Key Points
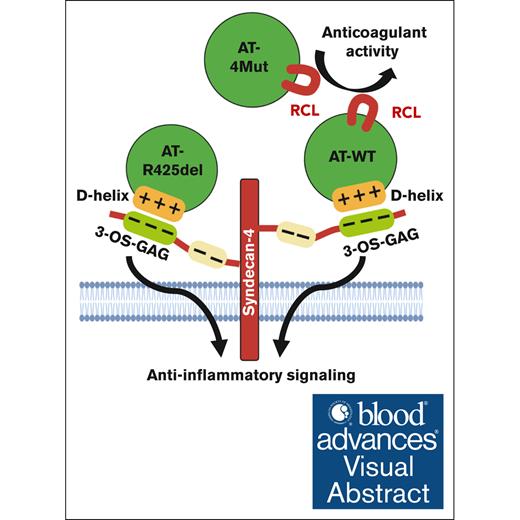
Interaction of D-helix of AT with therapeutic heparins promotes the protease-inhibitory function of the serpin.
Interaction of D-helix of AT with vascular GAGs unveils the anti-inflammatory signaling function of the serpin.
Visual Abstract
Antithrombin (AT) is an anticoagulant serpin involved in the regulation of proteolytic activities of coagulation proteases. AT also possesses a direct anti-inflammatory function. The anticoagulant function of AT is mediated through its reactive center loop–dependent inhibition of coagulation proteases, but anti-inflammatory function of AT is mediated via its D-helix–dependent interaction with vascular glycosaminoglycans (GAGs). In vitro assays have established that therapeutic heparins promote the anticoagulant function of AT by binding D-helix and activating the serpin, however, the contribution of vascular GAGs to D-helix–dependent anticoagulant function of AT has remained poorly understood in vivo. Here, we explored this question by using 2 AT mutants, (AT-4Mut), which exhibits neither affinity for heparin nor D-helix–dependent anti-inflammatory signaling but possesses normal protease-inhibitory function and an inactive signaling-selective AT mutant in which its P1-Arg425 is deleted (AT-R425del). In vivo properties of mutants were compared with wild-type AT (AT-WT) in a small interfering RNA (siRNA)-mediated AT-deficient mouse model. The siRNA knockdown efficiently reduced expression of AT and induced robust procoagulant and proinflammatory phenotypes in mice. Infusion of both AT-WT and AT-4Mut rescued the procoagulant phenotype of AT-deficient mice as evidenced by restoration of the plasma clotting time and inhibition of fibrin deposition. AT-WT also attenuated inflammation as evidenced by reduced VCAM-1 expression and leukocyte infiltration in the liver and lungs; however, AT-4Mut failed to attenuate inflammation. Interestingly, AT-R425del also effectively attenuated inflammation in AT-depleted mice. These results suggest that interaction of AT D-helix with vascular GAGs may primarily be responsible for anti-inflammatory signaling rather than protease-inhibitory function of the serpin.
Introduction
Antithrombin (AT) is a serine protease inhibitor of the serpin superfamily involved in regulation of proteolytic activities of procoagulant proteases of both intrinsic and extrinsic pathways.1,2 AT is a heparin-binding serpin with its basic D-helix interacting with a pentasaccharide sequence of heparin containing a 3-O-sulfate (3-OS) modification.3,4 The interaction leads to a conformational change in AT that activates it, thereby enhancing interaction of AT with coagulation proteases factor IXa (FIXa) and FXa by ∼300- to 500-fold.5-7 However, conformational activation of AT does not improve its reactivity with thrombin, rather longer chain heparins are required to promote AT reaction with thrombin by a template or bridging mechanism.8 The characteristic AT-binding 3-OS modification on therapeutic heparins has also been identified in a small population of glycosaminoglycans (GAGs; 1%-5%) attached to heparan sulfate proteoglycans on endothelium.9,10 Thus, it has been hypothesized that AT inhibition of coagulation proteases is also enhanced in vivo when AT binds vascular GAGs.
In addition to its anticoagulant function, D-helix–dependent interaction of AT with vascular GAGs leads to prostacyclin synthesis, thereby endowing anti-inflammatory and cytoprotective functions to AT.11,12 We have demonstrated AT exhibits a barrier-protective effect in cytokine-stimulated endothelial cells (ECs) by inducing prostacyclin synthesis and stabilizing vascular endothelial cadherin at cellular junctions.12,13 In vivo relevance for GAG-dependent protective signaling function of AT has been well established in different ischemia-reperfusion (I-R) injury models.11,14,15 We have demonstrated the cardioprotective effect of AT in an I-R injury model is mediated through AT activating the adenosine monophosphate kinase (AMPK) signaling pathway.14,15 Cardioprotective effect of AT required its D-helix–dependent interaction with heparan sulfate proteoglycans because the heparan sulfate antagonist, surfen, inhibited AMPK activation by AT.15 Moreover, no cardioprotective activity of AMPK activation was observed with D-helix mutant (AT-4Mut), which exhibits no affinity for heparin or vascular GAGs. By contrast, AMPK-dependent cardioprotective effect of AT was markedly higher with Asn-135 to Gln mutant of AT (AT-N135Q).15 This AT variant, as with the natural AT-β isoform, binds 3-OS–containing GAGs with 5- to 10-fold higher affinity.16 AT inhibited inflammatory c-Jun N-terminal protein kinase signaling in an I-R injury model.15 Anti-inflammatory signaling effect of AT during I-R injury was mediated solely through its D-helix–dependent interaction with cellular GAGs independent of its anticoagulant effect.14 We provided further support for this hypothesis by showing that engineering basic residues of D-helix of AT on α1-proteinase inhibitor (a non-heparin–binding serpin with an acidic D-helix) endows a GAG-dependent anti-inflammatory activity for chimeric serpin.17 In an in vitro study, we showed that AT binds syndecan-4 by its D-helix to exert protein kinase C-δ–dependent cytoprotective signaling.18
Unlike demonstration of GAG-dependent anti-inflammatory signaling for AT, there is, to our knowledge, no in vivo study on the direct role of vascular GAGs in promoting protease-inhibitory function of AT. In an interesting study, it was shown that mice deficient in heparan sulfate 3-O-sulfotransferase-1, which is required for 3-OS modification of GAGs in ECs, exhibit no hemostatic defect in response to prothrombotic challenge but show severe proinflammatory phenotype in response to lipopolysaccharide challenge.4,19 Based on this observation it has been hypothesized that AT interaction with 3-OS–containing GAGs is responsible for anti-inflammatory rather than protease-inhibitory function of AT. In this study, we investigated the possible contribution of vascular GAGs to protease-inhibitory function of AT by generating AT-deficient mice using a small interfering RNA (siRNA) knockdown strategy. siRNA knock down of AT effectively reduced plasma level of AT to 17% after 72 hours, which is within the range of siRNA-mediated AT depletion observed in treatment of patients with hemophilia in clinical trials.20 siRNA-mediated AT depletion promoted both procoagulant and proinflammatory pathways in AT-deficient mice. Characterization of phenotypes of AT-deficient mice before and after administration of AT derivatives (wild-type [AT-WT], AT-4Mut, or AT-R425del) suggests interaction with vascular GAGs is important for the signaling but not the protease-inhibitory function of the serpin.
Materials and methods
Reagents
Mice
C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed in compliance with institutional guidelines and were approved by the institutional animal care and use committee of Oklahoma Medical Research Foundation.
Generation of AT-deficient mice
C57BL6/J mice aged 6 to 8 weeks were used for the study as previously described.22 To generate AT deficiency, siRNA targeting mouse AT (AT siRNA; Ambion, Life Technologies, no. s62673) were complexed with Invivofectamine 3.0 and injected IV into 6- to 8-week-old mice at a dose of 3.5 μg/g bodyweight as previously described.23 Mice were euthanized 72 hours after AT siRNA injection and analyzed as described below.
Peripheral blood counts
Peripheral blood counts were measured using Hemavet 950 veterinary hematology analyzer (Drew Scientific, Inc).
Plasma clotting assays
Activated partial thromboplastin time (aPTT) was measured using Stago ST4 hemostasis analyzer (Diagnostica/Stago), according to the manufacturer’s instruction and as described.24
ELISA
AT and fibrinogen plasma levels were measured using direct enzyme-linked immunosorbent assay (ELISA) using specific antibodies. Purified mouse AT (catalog no. MCATIII-5120, Haematologic Technologies Inc, VT) and mouse fibrinogen (catalog no. ab92791, Abcam, MA) were used to generate a standard curve. Briefly, purified proteins and diluted plasma (1:16 000 for AT and 1:50 000 for fibrinogen) in phosphate-buffered saline were captured on high-binding microwell plates (Immulon 2 HB) overnight, washed, blocked with 2% bovine serum albumin and then detected using a specific anti-AT antibody and anti-fibrinogen antibody.
Scoring for morbidity
A sepsis scoring system was performed to determine the extent of morbidity in mice as previously described.25 The following parameters were analyzed for scoring morbidity: appearance, level of consciousness, activity, response to stimuli, and hemorrhage in eyes.
Quantitative real-time polymerase chain reaction analysis for gene expression
Quantitative real-time polymerase chain reaction analysis for gene expression was conducted as previously described.26
Statistical analysis
Data are presented as mean ± standard error of mean from ≥3 independent experiments. All data were analyzed using analysis of variance (analysis of variance) followed by Bonferroni post hoc test using Graph Pad Prism7 (Graph Pad Prism, San Diego, CA). A P value <.05 was considered statistically significant. Frequency of the eye hemorrhage between different groups was determined using the contingency table and the χ2 test.
Results
Generation and characterization of siRNA-mediated AT-deficient mice
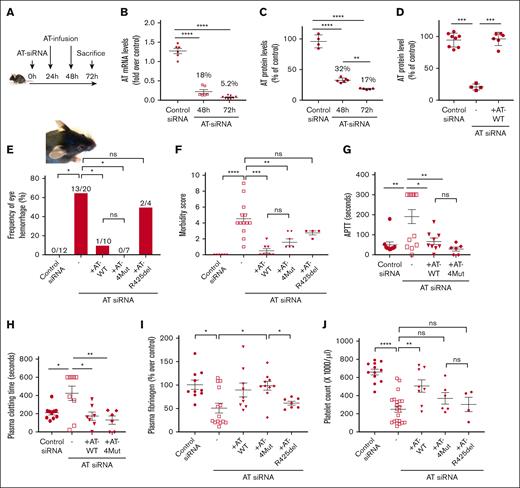
We generated AT-deficient mice using an siRNA knockdown strategy described by Safdar et al.23 In vivo gene silencing resulted in efficient AT knockdown with only 5.2% messenger RNA transcript remaining in the liver and 17% AT protein remaining in plasma after 72 hours (Figure 1A-C). AT infusion (twice 0.5 mg, determined to be optimal) yielded similar circulating plasma levels as in control siRNA-treated mice after 72 hours (Figure 1D). AT siRNA knockdown resulted in eye hemorrhage in significant number of female mice after 72 hours (Figure 1E), whereas this phenotype was not observed in male mice for duration of this time. This observation is consistent with findings of Safdar et al except they knocked down both AT and protein C to get this hemorrhagic phenotype in female mice.23 Infusion of either human AT-WT or its anticoagulant-selective D-helix mutant (AT-4Mut),15,21 both rescued hemorrhagic phenotype and restored morbidity associated with AT-depletion (Figure 1F). Signaling-selective AT mutant (AT-R425del), which lacks protease-inhibitory function, failed to effectively rescue hemorrhagic phenotype (Figure 1E); however, it exhibited improved morbidity score, although it did not reach statistical significance (Figure 1F). Clotting assays were performed to analyze activation of clotting pathways (plasma clotting time)24 and intrinsic pathway (aPTT) in AT siRNA–treated mice. In both assays, mice with AT depletion showed either prolonged clotting time or failed to clot (Figure 1G-H). This was the opposite of what 1 would expect if coagulation pathways were activated. Further analysis revealed consumption of plasma fibrinogen (Figure 1I) and thrombocytopenia (Figure 1J), suggesting that AT-deficient mice had likely consumptive coagulopathy. Infusion of either AT-WT or AT-4Mut restored plasma clotting time, aPTT, and plasma fibrinogen to control levels (Figure 1G-I). However, AT-4Mut was slightly less efficient than AT-WT in restoring platelet counts to normal levels (Figure 1J). AT-R425del rescued neither thrombocytopenia nor fibrinogen consumption (Figure 1I-J), suggesting that the lack of AT protease-inhibitory function is responsible for consumptive coagulopathy. It is worth noting that AT-R425del is known to exhibit improved heparin affinity.27 Here, we evaluated the AT-R425del capacity to bind heparin by monitoring its competitive effect on FXa inhibition by the AT–heparin complex and demonstrated that the variant effectively competes with AT, thus displacing heparin from the D-helix of the serpin, suggesting the variant is functional and properly folded (supplemental Figure 1).
Characterization of the procoagulant phenotype of AT-deficient mice. (A) Schematic diagram of experimental design, in which 6- to 8-week-old C57BL/6 mice were treated with IV injection of AT siRNA (3.5 μg/g bodyweight), complexed with Invivofectamine 3.0. After 24 and 48 hours of siRNA injection, AT variants (AT-WT, AT-4Mut, and AT-R425del) were administered IV at 500 μg per injection. Mice were euthanized 72 hours after siRNA injection. (B) Expression of AT gene in liver tissues were measured through quantitative real-time polymerase chain reaction after 48 and 72 hours of siRNA treatment. β-actin was used as the reference gene. (C) Plasma levels of AT in control siRNA and AT siRNA–treated mice were measured by ELISA after 48 and 72 hours of the siRNA treatment. (D) Plasma levels of AT at the 72-hour time point were measured by ELISA. (E) Frequency of periocular hemorrhage for different animal groups in female mice is presented. (F) Morbidity scores in different animal groups are determined as described in “Methods.” (G) aPTT in different animal groups was measured using Stago ST4 coagulation analyzer. (H) Plasma clotting time in different animal groups was measured using STart 4 coagulation analyzer. (I) Plasma fibrinogen level in different animal groups was measured by ELISA. (J) Peripheral blood counts were measured using Hemavet 950 veterinary hematology analyzer. Platelet counts of different animal groups are presented. All data are presented as mean ± standard error of the mean (SEM). n ≥ 7. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way analysis of variance (ANOVA), followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. ns, not significant.
Characterization of the procoagulant phenotype of AT-deficient mice. (A) Schematic diagram of experimental design, in which 6- to 8-week-old C57BL/6 mice were treated with IV injection of AT siRNA (3.5 μg/g bodyweight), complexed with Invivofectamine 3.0. After 24 and 48 hours of siRNA injection, AT variants (AT-WT, AT-4Mut, and AT-R425del) were administered IV at 500 μg per injection. Mice were euthanized 72 hours after siRNA injection. (B) Expression of AT gene in liver tissues were measured through quantitative real-time polymerase chain reaction after 48 and 72 hours of siRNA treatment. β-actin was used as the reference gene. (C) Plasma levels of AT in control siRNA and AT siRNA–treated mice were measured by ELISA after 48 and 72 hours of the siRNA treatment. (D) Plasma levels of AT at the 72-hour time point were measured by ELISA. (E) Frequency of periocular hemorrhage for different animal groups in female mice is presented. (F) Morbidity scores in different animal groups are determined as described in “Methods.” (G) aPTT in different animal groups was measured using Stago ST4 coagulation analyzer. (H) Plasma clotting time in different animal groups was measured using STart 4 coagulation analyzer. (I) Plasma fibrinogen level in different animal groups was measured by ELISA. (J) Peripheral blood counts were measured using Hemavet 950 veterinary hematology analyzer. Platelet counts of different animal groups are presented. All data are presented as mean ± standard error of the mean (SEM). n ≥ 7. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way analysis of variance (ANOVA), followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. ns, not significant.
Analysis of liver and lung tissues
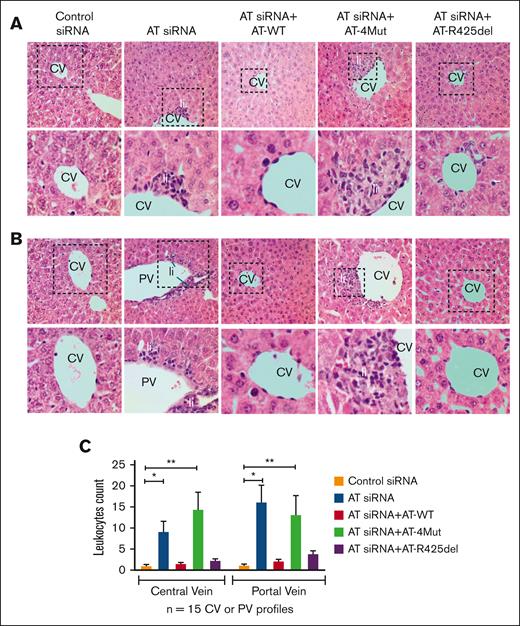
Hematoxylin and eosin staining of liver tissues indicated AT gene silencing leads to inflammation, characterized by leukocyte infiltration in areas surrounding central veins or portal veins of the liver (Figure 2A-B, representative liver sections and quantitation are presented in panel C). Infusion of AT-WT reversed pathological features induced by AT silencing. Conversely, infusion of AT-4Mut did not correct these pathologies, suggesting that AT D-helix–dependent binding to vascular GAGs is required for its anti-inflammatory function. In support of this hypothesis, AT-R425del, which lacks protease-inhibitory function of AT but possesses normal vascular GAG binding, inhibited infiltration of leukocytes to liver tissues as effective as AT-WT (Figure 2). These results suggest that AT signaling is primarily responsible for suppression of siRNA-mediated inflammation in mice liver, independent of the anticoagulant function of the serpin. Similarly, hematoxylin and eosin staining revealed AT gene silencing results in acute lung injury, as evidenced by leukocyte extravasation, alveolar integrity breakdown, intra-alveolar bleeding, and pulmonary edema formation (supplemental Figure 2). Infusion of AT-WT corrected both inflammation and bleeding. However, AT-4Mut rescued bleeding but not the inflammatory phenotype, and AT-R425del was only effective in partially reducing inflammation (supplemental Figure 2).
Analysis of liver histology. (A-B) Mice were treated with control siRNA, AT siRNA, AT siRNA + AT-WT, AT siRNA+AT-4Mut, or AT siRNA+AT-R425del as described in “Methods.” After 72 hours of siRNA treatment, mice were euthanized followed by perfusion with phosphate-buffered saline (PBS). Different organs were freshly collected and fixed with 10% neutral buffered formalin. The right lobe of the liver was collected and processed for histological analysis. Paraffin-embedded sections of the liver tissue were stained with hematoxylin and eosin and histopathological analysis was performed. Changes in the liver cytoarchitecture in animals treated with AT siRNA, with or without supplementation with AT-WT, AT-4Mut, or AT-R425del, were analyzed. Control siRNA–treated mice were used as controls. AT gene silencing (AT siRNA) leads to inflammation, characterized by leukocytic infiltration (Li) in areas surrounding the central veins (CV) or portal veins (PV). Inset boxes from each group are magnified. (C) Quantitation of leukocytes infiltration in areas surrounding the CV and PV. n ≥ 3. Scale bar, 50 μm.
Analysis of liver histology. (A-B) Mice were treated with control siRNA, AT siRNA, AT siRNA + AT-WT, AT siRNA+AT-4Mut, or AT siRNA+AT-R425del as described in “Methods.” After 72 hours of siRNA treatment, mice were euthanized followed by perfusion with phosphate-buffered saline (PBS). Different organs were freshly collected and fixed with 10% neutral buffered formalin. The right lobe of the liver was collected and processed for histological analysis. Paraffin-embedded sections of the liver tissue were stained with hematoxylin and eosin and histopathological analysis was performed. Changes in the liver cytoarchitecture in animals treated with AT siRNA, with or without supplementation with AT-WT, AT-4Mut, or AT-R425del, were analyzed. Control siRNA–treated mice were used as controls. AT gene silencing (AT siRNA) leads to inflammation, characterized by leukocytic infiltration (Li) in areas surrounding the central veins (CV) or portal veins (PV). Inset boxes from each group are magnified. (C) Quantitation of leukocytes infiltration in areas surrounding the CV and PV. n ≥ 3. Scale bar, 50 μm.
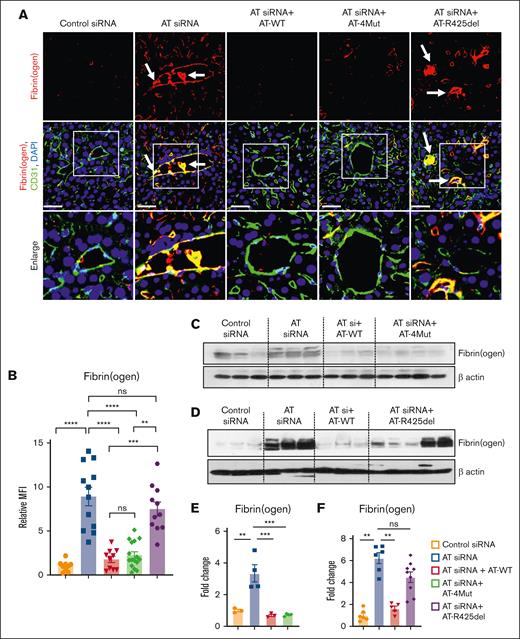
Immunofluorescence analysis revealed increased fibrin(ogen) deposition and presence of fibrin(ogen)-rich thrombus inside liver vessels of AT siRNA–treated mice, corroborating results presented in Figure 1 that AT depletion leads to a thrombotic phenotype (Figure 3A). This observation may also explain the underlying basis of consumptive coagulopathy and prolonged clotting time observed in AT-deficient mice. Infusion of either AT-WT or AT-4Mut but not AT-R425del to AT-deficient mice effectively eliminated intravascular thrombus formation and fibrin(ogen) deposition on hepatic vessels (Figure 3A, quantified in panel B). Western-blot analysis of whole liver cell lysates supported these results, showing that AT silencing leads to enhanced fibrin(ogen) deposition that is reversed by infusion of either AT-WT or AT-4Mut (Figure 3C,E), but not AT-R425del (Figure 3D,F). Anti-fibrin(ogen) antibody used in these experiments recognizes both fibrin and fibrinogen. Because liver tissues were extensively perfused with phosphate-buffered saline, bands on western blots represent fibrin deposition (this was also verified by molecular weight analysis). Immunofluorescence analysis of lung tissues revealed an essentially similar fibrin(ogen) deposition phenotype for all AT variants as observed for the liver (supplemental Figure 3).
Characterization of the prothrombotic phenotype of AT-deficient and AT-infused mice. Mice were treated with control siRNA or AT siRNA followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment as shown in Figure 1. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-fibrin(ogen) (rabbit) and anti-CD31 (goat) antibodies followed by Alexa Fluor 555–conjugated anti-rabbit and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI (4′,6-diamidino-2-phenylindole) was used to stain the nucleus. The arrows indicate intravascular thrombosis. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) Relative mean fluorescence intensity (MFI) of fibrinogen stain in (panel A) is presented. (C-D) The perfused left lobe of the liver was collected in the tissue lysis buffer and immunoblotted for fibrin(ogen). β-actin was used as a loading control. (E-F) Densitometric analysis of fibrin(ogen) deposition in the liver tissue sample is presented. All data are presented as mean ± SEM. n ≥ 3. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. MFI, mean fluorescence density; ns, not significant.
Characterization of the prothrombotic phenotype of AT-deficient and AT-infused mice. Mice were treated with control siRNA or AT siRNA followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment as shown in Figure 1. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-fibrin(ogen) (rabbit) and anti-CD31 (goat) antibodies followed by Alexa Fluor 555–conjugated anti-rabbit and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI (4′,6-diamidino-2-phenylindole) was used to stain the nucleus. The arrows indicate intravascular thrombosis. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) Relative mean fluorescence intensity (MFI) of fibrinogen stain in (panel A) is presented. (C-D) The perfused left lobe of the liver was collected in the tissue lysis buffer and immunoblotted for fibrin(ogen). β-actin was used as a loading control. (E-F) Densitometric analysis of fibrin(ogen) deposition in the liver tissue sample is presented. All data are presented as mean ± SEM. n ≥ 3. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. MFI, mean fluorescence density; ns, not significant.
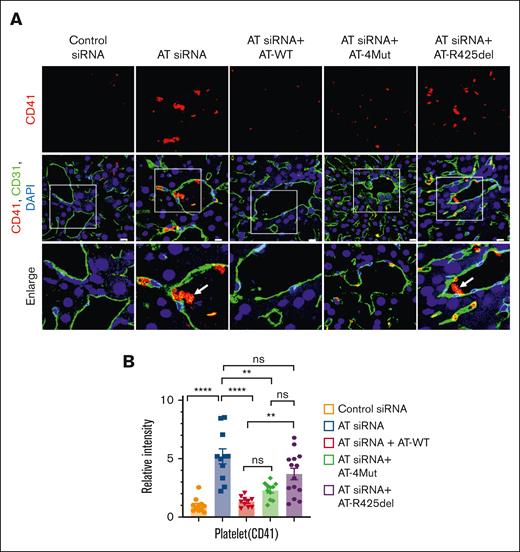
Platelet deposition
The effect of AT silencing on platelet deposition and thrombus formation was evaluated by CD41 and CD31 costaining of liver sections (Figure 4A-B). The formation of the platelet rich thrombus was confirmed in the AT siRNA group (Figure 4A, AT siRNA), which is consistent with aforementioned results showing AT silencing induces thrombocytopenia in AT-depleted mice. Infusion of either AT-WT or AT-4Mut rescued accumulation of intravascular platelet rich thrombus; however, relative to AT-WT, an increased platelet adhesion to ECs was observed in the case of AT-4Mut (Figure 4A, AT siRNA + AT-4Mut). This result may explain the basis for the moderate but not a full effect of AT-4Mut on attenuating thrombocytopenia in AT-deficient mice as compared with AT-WT as shown in Figure 1. As expected, unlike AT-WT and AT-4Mut, AT-R425del had no inhibitory effect on platelet-rich thrombus formation (Figure 4A), suggesting vascular D-helix–dependent interaction of AT does not contribute to rescuing procoagulant effects of AT silencing in this model. Further studies will be required to examine the role of AT on platelet function.
Characterization of the prothrombotic phenotype of AT-deficient and AT-infused mice. Mice were treated with control siRNA and AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-CD41 (rat; platelet marker) and anti-CD31 (goat) antibodies followed by Alexa Fluor 562–conjugated and anti-rat Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. The arrows indicate platelet-rich thrombus. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) Relative MFI of CD-41 staining is presented. All data are presented as mean ± SEM. n ≥ 3. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗∗P < .01; ∗∗∗∗P ≤ .0001. ns, not significant.
Characterization of the prothrombotic phenotype of AT-deficient and AT-infused mice. Mice were treated with control siRNA and AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-CD41 (rat; platelet marker) and anti-CD31 (goat) antibodies followed by Alexa Fluor 562–conjugated and anti-rat Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. The arrows indicate platelet-rich thrombus. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) Relative MFI of CD-41 staining is presented. All data are presented as mean ± SEM. n ≥ 3. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗∗P < .01; ∗∗∗∗P ≤ .0001. ns, not significant.
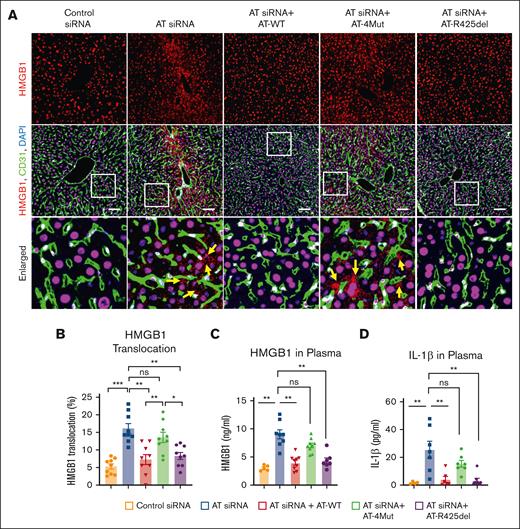
Signaling function of AT inhibits HMGB1 release and inflammation
We previously demonstrated AT negatively regulates VWF expression by ECs in response to proinflammatory stimuli (ie, histones and high mobility group box 1 [HMGB1]).28,29 HMGB1, a DNA binding protein, is primarily localized to the nucleus and can be translocated to the cytoplasm and released to extracellular space during inflammation.30 Translocation of the nuclear protein occurs when HMGB1 is acetylated on specific lysine residues within 2 clusters of nuclear-localization sites.31,32 Immunofluorescence analysis indicated that siRNA-mediated AT depletion leads to acetylation of HMGB1 in liver cells, and both AT-WT and AT-R425del but not AT-4Mut effectively inhibit modification responsible for the HMGB1 release (supplemental Figure 4). Nuclear staining further revealed that AT siRNA markedly enhances the nucleus to cytoplasmic translocation of HMGB1 in hepatocytes and sinusoidal ECs. Both AT-WT and AT-R425del but not AT-4Mut inhibited this process (Figure 5A, shown by yellow arrows on the enlarged panel). Quantitation of HMGB1 translocation from the nucleus to the extracellular space is shown in Figure 5B. In agreement with these results, analysis of plasma levels of HMGB1 by ELISA indicated AT silencing leads to significant increase in secretion of HMGB1 to circulation (Figure 5C). Interestingly, consistent with marked upregulation of inflammation by AT siRNA, plasma interleukin-1β (IL-1β), a marker of inflammasome activation, was also increased and like inhibition of HMGB1 translocation, both AT-WT and AT-R425del but not AT-4Mut, effectively inhibited IL-1β in AT-depleted mice (Figure 5D). Inflammasome activation and HMGB1 release are associated with inflammatory conditions, in which inflammasome activates HMGB1 release and HMGB1 induces IL-1β release.33,34 Our findings indicate that D-helix–dependent interaction of AT with GAGs induces cytoprotective signaling that negatively regulates HMGB1 translocation and inflammasome activation.
Analysis of HMGB1 translocation and release in AT-deficient and AT-infused mice. Mice were treated with control siRNA and AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-HMGB1 (rabbit) and anti-CD31 (goat) antibodies followed by Alexa Fluor 555–conjugated anti-rabbit and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. Arrows indicate nuclear to cytoplasmic translocation of HMGB1. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) The quantification of HMGB1 translocation from the nucleus to the cytoplasm. (C) Plasma level of HMGB1 was measured using commercial ELISA kit according to the manufacturer’s protocol. (D) Plasma level of IL-1β was measured using a commercial ELISA kit according to the manufacturer’s protocol. All data are presented as mean ± SEM. n ≥ 4. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001. ns, not significant.
Analysis of HMGB1 translocation and release in AT-deficient and AT-infused mice. Mice were treated with control siRNA and AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-HMGB1 (rabbit) and anti-CD31 (goat) antibodies followed by Alexa Fluor 555–conjugated anti-rabbit and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. Arrows indicate nuclear to cytoplasmic translocation of HMGB1. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) The quantification of HMGB1 translocation from the nucleus to the cytoplasm. (C) Plasma level of HMGB1 was measured using commercial ELISA kit according to the manufacturer’s protocol. (D) Plasma level of IL-1β was measured using a commercial ELISA kit according to the manufacturer’s protocol. All data are presented as mean ± SEM. n ≥ 4. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001. ns, not significant.
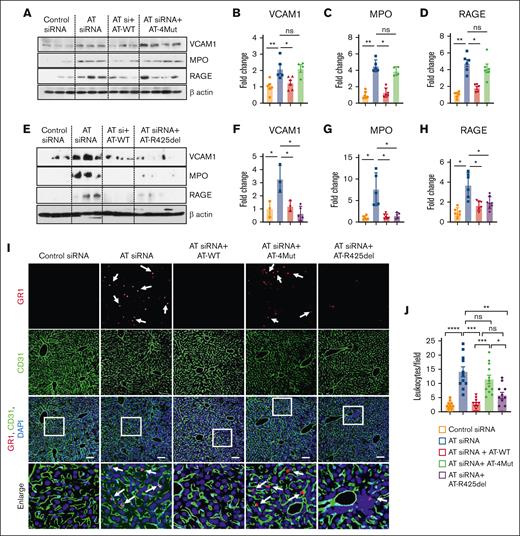
Western-blot analysis of whole liver cell lysates derived from AT-depleted mice further indicated that AT silencing leads to elevated expression of vascular cell adhesion molecule 1, myeloperoxidase, and receptor for advanced glycation end-products (RAGE; Figure 6). Infusion of AT-WT but not AT-4Mut reversed these inflammatory phenotypes (Figure 6A-D). Remarkably, AT-R425del was as effective as AT-WT in reversing these inflammatory phenotypes in AT-depleted mice (Figure 6E-H), suggesting D-helix–dependent interaction of AT with vascular GAGs is primarily responsible for its anti-inflammatory signaling.
Western blot analysis of signaling molecules and infiltration of GR1-positive leukocyte to the liver tissues. Mice were treated with control siRNA and AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) The perfused left lobe of the liver was harvested in the tissue lysis buffer and immunoblotted for VCAM1, myeloperoxidase (MPO), and RAGE. β-actin was used as a loading control. (B-D) Densitometric analysis of VCAM1, MPO, and RAGE expression in liver tissue samples. (E) The perfused left lobe of the liver was harvested in the tissue lysis buffer and immunoblotted for VCAM1, MPO, and RAGE expression in liver tissue samples. β-actin was used as a loading control. (F-H) Densitometric analysis of VCAM1, MPO, and RAGE in liver tissue samples. (I-J) Mice were treated with control siRNA or AT siRNA followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. (I) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-GR1 (rat) and anti-CD31 (goat) antibodies followed by Alexa Fluor 555–conjugated anti-rabbit and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. Inset boxes from each group are magnified. Scale bar, 50 μm. (J) Number of GR1-positive leukocytes per field is presented. All data are presented as mean ± SEM. n ≥ 4. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001. ns, not significant.
Western blot analysis of signaling molecules and infiltration of GR1-positive leukocyte to the liver tissues. Mice were treated with control siRNA and AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. Mice were euthanized after 72 hours of siRNA treatment and perfused with PBS. (A) The perfused left lobe of the liver was harvested in the tissue lysis buffer and immunoblotted for VCAM1, myeloperoxidase (MPO), and RAGE. β-actin was used as a loading control. (B-D) Densitometric analysis of VCAM1, MPO, and RAGE expression in liver tissue samples. (E) The perfused left lobe of the liver was harvested in the tissue lysis buffer and immunoblotted for VCAM1, MPO, and RAGE expression in liver tissue samples. β-actin was used as a loading control. (F-H) Densitometric analysis of VCAM1, MPO, and RAGE in liver tissue samples. (I-J) Mice were treated with control siRNA or AT siRNA followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. (I) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-GR1 (rat) and anti-CD31 (goat) antibodies followed by Alexa Fluor 555–conjugated anti-rabbit and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. Inset boxes from each group are magnified. Scale bar, 50 μm. (J) Number of GR1-positive leukocytes per field is presented. All data are presented as mean ± SEM. n ≥ 4. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001. ns, not significant.
Signaling function of AT inhibits infiltration of leukocytes to the liver and lungs
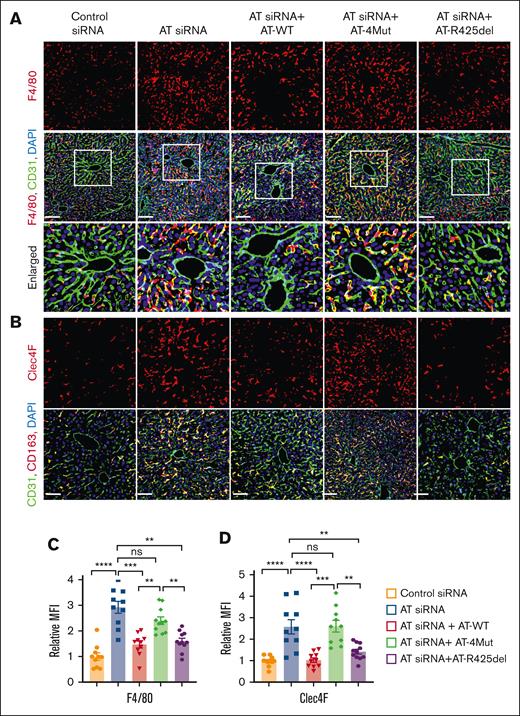
Immunofluorescence analysis indicated that AT silencing leads to infiltration of GR1-positive proinflammatory leukocytes to the liver and this effect was effectively inhibited by infusion of both AT-WT and AT-R425del but not by AT-4Mut (Figure 6I-J). Immunofluorescence analysis of liver tissues further suggested AT silencing significantly increases number of F4/80-positive macrophages in the liver when compared with control siRNA and that both AT-WT and AT-R425del but not AT-4Mut decreased the number of these macrophage populations in the liver (Figure 7A,C). To further characterize hepatic macrophages, liver sections were stained for CD68 (pan macrophage marker; supplemental Figure 5), Ly6C (proinflammatory monocyte-derived macrophages; supplemental Figure 6), and CD163 (alternatively activated macrophages; supplemental Figure 7). Results showed no significant differences in Ly6C- and CD163-positive macrophages in the liver after either AT depletion or infusion. However, CD68-positive population was found to be increased after AT depletion, which was attenuated by infusion of both AT-WT and AT-R425del but not AT-4Mut (supplemental Figure 5). These results suggest Kupfer-cell population and/or residential macrophages are increased after AT depletion. Immunostaining for Clec4F, which is a specific marker of Kupfer cells, confirmed that Clec4F-positive Kupfer cell population has been increased after AT depletion and that they were attenuated by infusion of both AT-WT and AT-R425del but not AT-4Mut (Figure 7B,D). Taken together, these results strongly suggest that D-helix–dependent interaction of AT with vascular GAGs is required for anti-inflammatory function of the serpin, but it does not appear to play a significant role on its protease-inhibitory function in the anticoagulant pathway in these studies. Immunofluorescence analysis revealed that, as in the liver, AT-WT but not AT-4Mut inhibits infiltration of GR1-positive proinflammatory leukocytes to the lung, and the inhibitory effect was partial with AT-R425del (supplemental Figure 8).
Analysis of liver tissue macrophages. Mice were treated with control siRNA or AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-F4/80 (rat) and anti-CD31 (goat) antibodies followed by Alexa Fluor 562–conjugated anti-rat and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) Perfused liver cryosections were fixed, permeabilized, and incubated with Clec4F (goat) and anti-CD31 (rat) antibodies followed by Alexa Fluor 562–conjugated anti-goat and Alexa Fluor 488–conjugated anti-rat antibodies. DAPI was used to stain the nucleus. Scale bar, 50 μm. (C-D) Relative intensity of F4/80 and Clec4F stain presented. All data presented as mean ± SEM. n ≥ 4. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001. MFI, mean fluorescence density; ns, not significant.
Analysis of liver tissue macrophages. Mice were treated with control siRNA or AT siRNA, followed by infusion of saline, AT-WT, AT-4Mut, or AT-R425del at 24 and 48 hours of siRNA treatment. (A) Perfused liver cryosections were fixed, permeabilized, and incubated with anti-F4/80 (rat) and anti-CD31 (goat) antibodies followed by Alexa Fluor 562–conjugated anti-rat and Alexa Fluor 488–conjugated anti-goat antibodies. DAPI was used to stain the nucleus. Inset boxes from each group are magnified. Scale bar, 50 μm. (B) Perfused liver cryosections were fixed, permeabilized, and incubated with Clec4F (goat) and anti-CD31 (rat) antibodies followed by Alexa Fluor 562–conjugated anti-goat and Alexa Fluor 488–conjugated anti-rat antibodies. DAPI was used to stain the nucleus. Scale bar, 50 μm. (C-D) Relative intensity of F4/80 and Clec4F stain presented. All data presented as mean ± SEM. n ≥ 4. Statistical analysis was performed using Graph Pad Prism 10. P values are determined by 1-way ANOVA, followed by Bonferroni multiple comparison test. ∗P < .05; ∗∗P < .01; ∗∗∗P ≤ .001. MFI, mean fluorescence density; ns, not significant.
Discussion
In addition to its anticoagulant function, AT exhibits anti-inflammatory signaling activities when it binds to 3-OS–modified vascular GAGs via its D-helix. Binding of heparins to the same D-helix of AT is responsible for promotion of its anticoagulant function. Whether interaction of AT D-helix with GAGs plays a similar role to that of heparin is not known. It has been reported that a small fraction of vascular GAGs contains 3-OS modification, possibly functioning as heparin-like cofactors to promote AT reactivity with coagulation proteases under different pathophysiological conditions. However, there is no in vivo data to show that D-helix interaction of AT with GAGs contributes to promotion of the protease-inhibitory function of the serpin.
The anticoagulant function of AT is mediated through the reactive center loop of the serpin with a characteristic P1-Arg (Arg425) that binds the catalytic pocket and irreversibly acylates the active-site serine (Ser195) of coagulation proteases, thereby rendering them catalytically inactive.1-3 Thus, for constructing an anticoagulant-defective (signaling-selective) AT, Arg425 residue was deleted and the resulting mutant, AT-R425del lost its protease-inhibitory function but retained a normal anti-inflammatory signaling function. D-helix of AT is solely responsible for the GAG-dependent signaling function of AT.17 Thus, we prepared a signaling-defective (anticoagulant-selective) AT mutant in which 4 basic residues of the D-helix are mutated, and the resulting mutant (AT-4Mut) exhibits normal protease-inhibitory function21 but is not capable of interacting with GAGs and/or heparins.28,35
To understand the physiological significance of the interaction of AT with vascular GAGs, we then developed an AT-deficient mouse model by a siRNA-silencing approach and infused the AT derivatives (AT-WT, AT-4Mut, and AT-R425del) to AT-depleted mice followed by their phenotypic characterization in different coagulation and inflammatory assays. The AT siRNA knockdown was highly efficient, and treated mice exhibited robust procoagulant and proinflammatory phenotypes, rendering this model suitable for characterization of properties of AT variants by replacement studies. Results indicate the protease-inhibitory function of AT is sufficient to rescue AT silencing-mediated procoagulant phenotype independent of heparin-like cofactor function of vascular GAGs. This is derived from observations that AT-4Mut exhibited normal anticoagulant activity and AT-R425del inhibited inflammatory responses as efficiently as AT-WT. These results suggest that D-helix–dependent interaction of AT with GAGs may primarily be required for its anti-inflammatory signaling but not promotion of its anticoagulant function. The mechanism through which AT silencing induces venous thrombosis and fibrin deposition in the liver has been found to require tissue factor and platelets with no contribution to the procoagulant phenotype from FXII or Ly6G-positive neutrophils.22 Thus, the procoagulant mechanism of AT silencing was not further investigated.
It was interesting to note that the plasma levels of HMGB1 and IL-1β were significantly elevated by the AT siRNA, suggesting that D-helix–dependent signaling by AT may play an important role in basal regulation of the vascular tone, and its absence leads to inflammation and endothelial dysfunction in AT-deficient mice. The mechanism of this regulation may be mediated by the AT interacting with vascular GAGs and inhibiting HMGB1-binding and RAGE signaling. In support of this hypothesis, we found that AT depletion leads to HMGB1 release and upregulation of RAGE and both AT-WT and AT-R425del but not AT-4Mut, inhibit elevated expression of both molecules. It should be noted that the binding of RAGE ligands including HMGB1 to heparan sulfates is essential for oligomerization of RAGE required for the signal transduction.36 We previously demonstrated AT interaction with GAGs not only protects barrier-permeability function of ECs but also competitively inhibits interaction of nuclear proteins with GAGs, thereby inhibiting RAGE signaling.13,35 Thus, it appears GAG-dependent signaling by AT plays an important protective role by inhibiting RAGE signaling in liver cells.
Another interesting finding was the observation that AT-R425del inhibited infiltration of inflammatory leukocytes both to the liver and lungs, suggesting that D-helix–dependent interaction of AT with GAGs may play key roles in regulating inflammatory responses. We think this finding may have clinical ramification for patients carrying AT mutations on the D-helix, which are classified as type 2 heparin-binding site (HBS) mutations. At least 12 such type 2 AT-HBS mutations in patients have been reported, which are associated with higher incidence of thrombosis.37 The underlying basis of thrombosis in carriers of these mutations has been largely attributed to their loss of heparin cofactor-dependent protease-inhibitory functions in patients with heterozygous type 2 HBS. However, considering our results here and the established concept that thrombosis is driven by interactions between thrombotic and inflammatory pathways,38 possible contribution of inflammation to the pathogenic mechanism of AT type 2 HBS mutations warrants further investigation.
The importance of GAG-dependent interaction of AT to its physiological function may also be gleaned from the observation that, as with homozygous AT deficiency, homozygous AT-HBS mutations, except for 2 (L99F and R47C) that predispose carriers to higher incidence of thrombosis, are not compatible with life.39-42 Moreover, targeting of the AT type 2 HBS Arg47-to-Cys mutation to the mouse genome results in life-threatening spontaneous thrombosis with only a fraction of mice surviving to adulthood, even though their thrombin-AT level has been found to be normal.43 Moreover, analysis of the plasma AT from 2 patients with homozygous L99F have indicated that a portion of the AT mutant exists as β-glycoform, thus exhibiting higher affinity for GAGs.44 The AT β-glycoform arises when Asn135 of AT is not posttranslationally glycosylated.16 The higher prevalence of AT-L99F β-glycoform reduces severity of thrombosis in these patients. Because both α and β isoforms of AT exhibit similar heparin-cofactor protease-inhibitory activities, our findings implicate L99F β-glycoform in the rescuing of AT’s anti-inflammatory defect, thereby leading to a milder prothrombotic phenotype. A higher incidence of AT-HBS mosaicism has only been proposed for L99F and R47C mutations.44
As with AT-4Mut, heparin-independent protease-inhibitory activities of HBS mutants have been minimally affected. The results presented here, together with findings that 3-O-sulfotransferase-1–deficient mice have normal hemostasis and exhibit comparable phenotypes as in wild-type mice in response to prothrombotic challenges but exhibit severe proinflammatory phenotypes in response to lipopolysaccharide challenge,4 provide further support for our hypothesis that interaction of AT with GAGs may primarily be responsible for signaling rather than protease-inhibitory function of AT. However, further studies with clinically relevant AT type 2 HBS mutants in the AT-deficient mouse model are required to validate this hypothesis. Another limitation is that it is not known whether the mechanism of GAG-dependent anticoagulant and anti-inflammatory function of AT in mice can also apply to the human system.
Finally, the siRNA-mediated AT depletion strategy has been successfully used for evaluating therapeutic potential of the siRNA knock down of AT for treating patients with hemophilia using fitusiran.20 The siRNA approach in our study depleted AT to 17% after 72 hours, which is within the range of fitusiran-mediated AT depletion observed for patients with hemophilia in clinical trials.20 The properties of fitusiran with respect to its safety and adverse effects have been extensively studied.20 Although the procoagulant effect of the siRNA-mediated AT-depletion strategy in animal models and human clinical trials has been thoroughly investigated in the previous studies,20,22,23,45 nevertheless, the proinflammatory effect of this strategy has not been fully addressed. This study is, to our knowledge, the first to demonstrate that siRNA-mediated AT depletion is associated with significant upregulation of the inflammatory pathways. The clinical data on patients with hemophilia have indicated that the dose of fitusiran for treatment must be optimized to alleviate the adverse effects of this strategy including increased aminotransferases and blood alkaline phosphatase in siRNA-treated patients,20,45 which are most likely arising from suboptimal vascular GAG-mediated AT signaling. We believe the therapeutic potential of AT-R425del, as an adjuvant therapy for AT siRNA, may be considered in some patients in long-term treatments.
Acknowledgment
This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL062565 and HL101917) (A.R.R.) and (GM141040) (F.L.).
Authorship
Contribution: I.B. and S.R.P designed and performed the study, and analyzed the data; F.L. assisted with histological and immunofluorescence analysis of the data; A.R.R. designed experiments, analyzed data, wrote the manuscript, and supervised the project; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alireza R. Rezaie, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; email: ray-rezaie@omrf.org.
References
Author notes
I.B. and S.R.P. contributed equally to this study.
Data are available on request from the corresponding author, Alireza R. Rezaie (ray-rezaie@omrf.org).
The full-text version of this article contains a data supplement.