Visual Abstract
Hodgkin lymphoma (HL) incidence is on the rise globally with higher morbidity and mortality rates in low- and middle-income countries (LMICs). The role of treatment patterns in these disparities is not well understood. We systematically reviewed the published literature on adult HL in LMICs with specific focus on treatment to identify potential gaps in access that may contribute to inequities in outcomes. We searched PubMed, Embase, Scopus, Global Health (EBSCOhost), and Global Index Medicus databases. Two independent reviewers screened articles for inclusion, performed data extraction, and assessed the quality of each study. The review included 111 studies from 34 different LMICs. Most cohorts of HL adults were young, predominantly male, and had advanced stage III/IV HL. Radiation therapy was widely used in only 75 studies (68%) overall. Among first-line studies, 59 (71%) used doxorubicin, bleomycin, vinblastine, and dacarbazine as their primary chemotherapy treatment regimen. Among relapsed/refractory (R/R) HL studies, autologous stem cell transplantation was primarily used in 18 studies (62%). Median 2-year overall survival (OS) was 91% in first-line studies compared with 83% in R/R HL studies. Median 5-year OS in frontline studies was 85% compared with 75% for R/R HL cohorts. There was a positive trend between 2-year OS and gross domestic product per capita. Data on adverse events and treatment-related mortality were underreported. Although we present, to our knowledge, the most comprehensive data on HL treatment options and outcomes in LMICs, our findings are limited by the few studies published in low-income countries and the lack of prospective, high-quality data. Further investigation of HL disparities in LMICs is needed.
Introduction
Hodgkin lymphoma (HL) is the 26th most common cancer globally, with an estimated 82 409 cases and 22 701 deaths annually.1 HL disproportionately affects children and young adults and its incidence, especially among women and adults aged 18 to 29 years, is on the rise globally.2,3 Importantly, HL is highly curable in most patients with basic cytotoxic chemotherapy with or without radiation therapy. In high-income countries (HICs), such as the United States, even advanced, late-stage HL has relatively high survival rates, with a 5-year survival exceeding 80% for stage IV disease.4 Such outstanding survival outcomes are because of sophisticated health systems that can administer intensive chemotherapy as well as the accessibility of treatments for relapsed/refractory (R/R) disease, such as autologous stem cell transplant and other innovative therapeutics.5 However, many of these advanced therapies for R/R HL are not available in low- and middle-income countries (LMICs); therefore, a comprehensive understanding of the burden, treatment capabilities, and outcomes is needed to improve global cancer equity.
Although previous publications have highlighted the global epidemiology of HL, including a higher mortality rate and greater disability-adjusted life-years lost because of HL in LMICs, there is a gap in the literature surrounding treatment patterns and what role treatment patterns play in these disparities.2,6 Furthermore, which treatment modalities are commonly used and available for HL across LMICs has not, to our knowledge, been comprehensively described previously. Treatment outcomes may vary because of differences in either efficacy or toxicity. Thus, understanding the underlying factors driving these disparities in HL treatment in LMICs is key in identifying and tailoring optimal therapies in different treatment environments. In individual studies, HL treatment in LMICs has been shown to be both effective and affordable, even without access to radiation in some cases.7,8 However, survival outcomes are variable and continue to lag behind those observed in high-resource settings. Potential factors that may contribute to worse outcomes in LMICs include differential access to treatment such as radiotherapy and stem-cell transplantation, health system infrastructure limitations, late or advanced HL presentation, HIV and other coinfections, and certain disease characteristics, although these contributing factors are understudied.9
In this systematic review, we aim to fill this gap by consolidating previously published studies conducted in LMICs with a specific focus on treatment patterns and outcomes to identify gaps in access that may contribute to inequities in outcomes.
Methods
Search strategy and selection criteria
A systematic review of the literature was performed to synthesize existing evidence on HL treatment in LMICs. This systematic review was conducted using the 2020 PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.10 The study protocol was registered on Prospero (CRD42023398618).
We conducted a systematic search of the following databases on 6 February 2023: PubMed, Embase (Embase), Scopus (Elsevier), Global Health (EBSCOhost), and Global Index Medicus. The search was designed to include relevant articles that encompassed 3 main concepts and related terms: “[LMICs]” AND “[Hodgkin lymphoma]” AND “[Therapeutics].” Articles were excluded that included reference to “[non-Hodgkin lymphoma].” A comprehensive search strategy (supplemental Table 1) was developed and adapted for each database under the facilitation of health science librarians. A second search using this strategy was completed on 2 July 2024 on the PubMed database to include recent articles published in 2023 or 2024.
Studies published in peer-reviewed journals were eligible for the review if they reported on adult HL treatment modalities and outcomes in LMICs. LMICs were classified as low income, lower-middle income, and upper-middle income in accordance with the 2021 World Bank income level classifications.11 The 2021 Human Development Index level (HDI) was also obtained for each country.12 Only articles written in English, Spanish, Portuguese, and French were included because of the team’s language skill limitations.
This systematic review excluded editorials, conference proceedings, reviews, case reports, and conference abstracts. Studies that did not include either treatment response or survival outcome data were excluded. Studies on children and adolescents (aged <18 years) only were excluded.
Quality assessment
The quality and bias of included studies was assessed using guidelines derived from the Critical Appraisal Skills Programme cohort study checklist.13 The checklist comprises 8 questions that address study design and methodology, treatment modalities and outcomes, and appropriate follow-up. Although follow-up in cohort studies in LMICs may be challenging, it has been reported that a “lost to follow-up” rate of >20% may compromise the validity of a study.14 Thus, studies with follow-up loss of ≥20% were categorized as lower quality. Articles were also deemed lower quality for unclear or unreported study design, treatment regimens, survival outcomes, or demographic characteristics (sex, age, and cancer staging). From a total possible score of 8, the overall quality scores were categorized as high (7-8), medium (6), and low (≤5). Two independent reviewers assessed the quality of all included studies. Disagreements between the reviewers were resolved by discussion and subsequent consensus. The quality assessment guidelines and associated point values are described in Appendix 1.
Data analysis
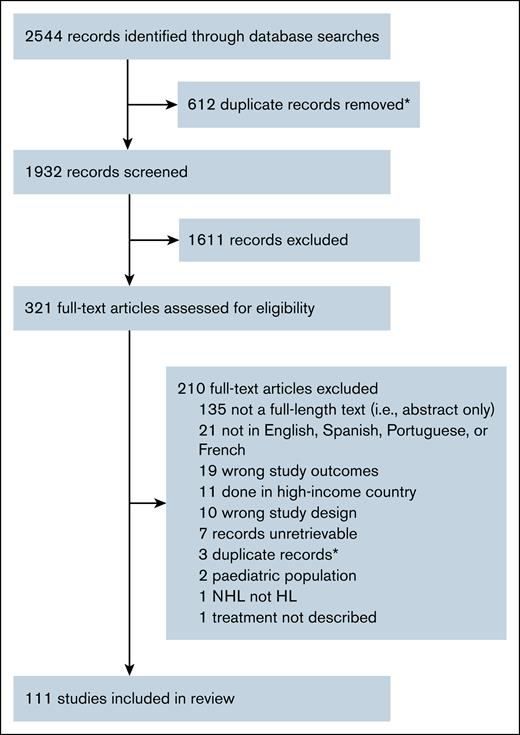
References retrieved from all databases were extracted to, and deduplicated, using Endnote ×9 software (Clarivate, Philadelphia, PA). We placed all unique references into Covidence (Veritas Health Innovation, Melbourne, Australia) systematic review software for screening.15 Two independent reviewers screened each reference for relevance by both titles/abstracts. After this process, full-text articles were screened by study inclusion criteria by 2 independent reviewers (Figure 1). For articles excluded from full-text review, reviewers had to agree on the reason for exclusion. Conflicts between reviewers were resolved by either a third-party vote or group consensus.
Study selection. ∗Duplicates identified via EndNote, version 9, software or in Covidence. NHL, non-Hodgkin lymphoma.
Study selection. ∗Duplicates identified via EndNote, version 9, software or in Covidence. NHL, non-Hodgkin lymphoma.
Data from included studies were extracted using a customized data extraction tool on Covidence. Extracted data included study design; years of data observation; country of study; as well as cohort-level data such as age, sample size, sex, HL stage and subtype, treatment methods, and survival or response outcomes. Chemotherapy was classified by the regimen given and grouped into unique or similar groups of treatment regimens. Radiation was classified by the number of participants receiving it during the study period as widely used (>10%), rarely used (<10%), or not used (0%). For each article, 2 researchers independently extracted data, and inconsistencies between reviewers were rectified by consensus. Studies that reported data for distinct cohorts across multiple regions/countries, time periods, or treatment groups (eg, chemotherapy alone vs combined chemotherapy and radiotherapy groups) were extracted separately. For example, 1 study provided survival outcomes by low vs high socioeconomic status without combined analysis, so we included these as 2 separate cohorts.16
Statistical analysis
For study outcome analysis, we calculated the median and interquartile range (IQR) for each data point across all cohorts, including 2-year overall survival (OS) and progression-free survival (PFS), 5-year OS/PFS, and response assessment (that is, complete response [CR], partial response, stable disease, and progressive disease). We performed unweighted linear regression analyses to assess the association between OS and PFS and both country gross domestic product (GDP; 2021) and publication year.
Results
The initial database search resulted in 1882 unique records that were screened for relevance by 2 independent reviewers of title/abstracts, after which 1611 were deemed irrelevant. After this process, 311 relevant and full-text articles were reviewed for eligibility by 2 independent reviewers, which garnered 101 studies for inclusion (Figure 1). The second search of 2023 to 2024 PubMed studies resulted in 50 unique records screened by 1 reviewer, which resulted in 10 studies for inclusion in this review, resulting in 111 studies, including a total of 120 cohorts (supplemental Table 2). Reasons for exclusion during the full-length article review were varied. Most records (n = 135) excluded were not full-length articles (ie, they were conference proceedings or abstracts). We excluded 21 articles for language, including those written in Mandarin Chinese (n = 16), Arabic (n = 3), Vietnamese (n = 1), and Bulgarian (n = 1). We excluded 19 studies that did not report survival or treatment response outcomes. Other reasons for exclusion were: conducted in a HIC (n = 11), wrong study design (n = 10; ie, review or case report), unretrievable or duplicate records (n = 10), pediatric population only (n = 2), non-HL (n = 1), and treatment not described (n = 1).
Study methods and quality
Of 111 studies, 84 (76%) were retrospective cohort studies, 21 (19%) were prospective cohort studies, 4 (3%) were registered clinical trials, 1 (1%) was an ambispective cohort study, and 1 (1%) study design could not be ascertained. We assessed the quality of each included study as described earlier. Most studies were determined to be of high quality (n = 89), several were medium quality (n = 16), and a few were of low quality (n = 6). The 6 studies of low quality were older studies with either significant loss to follow-up (n = 1) or lack of demographic data (n = 5). Most studies (n = 83 [76%]) either reported no funding or did not report their funding sources. Among the 28 studies that did report funding, government sources were the most common (13 studies [12%]). Eight studies (7%) were funded by a combination of government with either foundation or industry funding, and 3 studies (2%) were funded solely by industry or foundations, respectively. One study (1%) reported academic funding.
Economic and geographic distribution of studies
Included studies described data on 120 cohorts in 34 different countries from every World Health Organization region (Table 1; Figure 2A). Most studies were conducted in upper-middle-income countries (n = 63 [58%]), with the bulk of studies coming from only 5 different countries: Brazil (n = 16), China (n = 12), Mexico (n = 7), Turkey (n = 7), and Argentina (n = 6). A total of 42 (39%) studies were conducted in lower-middle-income countries, in which Southeast Asian countries such as India (n = 20) were predominantly represented. Only 4 studies (4%) were completed in low-income countries (Malawi [n = 2], Rwanda [n = 1], and Ethiopia [n = 1]; Figure 2B). Certain countries made up most studies conducted in certain regions: 92% of studies in Western Pacific were from China; 88% of studies in the Americas were from Brazil, Mexico, or Argentina; and 87% of studies in Southeast Asia were from India. Other regions were more diversely represented with all other countries having 1 to 3 studies included (Figure 2A). Most included studies were written in English (89%), 5 (5%) were written in Spanish, with 5 (3%) each written in French and Portuguese. Overall, 73 (67%) studies were conducted in countries with high or very high HDI, 27 (25%) were categorized as medium HDI, and 9 (8%) were low HDI. Of the 9 studies conducted in low-HDI countries, 6 were conducted in sub-Saharan Africa and the remaining 3 were from Pakistan. Two included studies were conducted in multiple countries and were thus not included in country-level data collection shown in Table 1.
Disribution of included studies by geography and income classification. (A) World map of included studies. Income classification is shown by shade of red, with darker colors indicating higher income countries and the number of included studies is shown in the text attached to each country name. (B) Studies published from 1976 to 2024 by 2021 GDP per capita of the country in which the study took place, regardless of the year of the study. Reference, lowest GDP per capita country was Malawi at $633. USD, United States dollars.
Disribution of included studies by geography and income classification. (A) World map of included studies. Income classification is shown by shade of red, with darker colors indicating higher income countries and the number of included studies is shown in the text attached to each country name. (B) Studies published from 1976 to 2024 by 2021 GDP per capita of the country in which the study took place, regardless of the year of the study. Reference, lowest GDP per capita country was Malawi at $633. USD, United States dollars.
Patient characteristics
Studies were published between 1976 and 2024, with the mid-year of cohort observation ranging from 1972 to 2021 (median mid-year observation was 2009; (IQR, 2000-2014; Table 2). Of 85 studies that reported cohort follow-up, the median follow-up time was 2.9 years (IQR, 1.8-4.4). Most cohorts (53%) included both adults and children, whereas 18 (16%) included adults and adolescents (aged ≥13 years), and 33 (29%) included only adults aged ≥18 years. The median age of cohorts was 30 years (IQR, 28-34). Cohort size ranged from 7 to 1254 participants, with a median number of 94 participants (IQR, 50-196). Male sex predominated in most cohorts with a median proportion of 60% (IQR, 53-68). Stage III/IV HL was common, with a median proportion of 57% (IQR, 44-71). Five studies focused on advanced stage HL only, whereas 6 studies reported on early-stage HL only; the remaining studies contained both groups. Only 78 (77%) studies reported the proportion of participants with B symptoms, with the median proportion being 58% (IQR, 43-70).
Treatment details
Cancer-directed treatments were almost all curative-intent, with 83 studies focused on first-line therapy, 28 studies focused on R/R HL, and 1 study included a section on both first-line and R/R HL treatment (Table 3). Among first-line studies, 59 (71%) used ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) as their primary chemotherapy treatment regimen; several studies published in 2005 or earlier used MOPP (mechlorethamin, vincristine, procarbazine, and prednisone), COPP (cyclophosphamide, vincristine, procarbazine, and prednisone), or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) chemotherapy regimens (n = 15), before the ABVD regimen became first-line treatment. Other therapies used were CVPP or AOPE in 1 study; EVAP in another study; and 2 studies gave variable treatments to each patient, including COPP, CVPP, MOPP, and other chemotherapies. Five (6%) studies did not specify the type of chemotherapy given to patients. Radiation treatment was widely used in 58 (70%) studies, rarely used in 10 (12%), and not used or not reported in 15 (18%).
In studies focused on R/R HL treatment, studies typically used a single primary advanced treatment that all or nearly all participants in the cohort received (Table 3). The most common therapy investigated was autologous stem cell transplantation (ASCT) in 18 (62%) studies. Other studies investigated targeted monoclonal antibody agents, including nivolumab (n = 3), brentuximab vedotin (n = 3), sintilimab (n = 1), and zimberelimab (n = 1). The remaining studies investigated use of ABVD (n = 1) and allogeneic SCT (n = 1), and 1 study reported outcomes after failure of ASCT (other miscellaneous treatments). In studies focused on R/R HL, radiotherapy was widely use in 17 studies (59%) and rarely used or not used in 12 studies (41%).
Importantly, only 4 of 111 included studies were conducted in low-income countries; 2 studies were prospective cohorts from Malawi, whereas 1 retrospective cohort study was from Rwanda and another from Ethiopia. All 4 studies used ABVD as their primary first-line treatment. Radiotherapy was not available in the studies conducted in Malawi and Rwanda. Conversely, radiotherapy was given to some patients in the Ethiopia study who were given a combined therapeutic regimen of ABVD and COPDAC (cyclophosphamide, vincristine sulfate, prednisone, and dacarbazine) with or without radiotherapy (n = 15).17 Of 15 patients given combined ABVD and COPDAC, 6 patients were given radiotherapy (only 5% of total cohort). In a subanalysis, the Ethiopian group found that the group given a combined chemotherapy/radiotherapy regimen had better 4-year OS (100%) than patients given ABVD only (77.9%).
Study outcomes
Most studies reported both OS and PFS in their results, with 75 (63%) reporting 2-year/5-year OS, and 63 (53%) reporting PFS (Table 4). Median 2-year OS was 91% (IQR, 86-96) in frontline studies compared with 83% (IQR, 72-90) in R/R HL studies. Median 5-year OS in frontline studies was 85% (IQR, 72-91) compared with 75% (IQR, 58-84) for R/R HL cohorts. OS and PFS were slightly better in cohorts treated with ABVD (2-year OS/PFS, 93%/83% and 5-year OS/PFS, 87%/78%) compared with all frontline studies (91%/82% and 85%/77%, respectively). There was no difference among R/R HL outcomes among those treated with ASCT (83%/64% and 76%/52%, respectively) compared with all R/R HL studies (83%/61% and 75%/51%, respectively). Median 2-year and 5-year PFS were 82% (IQR, 76-88) and 77% (IQR, 63-81), respectively, in frontline studies but much lower in R/R HL cohorts at 61% (IQR, 52-65) and 51% (IQR, 43-57), respectively.
For treatment responses, only 41 (65%) studies reported CR for ABVD and 14 (78%) reported CR for ASCT studies. ABVD had a median CR of 76% whereas ASCT had a CR of 62%; partial response was 13% and 26%, respectively for ABVD and ASCT; stable disease was 1% and 15%; and progressive disease was 6% and 11%, respectively. Safety and toxicity observation within included studies was low, with only 44 (40%) studies reporting any adverse event (AE) details and only 40 (36%) studies reporting treatment-related deaths.
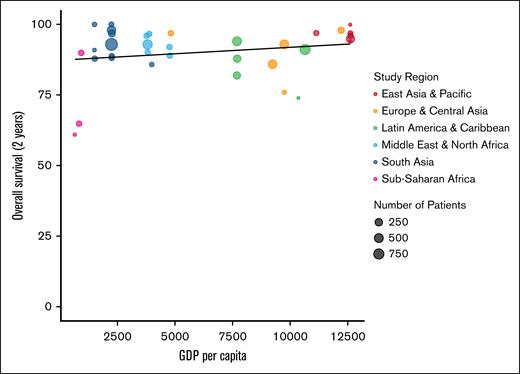
In the included studies, there is a positive correlation between 2-year OS and GDP per capita (P = .043; Figure 3). Notably, countries within sub-Saharan Africa and South Asia regions represented most lower income countries yet had a wide range of 2-year OS. Nevertheless, OS increased with GDP per capita. This positive trend between 2-year OS and GDP per capita was also present with first-line studies that used ABVD as their primary treatment (Figure 4). Year of publication was likewise positively correlated with 2-year OS (P = .016), including when stratifying specifically by pre-2010 and post-2010 as a marker for the introduction of ABVD and other advanced treatments for HL (Figure 5). However, it must be noted that there was marked heterogeneity in the adoption of ABVD across LMICs. Therefore, although outcomes improved over time in these studies, this improvement may reflect factors beyond the introduction of new treatments during the 2010s.
Two-year OS of all cohorts by country GDP per capita. All studies that reported OS (y-axis) plotted by country GDP per capita (x-axis).11 Each study is represented by a dot with varying size (number of patients in the study) and color (World Health Organization region).
Two-year OS of all cohorts by country GDP per capita. All studies that reported OS (y-axis) plotted by country GDP per capita (x-axis).11 Each study is represented by a dot with varying size (number of patients in the study) and color (World Health Organization region).
Two-year OS of cohorts treated with first-line ABVD by country GDP per capita. All first-line studies that used ABVD as primary treatment and reported OS (y-axis) plotted by country GDP per capita (x-axis).11 Each study is represented by a dot with varying size (number of patients in the study) and color (World Health Organization region).
Two-year OS of cohorts treated with first-line ABVD by country GDP per capita. All first-line studies that used ABVD as primary treatment and reported OS (y-axis) plotted by country GDP per capita (x-axis).11 Each study is represented by a dot with varying size (number of patients in the study) and color (World Health Organization region).
Two-year OS of all studies by country GDP per capita and stratified by publication date pre-2010 and post-2010. All studies that reported OS (y-axis) plotted by country GDP per capita (x-axis).11 Each study is represented by a dot with varying size (number of patients in the study) and color (pre-2010 and post-2010).
Two-year OS of all studies by country GDP per capita and stratified by publication date pre-2010 and post-2010. All studies that reported OS (y-axis) plotted by country GDP per capita (x-axis).11 Each study is represented by a dot with varying size (number of patients in the study) and color (pre-2010 and post-2010).
Discussion
In this systematic review, we synthesize the available evidence from 111 studies of adult HL across LMICs. Overall, we found that these predominantly retrospective studies include mostly young and male participants with advanced stage III/IV disease. Compared with population level data from HICs, our findings demonstrate relatively lower OS (2-year OS median, 90%) in LMICs. However, there are numerous confounders, which make comparisons difficult without a dedicated meta-analysis (especially, stage, age, and study quality).4 ABVD chemotherapy was common in first-line treatment regimens and high-dose chemotherapy with ASCT was common in R/R studies. Although most studies had radiotherapy widely available, at least 30% of first-line and 42% of R/R HL treatment cohorts only had radiotherapy rarely used or unused. In addition, we show a linear trend between HL 2-year OS and LMICs’ GDP per capita. Finally, data on AEs and treatment-related mortality are infrequently reported, making assessments of the cause of HL mortality in LMICs incomplete and emphasizing the need for high quality, prospective studies. Although we present, to our knowledge, the most comprehensive data on HL treatment options and outcomes in LMICs, our findings are limited by the few studies published in low-income countries, with only 4 publications coming from low-income countries in sub-Saharan Africa (eg, Malawi [n = 2], Ethiopia, and Rwanda).
Compared with contemporary studies from HICs, outcomes in similar treatment scenarios are similar but confounding variables make direct comparisons impossible. For example, in the ABVD-treated control arm of the recently completed ECHELON-1 trial,18 the 2-year and 6-year OS were 95% and 89%, respectively. This compares favorably to the median 93% and 87% 2-year and 5-year OS numbers, respectively, among all ABVD studies in this systematic review. Likewise, 2-year and 6-year PFS in ECHELON-1 were 78% and 74%, respectively, compared with median 2-year and 5-year PFS of 83% and 78% across studies in this review. However, direct comparison would require a more comprehensive meta-analysis because there are a number of confounders including age (median age in our studies was 30 years compared with 37 years in ECHELON-1), disease stage (ECHELON-1 contained exclusively patients with stage III/IV disease whereas the ABVD studies summarized here contains a mix of early-stage and advanced-stage disease studies), and other differences in underlying patient populations and study design and quality between a formal randomized controlled trial and the mix of prospective and retrospective studies summarized here. Likewise, ASCT in HICs cures ∼50% of patients with R/R HL, which is similar to numbers reported here, with 2-year and 5-year median PFS across studies of 64% and 52%, respectively. These data, with the aforementioned caveats, and especially that we are reporting the median values across studies and not at an individual patient level, speaks to the remarkable dissemination and implementation of both ABVD and ASCT for HL in LMICs, and points to areas in which uncertainty remains and in which continued health system improvements may allow for optimization of treatment for this highly curable cancer.
Access to radiotherapy is likely a contributing factor to HL survival outcome disparities across LMICs. In the United States, radiotherapy was associated with significantly improved survival in advanced stage III/IV HL.19,20 The high proportion of patients with advanced HL in our analyzed cohorts would likely benefit from access to radiotherapy. However, in countries such as Rwanda and Malawi in which radiotherapy was not used during the study period, survival was much lower than that in most studies from other LMICs.7,8 According to the International Atomic Energy Agency, ∼31% of LMICs lack access to radiotherapy.21 LMICs operate on ∼0.056 to 1.58 radiotherapy machines per million population, with almost half of low-income countries with no access to a single radiation center.21 To improve cancer equity efforts, especially in the management of the highly curable HL, increasing access to radiation therapy centers in LMICs is crucial.
Disparities in survival in LMICs relative to HICs may also be because of the high proportion of patients with advanced stage disease, differences in achieved intensity of therapy, or management of R/R HL. Many LMICs have centralized health care infrastructure and often have limited capacity for early disease detection in peripheral health centers, contributing to inequitable outcomes.22 Access to ASCT and other targeted therapies, such as monoclonal antibodies, is often limited. ASCT is frequently used to treat R/R HL in HICs, contributing to higher cure rates.23 Despite its importance as a therapeutic, many LMICs do not have access. Of 54 countries on the African continent, only 6 have access to SCT.24 Although there has been a push in LMICs to establish SCT capabilities,25 many challenges exist including lack of trained, specialized SCT doctors and staff; inadequate access to blood transfusion services; financial costs; and reliance on collaborations with institutions in HICs.
Additional considerations unique to LMIC populations, such as the HIV epidemic in sub-Saharan Africa specifically, may contribute significantly to the burden of HL. People living with HIV (PLWH) are 5 to 10 times more likely to develop HL compared with their counterparts who are HIV negative.26,27 Despite this, research on HIV-associated HL remains limited, which may further exacerbate outcome inequities. Prospective studies in Botswana and Malawi have reported that PLWH have better survival compared with patients not living with HIV.8,28 However, a study in South Africa reported the opposite association, with PLWH having lower survival, potentially because of extensive treatment delays or other unmeasured contributing factors.29 HICs have also investigated the association between HIV and HL and did not find that PLWH have an increased risk of death.30,31
In a similar systematic review on treatment-related mortality in childhood cancers in LMICs, investigators found that a significantly greater proportion of deaths were treatment related in children with hematologic malignancies. They estimated treatment-related mortality to be 6.78% (95% confidence interval, 0.00-15.06) for children with HL.32 However, very few of the studies we included reported treatment-related deaths (36%) or AEs (40%). A better understanding of how HL treatment may adversely affect patient quality of life and survival in LMICs is an important pursuit to decrease the overall burden of HL mortality in these regions of the world. Thus, studies investigating HL in LMICs ought to include achieved intensity of therapy, causes of death, and track AEs, ideally through prospective observational cohort data to tease apart the proportion of patients who are indeed dying from HL progression vs AEs of therapy.
In conclusion, LMICs have disproportionately limited access to radiation and treatments for R/R HL and patient in LMICs may have somewhat lower survival. Although there seem to be improvements to HL care over the decades and health care systems are prioritizing the development of radiotherapy centers and SCT capacity, further investigation into other factors that may contribute to HL outcome disparities in LMICs is needed. The generalizability of this analysis is limited because there is significant heterogeneity in the studies (both in terms of age populations sometimes including adults and children, as well as heterogenous treatments), we did not have complete data to conduct a meta-analysis, and there were very few studies from low-income countries. In LMICs, in which resources are limited, and health care infrastructure is rapidly changing, providing comprehensive data on survival, access to treatments, and affordability is an important pursuit to increase cancer treatment equity. We urge researchers to continue to measure and publish outcomes research on HL in their respective real-world settings. By monitoring outcomes across countries and institutions, continuous implementation of improvements can be made and may contribute to improved survival and quality of life for patients who are vulnerable with highly curable HL. Multidisciplinary cancer care is complex and by learning from successes and limitations of others, we will not only improve outcomes for patients with HL but likely strengthen the health system, with benefits expanding far beyond patients with HL.
Acknowledgments
The authors are exceedingly grateful to the University of North Carolina Project Malawi (Lilongwe, Malawi) for their support in conducting this study.
This study was supported by funding from the Lineberger Comprehensive Cancer Center (The University of North Carolina at Chapel Hill) and the National Institutes of Health (U54CA254564, D43CA260641, and K01TW011470).
The funding agencies had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit it for publication. The opinions expressed in this article are those of the authors and do not reflect the views of their affiliated institutions.
Authorship
Contribution: D.M.C. and M.S.P. conceived and designed the study; D.M.C., K.S.G., and H.B. planned and completed systemic review searches; D.M.C., M.J.H., G.M.S., T.C., M.M., and C.T.P. acquired and verified systematic review data; D.M.C., M.J.H., G.M.S., and M.S.P. analyzed, accessed, and interpreted data, and drafted and revised the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew S. Painschab, Department of Medicine, The University of North Carolina at Chapel Hill, Physicians Office Building, Third Floor, Chapel Hill, NC 27599; email: matthew_painschab@med.unc.edu.
References
Author notes
M.J.H. and G.M.S. contributed equally to this study and are joint second authors.
Extracted data from this systematic review are available on request from the corresponding author, Matthew S. Painschab (matthew_painschab@med.unc.edu).
The full-text version of this article contains a data supplement.