease-specific role of HMGA2 in MDS and indicate that elevated expression of HMGA2 in MDS clones promotes PNC formation and neutrophil cell death, thereby triggering pulmonary tissue damage. Our findings provide new insights into the mechanistic basis of systemic complications in patients with MDS.
Acknowledgments
This work was supported by Japan Science and Technology Agency, Support for Pioneering Research Initiated by the Next Generation grant JPMJSP2134 (N.M.) and Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research grant JP21K07073 (H.K.).
Authorship
Contribution: Y. Hayashi and H.H. contributed to study design and conception; N.M., Y. Hayashi., M.F., K.Y., Y.K.-A., H.K., and Y. Harada performed experiments; Y. Hayashi, N.S., Y. Harada, and H.H. acquired and interpreted the data; and Y. Hayashi, Y. Harada, and H.H. wrote and revised the manuscript.
Conflict-of-interest disclosure: H.H. reports consultancy for Novartis. The remaining authors declare no competing financial interests.
Correspondence: Yoshihiro Hayashi, Laboratory of Oncology, Tokyo University of Pharmacy and Life Sciences, 1432-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan; email: yshrhys@toyaku.ac.jp; and Hironori Harada, Laboratory of Oncology, Tokyo University of Pharmacy and Life Sciences, 1432-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan; email: hharada@toyaku.ac.jp.
References
Author notes
Data on the following were deposited in the Gene Expression Omnibus: BM c-Kit+ cells from HMGA2-overexpressing (HMGA2-OE) and RUNX1S291fs/HMGA2-OE mice as well as Ly6G+ neutrophils (GSE242015), and BM c-Kit+ cells from control and RUNX1S291fs mice (GSE151694). The following secure token has been created to allow review of record GSE242015 while it remains in private status: qxwdkouafbwlnkx. All data that support the findings of this study are available, upon request, from the corresponding authors, Yoshihiro Hayashi (yshrhys@toyaku.ac.jp) or Hironori Harada (hharada@toyaku.ac.jp).
The full-text version of this article contains a data supplement.