Key Points
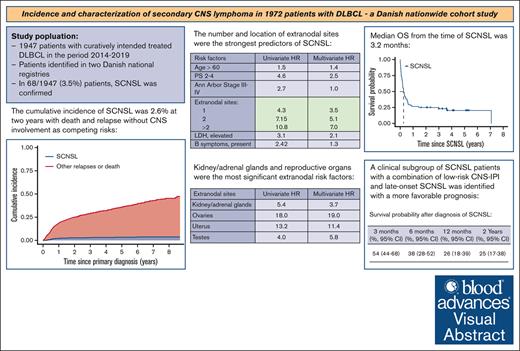
The cumulative incidence of SCNSL was 2.6% at 2 years with death and relapse without CNS involvement as competing risks.
The most significant predictors of SCNSL were the number and location of EN sites.
Visual Abstract
Secondary central nervous system lymphoma (SCNSL) is a rare manifestation of diffuse large B-cell lymphoma (DLBCL) with a poor prognosis. We present updated data from a nationwide study on the incidence and clinical characteristics of SCNSL. The incidence of SCNSL was calculated considering death or relapse without SCNSL as competing risks. Risk factors associated with SCNSL were identified using a cause-specific Cox proportional hazards model. A total of 1972 patients with DLBCL were included, of which 68 (3.4%) experienced SCNSL at the first relapse. The crude 1- and 2-year cumulative incidence of SCNSL was 2.0% (95% confidence interval [CI], 1.5-2.7) and 2.6% (95% CI, 2.0-3.4), respectively. For patients with a high-risk central nervous system international prognostic index (CNS-IPI) score, the 1- and 2-year cumulative incidence was 6.4% and 7.5%, respectively. The number and location of extranodal (EN) sites were the most significant predictors of SCNSL. Specific EN sites associated with an increased risk were the bone marrow, heart, kidneys/adrenal glands, ovaries, testes, and uterus. The median overall survival (OS) after SCNSL was 3.2 months. SCNSL within 6 months after the end of treatment (EOT) was associated with a higher baseline CNS-IPI score and worse OS than SCNSL >6 months after EOT. Patients with a combination of low-risk CNS-IPI and late-onset SCNSL had the most favorable prognosis. In conclusion, updated real-world population-based data on SCNSL at first relapse, adjusted for competing risks, demonstrated a lower incidence of SCNSL than previously reported, with the number and location of EN sites being the most significant predictors of SCNSL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma, and most patients are cured with frontline chemoimmunotherapy.1 However, ∼20% of patients are either refractory to first-line therapy or experience subsequent relapse, which is associated with a poor prognosis.2,3 Relapse involving the central nervous system (CNS) confers a particularly dismal prognosis.3,4
The incidence of secondary CNS lymphoma (SCNSL) in patients with DLBCL has been reported to range from 3% to 5%, depending on different study populations and definitions of SCNSL.5,6 In recent years, the incidences of SCNSL have been reported accounting for competing risks in larger epidemiological studies. El-Galaly et al7 covering cohorts from 2001 to 2013 reported 3-year incidences of SCNSL at first or later relapse according to the CNS international prognostic index (CNS-IPI) low-, intermediate-, and high-risk groups of 0.4%, 3.1%, and 11.2%, respectively. Harrysson et al2 conducted a population-based study from 2007 to 2014 with a 2-year incidence of SCNSL at first relapse of 3.0%. Most cases occur within the first year after primary DLBCL diagnosis8 and the majority occur as isolated SCNSL with two-thirds of cases located in the brain parenchyma.7,9 The median overall survival (OS) from the time of SCNSL is consistently reported to be <6 months.6,8
Since the introduction of the CNS-IPI in 2016,5 this risk stratification tool has become the standard for categorizing the risk of SCNSL in newly diagnosed DLBCL. The CNS-IPI includes age, performance status (PS), Ann Arbor stage, serum lactate dehydrogenase (LDH) level, number of extranodal (EN) sites, and involvement of the kidneys/adrenal glands as independent predictors of SCNSL. In addition to the CNS-IPI, specific EN sites have been reported as risk factors for SCNSL, with substantial evidence supporting kidneys/adrenal glands10,11 and testes12 as high-risk sites. The bone marrow,13 breast,14,15 and uterus16 have also been suggested as potential high-risk sites, as well as the presence of double-/triple-hit cytogenetic abnormalities. The potential of adding biological markers to the CNS-IPI has been explored,17 but it remains to be definitively established. According to treatment guidelines, CNS prophylaxis should be considered in patients at high risk of SCNSL, but recent studies have not been able to demonstrate a clear beneficial effect of this strategy.18-20
Here, we present updated data from a nationwide population-based study of patients with DLBCL from 2014 to 2019, including the incidence, independent predictors, and outcomes of SCNSL, estimated using a competing risk approach.
Methods
Registries and data collection
We conducted a nationwide retrospective observational cohort study on secondary CNS involvement after primary diagnosis of systemic DLBCL. Patients with DLBCL were identified in the Danish National Lymphoma Registry (LYFO)21 and the Danish National Patient Registry (DNPR).22 LYFO is a clinical database that covers all Danish centers treating patients with lymphoma, ensuring the consecutive registration of all newly diagnosed patients with lymphoma since 2000. The coverage of the LYFO has been validated.23 The DNPR has nationwide coverage with extensive data on hospital-related administrative, diagnostic, and procedural activities. It was fully updated until March 2019, at the time of data collection for this study. Clinical baseline characteristics at the primary diagnosis of DLBCL were retrieved from the LYFO for all patients. For those with SCNSL, we reviewed medical records to extract information on the date and localization of CNS involvement, concomitant systemic lymphoma, and history of CNS prophylaxis.
The study was approved by The Danish National Research Ethics Committee (D.nr. 1316405), under the Research Biobank and Clinical Database of patients with Lymphoproliferative Malignancies, known as the Danish Lymphoid Cancer Research protocol. Danish Lymphoid Cancer Research has been approved by the Danish Data Protection Agency and the National Ethics Committee (approvals P-2020-561 and 1804410, respectively).
Study population
Patients registered in the LYFO database diagnosed with DLBCL between January 2014 and March 2019, who received curatively intended treatment with front-line chemoimmunotherapy were included, and medical records were screened for the occurrence of SCNSL. Patients with discordant/transformed lymphomas were also included, whereas those with CNS involvement at diagnosis were excluded.
SCNSL was defined as CNS involvement detected after the primary diagnosis of DLBCL and initiation of front-line therapy. SCNSL diagnosis required either (1) the presence of malignant cells with DLBCL immunophenotype in the cerebrospinal fluid (CSF) detected by cytological examination and flow cytometry, (2) DLBCL in a stereotactic brain biopsy, or (3) CNS imaging (magnetic resonance imaging [MRI] or computed tomography scan) compatible with lymphoma involvement. Neuroimaging with lymphoma extending to the CNS from a systemic site was defined as CNS involvement only when there was a direct parenchymal or meningeal invasion. Only patients with SCNSL at first relapse were included in the analyses. To identify patients with SCNSL, we conducted a detailed review of the medical records for all patients recorded with SCNSL in the LYFO database and for all patients in the LYFO database who had a brain MRI procedure registered in the DNPR.
Some patients with SCNSL, diagnosed during or shortly after first-line treatment, are likely to have had subclinical CNS disease at the time of their primary DLBCL diagnosis. To further investigate very early CNS events, we compared the clinical characteristics and survival outcomes of our SCNSL cohort with those of patients diagnosed with de novo DLBCL who presented with concurrent CNS and systemic involvement at the time of primary diagnosis before starting first-line treatment.
Primary and secondary outcomes
The primary outcome was the cumulative incidence of SCNSL, with death and relapse without CNS involvement as competing risks. Secondary outcomes included (1) the frequency of early vs late SCNSL (occurring before and after 6 months from the end of primary lymphoma treatment, respectively), (2) the frequency of isolated SCNSL vs SCNSL with concomitant systemic relapse, (3) OS from the time of primary DLBCL diagnosis, (4) OS from the time of SCNSL diagnosis, and (5) prediction of SCNSL by the CNS-IPI risk factors.
Statistical analyses
Clinical characteristics were reported using descriptive statistics. For comparisons between groups, Fisher exact test was used for categorical variables and the Mann-Whitney U-test for continuous variables. The median follow-up time (interquartile range [IQR]) was calculated as the median time to relapse, death, or end of the study, whichever came first. OS was calculated from the date of SCNSL diagnosis to the date of death from any cause or the date of the last follow-up (15 September 2022) using the Kaplan-Meier (KM) method. Differences were tested using the log-rank test. The time to SCNSL was calculated from the primary DLBCL diagnosis to the time of SCNSL. The cumulative incidence of SCNSL was estimated using competing risk analyses, with death without SCNSL and relapse without CNS involvement as the competing risks. For comparison, incidence rates calculated using KM estimates without accounting for competing risks were also reported. Univariate and multivariate analyses were performed using a cause-specific Cox proportional hazards regression model censoring upon relapse and death. Schoenfeld residuals were applied to determine whether the variables independently satisfied the assumptions of the Cox model. P values < .05 were considered statistically significant. Statistical analyses were performed using R software version 4.3.2.24
Results
Study population characteristics
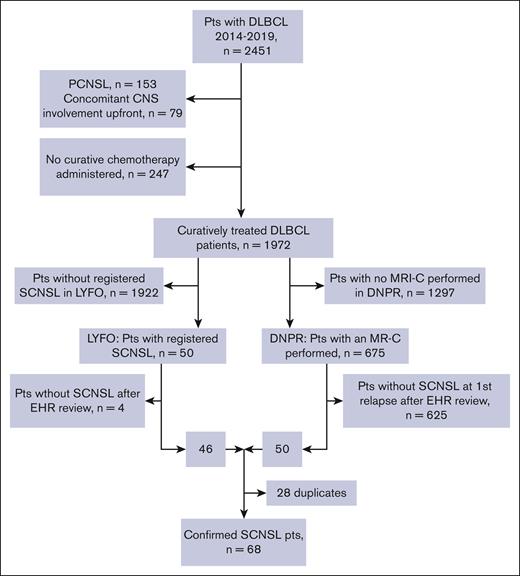
We identified 2451 patients registered in the LYFO database diagnosed with DLBCL between January 2014 and March 2019 (Figure 1; supplemental Table 1). Of the total cohort, 232 (9%) patients with primary CNS lymphoma (PCNSL) (n = 153) and concomitant CNS involvement at diagnosis (n = 79), and 247 (10%) who did not receive curatively intended therapy were excluded. The remaining 1972 patients were screened for the occurrence of SCNSL, and 50 cases of subsequent SCNSL were identified in the LYFO database. In the DNPR, 675 of 1972 patients with DLBCL were registered using a brain MRI procedure. In a review of medical records, we verified that 46 of 50 LYFO-registered patients with SCNSL and 50 cases of SCNSL were identified among 675 patients with MRI registered in the DNPR. Twenty-eight duplicates were identified using both screening procedures, resulting in a study cohort of 68 patients (3.4%) diagnosed with SCNSL. The median follow-up time for the entire cohort was 57.2 months (IQR, 25.6-77.8). Among the 1904 patients without SCNSL, 703 experienced a competing event (relapses without CNS involvement [n = 268] or death [n = 435]).
Consort diagram of the study cohort. EHR, electronic health record; MR-C, magnetic resonance imaging of the cerebrum; Pts, patients.
Consort diagram of the study cohort. EHR, electronic health record; MR-C, magnetic resonance imaging of the cerebrum; Pts, patients.
The baseline characteristics of the study cohort, stratified according to the presence of SCNSL, are shown in Table 1. Patients with subsequent SCNSL were characterized by a higher frequency of clinical predictors of poor outcomes. We found no differences in baseline treatment, with most (∼95%) receiving R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP–like therapy (supplemental Table 1). More patients achieved complete remission (CR) after first-line therapy in the group without SCNSL than in the group with SCNSL, whereas primary refractory disease with posttreatment non-CR was more commonly observed in the SCNSL group.
Baseline characteristics of patients with DLBCL with and without SCNSL
| Variables . | Patients with SCNSL (N = 68) . | Patients without SCNSL (N = 1904) . | P value . |
|---|---|---|---|
| Age, median (IQR), y | 71.2 (65-77) | 69.5 (59-76) | .28 |
| Age >60, n (%) | |||
| Yes | 53 (77.9) | 1404 (73.7) | .49 |
| No | 15 (22.1) | 500 (26.2) | |
| Sex, n (%) | |||
| Female | 20 (29.4) | 799 (42) | .045 |
| Male | 48 (70.6) | 1105 (58) | |
| ECOG PS, n (%) | |||
| 0-1 | 44 (64.7) | 1636 (85.9) | <.001 |
| >1 | 24 (35.3) | 268 (14.1) | |
| EN sites, n (%) | |||
| 0 | 6 (8.8) | 672 (35.3) | <.001 |
| 1 | 24 (35.3) | 673 (35.5) | |
| 2 | 17 (25) | 298 (15.6) | |
| >2 | 21 (30.9) | 261 (13.7) | |
| Stage, n (%) | |||
| I-II | 13 (19.1) | 686 (36.0) | .004 |
| III-IV | 55 (80.9) | 1218 (64.0) | |
| LDH,∗n (%) | |||
| Normal | 17 (25.0) | 889 (47.4) | <.001 |
| Elevated | 51 (75.0) | 985 (52.6) | |
| CNS-IPI, n (%) | |||
| 0-1 | 11 (16.2) | 547 (28.7) | <.001 |
| 2-3 | 20 (29.4) | 969 (50.9) | |
| 4-6 | 37 (54.4) | 388 (30.4) | |
| First-line treatment, n (%) | |||
| R-CHOP or similar (supplemental Table 1) | 67 (98.5) | 1884 (98.9) | .19 |
| Other regimens | 1 (1.5) | 20 (1.1) | |
| Response group, n (%) | |||
| CR | 32 (47.1) | 1515 (79.6) | <.001 |
| Non-CR | 36 (52.9) | 389 (20.4) | |
| CNS prophylaxis, HD-MTX, n (%) | <.001 | ||
| Yes | 29 (42.6) | 368 (19.3) | |
| No† | 39 (57.4) | 1536 (80.7) | |
| Cycles of HD-MTX,‡ median (IQR) | 2.0 (2-2) | 2.0 (2-2) | .22 |
| CNS prophylaxis, HD-MTX, CNS-IPI 4-6, n (%) | .031 | ||
| Yes | 20 (54) | 135 (34.7) | |
| No | 17 (46) | 253 (65.2) |
| Variables . | Patients with SCNSL (N = 68) . | Patients without SCNSL (N = 1904) . | P value . |
|---|---|---|---|
| Age, median (IQR), y | 71.2 (65-77) | 69.5 (59-76) | .28 |
| Age >60, n (%) | |||
| Yes | 53 (77.9) | 1404 (73.7) | .49 |
| No | 15 (22.1) | 500 (26.2) | |
| Sex, n (%) | |||
| Female | 20 (29.4) | 799 (42) | .045 |
| Male | 48 (70.6) | 1105 (58) | |
| ECOG PS, n (%) | |||
| 0-1 | 44 (64.7) | 1636 (85.9) | <.001 |
| >1 | 24 (35.3) | 268 (14.1) | |
| EN sites, n (%) | |||
| 0 | 6 (8.8) | 672 (35.3) | <.001 |
| 1 | 24 (35.3) | 673 (35.5) | |
| 2 | 17 (25) | 298 (15.6) | |
| >2 | 21 (30.9) | 261 (13.7) | |
| Stage, n (%) | |||
| I-II | 13 (19.1) | 686 (36.0) | .004 |
| III-IV | 55 (80.9) | 1218 (64.0) | |
| LDH,∗n (%) | |||
| Normal | 17 (25.0) | 889 (47.4) | <.001 |
| Elevated | 51 (75.0) | 985 (52.6) | |
| CNS-IPI, n (%) | |||
| 0-1 | 11 (16.2) | 547 (28.7) | <.001 |
| 2-3 | 20 (29.4) | 969 (50.9) | |
| 4-6 | 37 (54.4) | 388 (30.4) | |
| First-line treatment, n (%) | |||
| R-CHOP or similar (supplemental Table 1) | 67 (98.5) | 1884 (98.9) | .19 |
| Other regimens | 1 (1.5) | 20 (1.1) | |
| Response group, n (%) | |||
| CR | 32 (47.1) | 1515 (79.6) | <.001 |
| Non-CR | 36 (52.9) | 389 (20.4) | |
| CNS prophylaxis, HD-MTX, n (%) | <.001 | ||
| Yes | 29 (42.6) | 368 (19.3) | |
| No† | 39 (57.4) | 1536 (80.7) | |
| Cycles of HD-MTX,‡ median (IQR) | 2.0 (2-2) | 2.0 (2-2) | .22 |
| CNS prophylaxis, HD-MTX, CNS-IPI 4-6, n (%) | .031 | ||
| Yes | 20 (54) | 135 (34.7) | |
| No | 17 (46) | 253 (65.2) |
Boldface values represent statistically significant results.
ECOG, Eastern Cooperative Oncology Group.
Missing values in 30 patients in the group of patients without SCNSL.
High-dose cytarabine (HD-AraC) was administered to 4 patients in the group of patients without SCNSL.
Missing values in 6 patients in the group of patients without SCNSL.
A higher proportion of patients in the SCNSL cohort received prophylactic high-dose methotrexate (HD-MTX) (Table 1). Specific data on the dose of HD-MTX are not available in the LYFO database. However, in Danish centers, the dose typically ranges between 3.0 and 3.5 g/m2, in accordance with national treatment guidelines. Among those who received HD-MTX, patients with and without SCNSL were administered a median of 2 cycles. Patients with baseline CNS-IPI scores of 4 to 6 and those who subsequently developed SCNSL were more frequently treated with HD-MTX prophylaxis. In 28 of 29 patients with SCNSL receiving prophylaxis, HD-MTX was administered early before the third cycle of R-CHOP (or similar) treatment. Early administration of HD-MTX did not interfere with the planned immunochemotherapy, as 25 out of 29 patients received ≥5 cycles of R-CHOP, whereas the remaining 4 patients received 4 cycles.
SCNSL incidence and characteristics
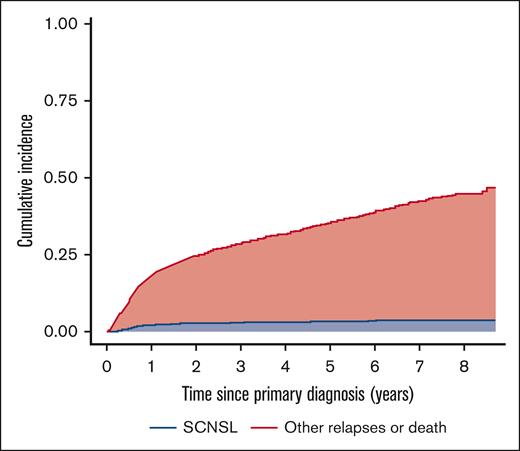
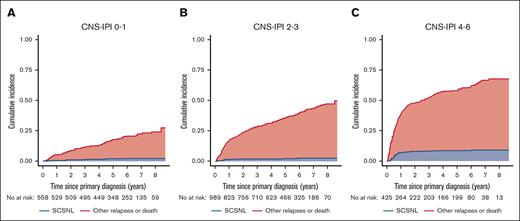
The median time from primary diagnosis of DLBCL to SCNSL was 8.3 months (IQR, 5.9-17.8). The 1- and 2-year cumulative incidences of SCNSL were 2.0% (95% confidence interval [CI], 1.5-2.7) and 2.6% (95% CI, 2.0-3.4), respectively, with death and relapses without CNS involvement as competing risks (Figure 2). For comparison, the cumulative incidence of SCNSL without competing risks was 2.2% (1.5-2.9) at 1 year and 2.9% (2.1-3.7) at 2 years (supplemental Figure 1). The cumulative incidence of SCNSL according to the CNS-IPI group is shown in Figure 3. There were no statistically significant differences in the 1- and 2-year incidences of SCNSL between the CNS-IPI 0 to 1 and 2 to 3 groups. However, the cumulative incidences of SCNSL for patients with CNS-IPI 4 to 6 at 1 year (6.4% [95% CI, 4.3- 8.9]) and 2 years (7.5% [95% CI, 5.3-10]) were significantly higher than those for patients with CNS-IPI 2 to 3 (1.1% [95% CI, 0.59-1.9] and 1.5% (95% CI, 0.89-2.4), respectively (supplemental Table 2).
Incidence of SCNSL with other relapses and death as competing events.
Incidence of SCNSL according to CNS-IPI risk groups with other relapses and death as competing events.
Incidence of SCNSL according to CNS-IPI risk groups with other relapses and death as competing events.
The diagnosis of SCNSL was verified using stereotactic biopsy or CSF evaluation in 49 (72%) patients. The remaining 19 (28%) were identified by neuroimaging. The tumor was localized to the brain parenchyma in 45 (66%) patients (Table 2). Most cases presented with isolated CNS involvement (69%), whereas 31% had concomitant systemic relapse. In 57% of patients with SCNSL and concomitant systemic relapse, relapse occurred at the same site(s) as the primary DLBCL diagnosis.
Clinical features of patients with SCNSL in the entire cohort, patients with early-onset SCNSL vs late-onset SCNSL, and patients with isolated SCNSL vs concomitant systemic disease
| Variables . | All SCNSL . | Early SCNSL (≤6 months from EOT) . | Late SCNSL (>6 months from EOT) . | P value . | Isolated SCNSL . | Concomitant systemic disease . | P value . |
|---|---|---|---|---|---|---|---|
| No. of patients | 68 | 39 | 29 | 47 | 21 | ||
| Age, median (IQR), y | 71 (65-77) | 71.6 (60-76) | 70.7 (66-77) | .427 | 72.4 (65-77) | 68.3 (59-75) | .29 |
| CNS-IPI, n (%) | .008 | .26 | |||||
| 0-1 | 11 (16.2) | 2 (5.1) | 9 (31.0) | 10 (21.2) | 1 (4.7) | ||
| 2-3 | 20 (29.4) | 11 (28.2) | 9 (31.0) | 13 (27.6) | 7 (33.3) | ||
| 4-6 | 37 (54.4) | 26 (66.7) | 11 (38.0) | 24 (51.0) | 13 (62.0) | ||
| EN sites, n (%) | .04 | 2 (9.5) | .53 | ||||
| 0 | 6 (9) | 1 (2.6) | 5 (17.2) | 4 (8.5) | 5 | ||
| 1 | 24 (35) | 11 (28.2) | 13 (44.8) | 19 (40.4) | (23.9) | ||
| 2 | 17 (25) | 12 (30.8) | 5 (17.2) | 10 (21.3) | 7 (33.3) | ||
| >2 | 21 (31) | 15 (38.4) | 6 (20.6) | 14 (29.8) | 7 (33.3) | ||
| Testes, n (%) | .44 | 1 | |||||
| Yes | 8 (20) | 3 (11.5) | 5 (22.7) | 6 (17.6) | 2 (14.3) | ||
| No | 40 (80) | 23 (88.5) | 17 (77.3) | 28 (82.3) | 12 (85.7) | ||
| Treated with first-line R-CHOP–like therapy, n (%) | .5 | 1 | |||||
| Yes | 67 (98.5) | 38 (97.4) | 29 (100) | 46 (97.9) | 21 (100) | ||
| No | 1 (1.5) | 1 (2.6) | 0 (0) | 1 (2.1) | 0 (0) | ||
| Response to first-line treatment, n (%) | <.001 | .06 | |||||
| CR | 32 (47.1) | 10 (25.6) | 22 (75.9) | 26 (55.3) | 6 (28.6) | ||
| Non-CR | 36 (52.9) | 29 (74.4) | 7 (24.1) | 21 (44.7) | 15 (71.4) | ||
| HD-MTX CNS prophylaxis, n (%) | .32 | .61 | |||||
| Yes | 29 (42.6) | 19 (48.7) | 10 (34.5) | 19 (40.4) | 10 (47.6) | ||
| No | 39 (57.4) | 20 (51.3) | 19 (65.5) | 28 (59.6) | 11 (52.4) | ||
| EOT to SCNSL, n (%) | .06 | ||||||
| ≤6 months | 39 (57.3) | 23 (48.9) | 16 (76.2) | ||||
| >6 months | 29 (43.7) | 24 (51.1) | 5 (23.8) | ||||
| Type of SCNSL, n (%) | .06 | ||||||
| Isolated | 47 (69.1) | 23 (59.0) | 24 (82.8) | ||||
| Concomitant systemic disease | 21 (30.9) | 16 (41.0) | 5 (17.2) | ||||
| Detection of SCNSL, n (%) | .323 | .11 | |||||
| Neuroimaging∗ | 19 (27.9) | 8 (20.5) | 11 (37.9) | 13 (28.7) | 6 (28.5) | ||
| CSF examination | 26 (38.2) | 16 (41.0) | 10 (34.5) | 16 (34.0) | 10 (47.6) | ||
| Both | 23 (33.8) | 15 (38.5) | 8 (27.6) | 18 (38.3) | 5 (23.8) | ||
| Site of SCNSL, n (%) | .2 | .051 | |||||
| Parenchymal | 45 (66.2) | 23 (59) | 22 (75.9) | 35 (74.5) | 10 (47.6) | ||
| unifocal | 26 | 4 | 15 | 17 | 2 | ||
| multifocal | 19 | 19 | 7 | 18 | 8 | ||
| Nonparenchymal | 23 (33.8) | 16 (41) | 7 (24.1) | 12 (25.5) | 11 (52.4) | ||
| Time to death after SCNSL, median (IQR), mo | 3.2 (1-18) | 2.5 (1-7) | 7.1 (2-25) | .05 | 5.0 (1-27) | 2.4 (1-4) | .09 |
| Variables . | All SCNSL . | Early SCNSL (≤6 months from EOT) . | Late SCNSL (>6 months from EOT) . | P value . | Isolated SCNSL . | Concomitant systemic disease . | P value . |
|---|---|---|---|---|---|---|---|
| No. of patients | 68 | 39 | 29 | 47 | 21 | ||
| Age, median (IQR), y | 71 (65-77) | 71.6 (60-76) | 70.7 (66-77) | .427 | 72.4 (65-77) | 68.3 (59-75) | .29 |
| CNS-IPI, n (%) | .008 | .26 | |||||
| 0-1 | 11 (16.2) | 2 (5.1) | 9 (31.0) | 10 (21.2) | 1 (4.7) | ||
| 2-3 | 20 (29.4) | 11 (28.2) | 9 (31.0) | 13 (27.6) | 7 (33.3) | ||
| 4-6 | 37 (54.4) | 26 (66.7) | 11 (38.0) | 24 (51.0) | 13 (62.0) | ||
| EN sites, n (%) | .04 | 2 (9.5) | .53 | ||||
| 0 | 6 (9) | 1 (2.6) | 5 (17.2) | 4 (8.5) | 5 | ||
| 1 | 24 (35) | 11 (28.2) | 13 (44.8) | 19 (40.4) | (23.9) | ||
| 2 | 17 (25) | 12 (30.8) | 5 (17.2) | 10 (21.3) | 7 (33.3) | ||
| >2 | 21 (31) | 15 (38.4) | 6 (20.6) | 14 (29.8) | 7 (33.3) | ||
| Testes, n (%) | .44 | 1 | |||||
| Yes | 8 (20) | 3 (11.5) | 5 (22.7) | 6 (17.6) | 2 (14.3) | ||
| No | 40 (80) | 23 (88.5) | 17 (77.3) | 28 (82.3) | 12 (85.7) | ||
| Treated with first-line R-CHOP–like therapy, n (%) | .5 | 1 | |||||
| Yes | 67 (98.5) | 38 (97.4) | 29 (100) | 46 (97.9) | 21 (100) | ||
| No | 1 (1.5) | 1 (2.6) | 0 (0) | 1 (2.1) | 0 (0) | ||
| Response to first-line treatment, n (%) | <.001 | .06 | |||||
| CR | 32 (47.1) | 10 (25.6) | 22 (75.9) | 26 (55.3) | 6 (28.6) | ||
| Non-CR | 36 (52.9) | 29 (74.4) | 7 (24.1) | 21 (44.7) | 15 (71.4) | ||
| HD-MTX CNS prophylaxis, n (%) | .32 | .61 | |||||
| Yes | 29 (42.6) | 19 (48.7) | 10 (34.5) | 19 (40.4) | 10 (47.6) | ||
| No | 39 (57.4) | 20 (51.3) | 19 (65.5) | 28 (59.6) | 11 (52.4) | ||
| EOT to SCNSL, n (%) | .06 | ||||||
| ≤6 months | 39 (57.3) | 23 (48.9) | 16 (76.2) | ||||
| >6 months | 29 (43.7) | 24 (51.1) | 5 (23.8) | ||||
| Type of SCNSL, n (%) | .06 | ||||||
| Isolated | 47 (69.1) | 23 (59.0) | 24 (82.8) | ||||
| Concomitant systemic disease | 21 (30.9) | 16 (41.0) | 5 (17.2) | ||||
| Detection of SCNSL, n (%) | .323 | .11 | |||||
| Neuroimaging∗ | 19 (27.9) | 8 (20.5) | 11 (37.9) | 13 (28.7) | 6 (28.5) | ||
| CSF examination | 26 (38.2) | 16 (41.0) | 10 (34.5) | 16 (34.0) | 10 (47.6) | ||
| Both | 23 (33.8) | 15 (38.5) | 8 (27.6) | 18 (38.3) | 5 (23.8) | ||
| Site of SCNSL, n (%) | .2 | .051 | |||||
| Parenchymal | 45 (66.2) | 23 (59) | 22 (75.9) | 35 (74.5) | 10 (47.6) | ||
| unifocal | 26 | 4 | 15 | 17 | 2 | ||
| multifocal | 19 | 19 | 7 | 18 | 8 | ||
| Nonparenchymal | 23 (33.8) | 16 (41) | 7 (24.1) | 12 (25.5) | 11 (52.4) | ||
| Time to death after SCNSL, median (IQR), mo | 3.2 (1-18) | 2.5 (1-7) | 7.1 (2-25) | .05 | 5.0 (1-27) | 2.4 (1-4) | .09 |
Boldface values represent statistically significant results.
MRI of the brain and spinal cord.
Patients with an early occurrence of SCNSL <6 months from the end of treatment (EOT) had a lower rate of CR than patients with late events of SCNSL (Table 2). The frequency of baseline CNS-IPI scores of 4 to 6 was also significantly higher in patients with early SCNSL than in those with late SCNSL. This finding remained significant when excluding patients with testis involvement confined to the immune-privileged site (n = 3) or systemic disease involving the testis (n = 5), which has been associated with late SCNSL despite a low CNS-IPI (P = .03).
The median OS from the time of SCNSL was 3.2 months (95% CI, 2.4-6.6). OS rates at 6, 12, and 36 months after SCNSL were 38%, 26%, and 23%, respectively, indicating that patients surviving >1 year after SCNSL were likely to become long-term survivors. Patients with early relapse had worse survival after SCNSL than patients with late occurring relapses, with a median of 2.5 months (95% CI, 1.4-4.7) vs 7.1 months (95% CI, 3.1 to not computable; P = .03; supplemental Figure 2). Patients with concomitant systemic relapse also had worse OS than patients with isolated SCNSL (median, 2.4 months [95% CI, 1.1-4.7] vs 5.0 months [95% CI. 2.4-11]; P = .04) (supplemental Figure 3).
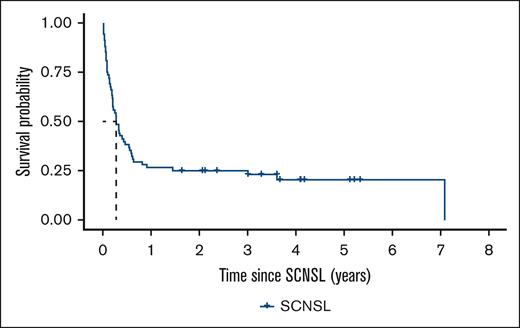
To better characterize long-term survivors, we compared the clinical characteristics of those alive 1 year after the diagnosis of SCNSL, as outlined in Table 3. This analysis demonstrated that a lower CNS-IPI score, longer time to SCNSL, and receiving SCNSL-directed treatment were strongly correlated with survival beyond the first year after SCNSL diagnosis. Among the individual factors within the CNS-IPI, the number of EN sites along with the PS and increased LDH were significantly associated with survival time. SCNSL as a very late event >2 years after the primary diagnosis of DLBCL was recorded in 16 of 68 patients (23.5%) with a median OS of 7.1 years and 14 of 68 patients (20.6%) still alive at the end of the follow-up (Figure 4; supplemental Table 3).
Clinical characteristics of patients who survived 1 year or less vs more than 1 year after SCNSL diagnosis
| Variables . | Survival ≤1 y (N = 50) . | Survival >1 y (N = 18) . | P value . |
|---|---|---|---|
| Age at diagnosis, median (IQR), y | 71 (65-78) | 71 (56-76) | .36 |
| Age at SCNSL, median (IQR), y | 71 (66-79) | 74 (58-77) | .46 |
| Time to SCNSL, median (IQR), mo | 7.8 (5.5-15) | 19.2 (7-37) | .037 |
| Type of SCNSL, n (%) | .15 | ||
| Isolated SCNSL | 32 (64.0) | 15 (83.3) | |
| Concomitant systemic relapse | 18 (36.0) | 3 (16.7) | |
| Site of SCNSL, n (%) | 1 | ||
| Parenchymal | 33 (66.0) | 12 (66.7) | |
| Nonparenchymal | 17 (34.0) | 6 (33.3) | |
| CNS-IPI, n (%) | <.001 | ||
| 0-1 | 3 (6) | 8 (44.4) | |
| 2-3 | 15 (30) | 5 (27.8) | |
| 4-6 | 32 (64.0) | 5 (27.8) | |
| Age at diagnosis >60, n (%) | .196 | ||
| Yes | 41 (82) | 12 (67) | |
| No | 9 (18) | 6 (33) | |
| ECOG PS at diagnosis >1, n (%) | .027 | ||
| Yes | 22 (44) | 2 (11) | |
| No | 28 (56) | 16 (89) | |
| Stage III-IV at diagnosis, n (%) | .089 | ||
| Yes | 43 (86) | 12 (67) | |
| No | 7 (14) | 6 (33) | |
| Baseline LDH elevated, n (%) | .009 | ||
| Yes | 42 (84) | 9 (50) | |
| No | 8 (16) | 9 (50) | |
| EN sites at diagnosis >1, n (%) | .007 | ||
| Yes | 33 (66) | 5 (28) | |
| No | 17 (34) | 13 (72) | |
| Kidney/adrenal gland involvement, n (%) | .205 | ||
| Yes | 14 (28) | 2 (11) | |
| No | 36 (72) | 16 (89) | |
| CNS prophylaxis, n (%) | .17 | ||
| Yes | 24 (48.0) | 5 (27.8) | |
| No | 26 (52.0) | 13 (72.2) | |
| SCNSL treatment, n (%) | .08 | ||
| Yes | 27 (81.8) | 17 (100) | |
| No | 6 (18.2) | 0 (0) | |
| Missing | 17 | 1 |
| Variables . | Survival ≤1 y (N = 50) . | Survival >1 y (N = 18) . | P value . |
|---|---|---|---|
| Age at diagnosis, median (IQR), y | 71 (65-78) | 71 (56-76) | .36 |
| Age at SCNSL, median (IQR), y | 71 (66-79) | 74 (58-77) | .46 |
| Time to SCNSL, median (IQR), mo | 7.8 (5.5-15) | 19.2 (7-37) | .037 |
| Type of SCNSL, n (%) | .15 | ||
| Isolated SCNSL | 32 (64.0) | 15 (83.3) | |
| Concomitant systemic relapse | 18 (36.0) | 3 (16.7) | |
| Site of SCNSL, n (%) | 1 | ||
| Parenchymal | 33 (66.0) | 12 (66.7) | |
| Nonparenchymal | 17 (34.0) | 6 (33.3) | |
| CNS-IPI, n (%) | <.001 | ||
| 0-1 | 3 (6) | 8 (44.4) | |
| 2-3 | 15 (30) | 5 (27.8) | |
| 4-6 | 32 (64.0) | 5 (27.8) | |
| Age at diagnosis >60, n (%) | .196 | ||
| Yes | 41 (82) | 12 (67) | |
| No | 9 (18) | 6 (33) | |
| ECOG PS at diagnosis >1, n (%) | .027 | ||
| Yes | 22 (44) | 2 (11) | |
| No | 28 (56) | 16 (89) | |
| Stage III-IV at diagnosis, n (%) | .089 | ||
| Yes | 43 (86) | 12 (67) | |
| No | 7 (14) | 6 (33) | |
| Baseline LDH elevated, n (%) | .009 | ||
| Yes | 42 (84) | 9 (50) | |
| No | 8 (16) | 9 (50) | |
| EN sites at diagnosis >1, n (%) | .007 | ||
| Yes | 33 (66) | 5 (28) | |
| No | 17 (34) | 13 (72) | |
| Kidney/adrenal gland involvement, n (%) | .205 | ||
| Yes | 14 (28) | 2 (11) | |
| No | 36 (72) | 16 (89) | |
| CNS prophylaxis, n (%) | .17 | ||
| Yes | 24 (48.0) | 5 (27.8) | |
| No | 26 (52.0) | 13 (72.2) | |
| SCNSL treatment, n (%) | .08 | ||
| Yes | 27 (81.8) | 17 (100) | |
| No | 6 (18.2) | 0 (0) | |
| Missing | 17 | 1 |
Boldface values represent statistically significant results.
Among the total population of patients with de novo DLBCL (n = 2451), excluding those with PCNSL (n = 153), 79 of 2298 patients (3.4%) presented with concomitant CNS involvement and systemic disease at baseline before the initiation of first-line therapy. Supplemental Table 4 provides a comparison of the clinical characteristics between these patients and those who had SCNSL confirmed after starting first-line treatment. The clinical characteristics were comparable between the 2 groups. However, patients with early involvement at the time of primary DLBCL diagnosis exhibited a markedly better prognosis than those with later onset of SCNSL. Among the 247 excluded patients who received only palliative care, CNS relapse was documented in just 2 cases in the lymphoma registry. However, 210 of these 247 patients were recorded as deceased.
Predictors of SCNSL
To investigate the impact of the potential risk factors for SCNSL, a cause-specific Cox regression model was constructed. Of these, the following were associated with an increased risk of SCNSL in both the unadjusted and adjusted analyses: poor PS, number of EN sites, elevated LDH levels, and involvement of the kidneys/adrenal glands (Table 4). The risk of SCNSL increased with the number of EN sites, with a hazard ratio (HR) of 3.5 with the involvement of 1 site, HR 5.1 with 2 sites; and HR 7.0 with >2 sites. The impact of specific EN sites on the risk of SCNSL is shown in Table 4. Involvement of the skin, heart, and bone marrow was associated with increased risk in the unadjusted analyses, and the association remained significant for heart and bone marrow involvement in the adjusted analyses. For female patients, unadjusted analyses showed that involvement of the breast, ovaries, and uterus was associated with an increased risk of SCNSL. In adjusted analyses, the significance of ovarian and uterine involvement persisted, with all cases showing concurrent involvement of both organs. Among male patients, testis involvement was associated with an increased risk of SCNSL in both unadjusted and adjusted analyses.
Cause-specific Cox regression model of risk factors for SCNSL with death without SCNSL and non-CNS relapse as competing events
| Risk factors . | Univariate (HR [95% CI]) . | Multivariate (HR [95% CI])∗ . |
|---|---|---|
| Age >60 | 1.5 (0.8-2.6) | 1.4 (0.7-2.6) |
| PS 2-4 | 4.6 (2.8-7.6) | 2.5 (1.4-4.3) |
| Ann Arbor stage III-IV | 2.7 (1.5-5.0) | 1.0 (0.5-2.1) |
| EN sites | ||
| 1 | 4.3 (1.8- 10.5) | 3.5 (1.4-8.6) |
| 2 | 7.15 (2.8-18.2) | 5.1 (1.9-13.7) |
| >2 | 10.8 (4.4-26.9) | 7.0 (2.6-18.5) |
| LDH, elevated | 3.1 (1.8-5.3) | 2.1 (1.5-3.7) |
| B symptoms, present | 2.42 (1.5-4.0) | 1.3 (0.8-2.3) |
| EN sites | Univariate (HR [95% CI]) | Multivariate (HR [95% CI])† |
| Orbits | 1.7 (0.2-12.5) | 2.7 (0.4-20.0) |
| Nasal cavity | 0.9 (0.1-6.5) | 0.5 (0.1-3.7) |
| Oral cavity | 2.0 (0.6-6.3) | 2.8 (0.9-9.2) |
| Salivary glands | 1.0 (0.1-7.1) | 1.5 (0.2-11.3) |
| Thyroid gland | 2.2 (0.8-6.1) | 2.0 (0.7-5.6) |
| Heart | 4.9 (1.5-15.6) | 3.7 (1.2-12.0) |
| Lungs | 1.5 (0.8-3.0) | 1.1 (0.5-2.1) |
| Stomach | 1.7 (0.8-3.8) | 1.5 (0.7-3.3) |
| Small intestine | 1.7 (0.7-4.0) | 1.7 (0.7-3.9) |
| Large intestine | 1.6 (0.7-3.8) | 1.3 (0.6-3.1) |
| Pancreas | 1.9 (0.7-5.2) | 1.3 (0.5-3.7) |
| Kidney/adrenal glands | 5.4 (3.0-9.4) | 3.7 (2.0-6.8) |
| Liver | 1.1 (0.5-2.5) | 0.6 (0.2-1.4) |
| Ascites | 2.2 (0.3-16.0) | 1.0 (0.1-6.9) |
| Bladder | 3.0 (0.4-22.0) | 2.3 (0.3-17.0) |
| Skin | 2.2 (1.0-4.6) | 2.0 (0.9-4.4) |
| Muscle | 1.0 (0.4-2.4) | 0.8 (0.3-1.9) |
| Bone | 1.3 (0.74-2.4) | 0.9 (0.5-1.7) |
| Bone marrow | 4.0 (2.4-6.5) | 3.2 (1.9-5.5) |
| Breast | 4.4 (1.3-15.0) | 2.8 (0.6-12.5) |
| Ovaries | 18.0 (6.0-53.9) | 19.0 (5.8-60.8) |
| Uterus | 13.2 (4.4-39.4) | 11.4 (3.5-36.8) |
| Testes | 4.0 (1.9-8.5) | 5.8 (2.7-12.7) |
| Risk factors . | Univariate (HR [95% CI]) . | Multivariate (HR [95% CI])∗ . |
|---|---|---|
| Age >60 | 1.5 (0.8-2.6) | 1.4 (0.7-2.6) |
| PS 2-4 | 4.6 (2.8-7.6) | 2.5 (1.4-4.3) |
| Ann Arbor stage III-IV | 2.7 (1.5-5.0) | 1.0 (0.5-2.1) |
| EN sites | ||
| 1 | 4.3 (1.8- 10.5) | 3.5 (1.4-8.6) |
| 2 | 7.15 (2.8-18.2) | 5.1 (1.9-13.7) |
| >2 | 10.8 (4.4-26.9) | 7.0 (2.6-18.5) |
| LDH, elevated | 3.1 (1.8-5.3) | 2.1 (1.5-3.7) |
| B symptoms, present | 2.42 (1.5-4.0) | 1.3 (0.8-2.3) |
| EN sites | Univariate (HR [95% CI]) | Multivariate (HR [95% CI])† |
| Orbits | 1.7 (0.2-12.5) | 2.7 (0.4-20.0) |
| Nasal cavity | 0.9 (0.1-6.5) | 0.5 (0.1-3.7) |
| Oral cavity | 2.0 (0.6-6.3) | 2.8 (0.9-9.2) |
| Salivary glands | 1.0 (0.1-7.1) | 1.5 (0.2-11.3) |
| Thyroid gland | 2.2 (0.8-6.1) | 2.0 (0.7-5.6) |
| Heart | 4.9 (1.5-15.6) | 3.7 (1.2-12.0) |
| Lungs | 1.5 (0.8-3.0) | 1.1 (0.5-2.1) |
| Stomach | 1.7 (0.8-3.8) | 1.5 (0.7-3.3) |
| Small intestine | 1.7 (0.7-4.0) | 1.7 (0.7-3.9) |
| Large intestine | 1.6 (0.7-3.8) | 1.3 (0.6-3.1) |
| Pancreas | 1.9 (0.7-5.2) | 1.3 (0.5-3.7) |
| Kidney/adrenal glands | 5.4 (3.0-9.4) | 3.7 (2.0-6.8) |
| Liver | 1.1 (0.5-2.5) | 0.6 (0.2-1.4) |
| Ascites | 2.2 (0.3-16.0) | 1.0 (0.1-6.9) |
| Bladder | 3.0 (0.4-22.0) | 2.3 (0.3-17.0) |
| Skin | 2.2 (1.0-4.6) | 2.0 (0.9-4.4) |
| Muscle | 1.0 (0.4-2.4) | 0.8 (0.3-1.9) |
| Bone | 1.3 (0.74-2.4) | 0.9 (0.5-1.7) |
| Bone marrow | 4.0 (2.4-6.5) | 3.2 (1.9-5.5) |
| Breast | 4.4 (1.3-15.0) | 2.8 (0.6-12.5) |
| Ovaries | 18.0 (6.0-53.9) | 19.0 (5.8-60.8) |
| Uterus | 13.2 (4.4-39.4) | 11.4 (3.5-36.8) |
| Testes | 4.0 (1.9-8.5) | 5.8 (2.7-12.7) |
Boldface values represent statistically significant results.
Adjusted for age, PS, Ann Arbor stage, EN sites, LDH, and B symptoms.
Adjusted for age >60, PS, Ann Arbor stage, elevated LDH, and B symptoms.
The distribution of individual CNS-IPI risk factors across the CNS-IPI risk categories is shown in supplemental Figure 4. Among patients in low-risk CNS-IPI groups, age, and advanced Ann Arbor stage were commonly observed as predominant risk factors. In the Cox regression model, neither age nor the Ann Arbor stage correlated with an increased risk of SCNSL.
Discussion
We presented data from a nationwide population-based study that included 1972 patients with DLBCL who received curatively intended first-line therapy. SCNSL was detected in 68 (3.4%) patients and the median time to the occurrence of SCNSL was 8.3 months from primary DLBCL diagnosis. In our competing risk model, counting death and relapses without CNS involvement as competing events, the cumulative incidence of SCNSL was 2.0% at 1 year and 2.6% at 2 years after primary DLBCL diagnosis. Almost a quarter of patients with SCNSL presented with CNS relapse >2 years after the initial DLBCL diagnosis, and most of these cases had isolated CNS involvement in the brain parenchyma. Early SCNSL (<6 months after EOT) occurred more frequently in the setting of primary refractory disease, concomitant systemic lymphoma relapse, EN manifestations, and baseline high CNS-IPI scores. The majority of patients who experienced early CNS disease events failed to achieve CR after front-line therapy, suggesting that CNS involvement is often associated with intrinsically chemorefractory disease. This points to an underlying biologically unfavorable disease entity in patients who experience CNS dissemination. The number of EN sites at diagnosis and involvement of the bone marrow, heart, kidneys/adrenal glands, ovaries, testes, and uterus had a significant and independent negative impact on the risk of SCNSL. The occurrence of SCNSL carried a dismal prognosis with a median OS of 3.2 months.
Previously reported incidences of SCNSL have been based mainly on cohorts of patients in clinical trials or from tertiary centers treating more high-risk patients which could result in biased estimates.25 Our population-based data from a more recent period revealed a 2-year cumulative incidence of 2.6% of SCNSL, lower than previously reported incidences, which could be attributed to the unselected cohort and/or the chosen statistical approach.26 The cumulative incidence of SCNSL has traditionally been reported by means of KM estimation of the primary event without considering competing risks.27,28 In this study, a competing risk analysis was applied to reduce the inherent risk of overestimating events using the KM method. Population-based Swedish data from an earlier period, which also accounted for death and other relapses as competing risks, revealed a comparable 2-year cumulative incidence of 3%.2 In addition, we have specifically addressed SCNSL as the first event only, thus reporting SCNSL in second or later relapses would increase the overall incidence.
Consistent with previous studies on SCNSL, we excluded patients who presented with CNS involvement at the time of primary DLBCL diagnosis, before the initiation of first-line therapy.2,7,8 The exclusion of baseline CNS cases also ensured the validity of the risk factor analysis based on the CNS-IPI, as the original report did not include patients with known CNS disease at diagnosis.5 However, CNS involvement discovered during or shortly after chemoimmunotherapy likely reflects subclinical disease also present at diagnosis. In our cohort, baseline CNS involvement with concomitant systemic disease was observed in 3.4% of the patients. These patients had clinical characteristics similar to those of patients who developed SCNSL after the initiation of the first-line therapy. Notably, patients with early detection of CNS involvement at the time of primary DLBCL diagnosis demonstrated significantly better survival rates after a CNS event. This suggests that timely CNS-directed treatment may play a role in improving survival outcomes or that SCNSL development later may possess inherently poor biological features that adversely affect prognosis. In the noncuratively treated group (n = 247), only 2 cases of CNS relapse were documented. However, the diagnostic work up of CNS involvement in this frail group of patients was likely low due to their poor general condition, expected toxicity to MTX, and very limited life expectancy. SCNSL is a prognostically serious event and median survival after SCNSL has been reported to be in the range of 2.5 to 7 months.26 In our unselected, population-based cohort, the median OS was only 3.2 months. However, a subgroup of patients surviving more than a year after SCNSL was characterized by appropriate SCNSL-directed treatments, late occurrence of SCNSL, and low baseline CNS-IPI. These characteristics of long-term survivors after SCNSL are similar to the prognostic features seen in other patients with relapsed/refractory (R/R) DLBCL29 and our data suggest the existence of a subgroup of patients with SCNSL being more likely to achieve a lasting remission with curatively intended treatment.
Our study population was characterized by a median age of >70 years, which was higher than that in other studies.5,30 We did not find age >60 years to be a significant risk factor for SCNSL in accordance with other studies.2,7 Our study showed an incidence of SCNSL among CNS-IPI high-risk patients (8%), which is lower than that reported in the original CNS-IPI publication,5 but similar to the results in the study by Harrysson et al2 applying a competing risk approach with both deaths and non-CNS relapses as competing events. In the study by El-Galaly et al,7 using only death as a competing event, the distribution of the risk classification remained similar to that in the original CNS-IPI study, with a cumulative incidence of 11% among high-risk patients.5 Therefore, the addition of relapses not including the CNS as a competing event reduces the risk estimation in CNS-IPI high-risk patients. From a clinical perspective, the inclusion of systemic relapse without SCNSL is meaningful, as risk stratifications are used predominantly to select patients for CNS prophylaxis. This treatment aims at reducing SCNSL as the first event or as part of a systemic relapse but not to prevent SCNSL that occurs after multiple relapses. In these cases, the outcomes are dismal regardless of whether the CNS is involved. Recent studies suggest that the effectiveness of CNS prophylaxis in preventing CNS relapse is modest.20 Therefore, the primary objective of assessing CNS risk is to identify patients who may already have occult CNS involvement at the time of DLBCL diagnosis, allowing for an upfront thorough CNS examination and tailored treatment to address early CNS events.
When stratifying according to the CNS-IPI risk groups, no clinically relevant differences were found between the low- and intermediate-risk groups. This lack of discrimination was also observed in patients included in the GOYA trial,17 as well as in a large multicenter study from the United States that applied death as a competing event.30 When considering the distribution of the CNS-IPI risk factors in the present cohort, a CNS-IPI score of 1 and 2 was mainly based on the presence of the risk factors age >60 and stage III to IV, and to some extent, elevated LDH. In the Cox regression analysis, neither age >60 nor stage III to IV were found to carry an increased risk of SCNSL. In contrast, low-risk patients showed less frequent involvement of EN sites, suggesting that EN sites and, in particular, the number of EN sites, are the main drivers in the prediction of SCNSL risk. Our data suggest that >2 EN sites are the strongest single risk factor for SCNSL (HR, 7.0), which supports previous findings of a gradual increase in the risk of SNCSL with an increasing number of EN sites.2,7,30 We found that involvement of the kidney/adrenal glands, testes, bone marrow, heart, ovaries, and uterus was significantly associated with SCNSL. It is well established that involvement of the kidneys/adrenal glands and testes carries a significant risk, and bone marrow involvement is also often classified as a high-risk site for later SCNSL.13,31 Involvement of the heart in relation to SCNSL has not been well described, but pericardial involvement has been shown to carry an increased risk.5 Primary cardiac involvement is rare, whereas secondary involvement, on the contrary, is more common.32 Patients with SCNSL and cardiac involvement all had CNS-IPI 4 to 6 and involvement of >1 EN site. Regarding the female reproductive organs, all patients had simultaneous ovarian and uterine involvement. Although both sites have been independently suspected of carrying an increased risk, uterine involvement appears to primarily elevate the risk of SCNSL16,33
Patients with SCNSL within the first 6 months after EOT had a significantly higher baseline CNS-IPI score. Patients with testicular lymphoma (n = 8) have been reported to relapse later and have a lower CNS-IPI34; however, the association between the early appearance of SCNSL and high-risk CNS-IPI remained after excluding testicular lymphoma. Patients with early onset SCNSL also had more frequent EN involvement and a trend toward more concomitant systemic diseases. This distinct early SCNSL phenotype may indicate an underlying DLBCL subtype that is clinically more aggressive, treatment-resistant, and exhibits a clinical presentation similar to that of primary refractory DLBCL cases. An overlapping explanation could be that patients with early SCNSL harbor subclinical involvement of the CNS at diagnosis. Others have shown a better prognosis in late-occurring relapses and the timing of relapse has been associated with clonal evolutionary patterns in a general R/R disease setting.35,36 This is also consistent with observations in patients with R/R disease, in which time to relapse is an important predictor of outcomes.37
More than half of patients with SCNSL did not achieve CR (52.9%) after primary treatment and, with a median time to SCNSL of 8.3 months, early subclinical CNS involvement was suspected in this cohort, in agreement with previous reports.26 A recent study identified asymptomatic leptomeningeal involvement by flow cytometry of CSF in 10% of their cohort of high-risk patients and advocated for baseline CSF screening.38 In other studies, CNS involvement was identified with a high sensitivity by analyzing circulating tumor DNA in the CSF and cases with parenchymal involvement were detected in patients with negative conventional CSF analyses.39,40 Recently, a larger study involving 67 patients with CNS lymphoma reproduced these findings.41 The results of these studies point toward the need for improved early diagnostic work up of CNS involvement to facilitate a more personalized treatment approach.
Our national lymphoma registry does not include molecular data; however, the molecular characteristics of SCNSL have been addressed by others.42 Stratified by cell-of-origin (COO), patients with the activated B-cell (ABC) subtype seem to have a higher risk of SCNSL. In a subset of patients in the GOYA trial,17 a predictive model combining CNS-IPI and COO status found a 2-year incidence of 15.2% among patients with CNS-IPI 4 to 6 and ABC/unclassified COO. Furthermore, molecular studies have also demonstrated an overlap with the ABC subtype and a distinct genetic signature including concordant MYD88L265P/CD79B mutations with a higher affinity for EN sites such as the CNS and testes.43 SCNSL likely develops through immune evasion mechanisms similar to PCNSL, with loss of immune surveillance and antigen presentation driving CNS spread.44 SCNSL shares genetic features with PCNSL and systemic DLBCL, including frequent driver mutations in MYD88, CD79B, and TP53. Approximately 40% of cases mirror PCNSL, whereas others show diverse genomic profiles.44 Finally, double-expressor and double-hit lymphomas have also been reported to be associated with the later occurrence of SCNSL.45 These findings emphasize that the molecular characteristics of DLBCL have a major influence on the risk of relapse involving the CNS and should be considered in future risk prediction models.
Given the poor outcomes of SCNSL and the limited benefit of current CNS prophylaxis, it is essential to reconsider how we manage patients with DLBCL who are at risk for SCNSL. Our findings indicate that most SCNSL cases arise either at the time of the initial DLBCL diagnosis or shortly after EOT. Our data also clearly demonstrate that the involvement of specific EN sites plays a critical role in determining a patient's risk of CNS dissemination. In light of this, screening should specifically target patients with involvement in high-risk EN sites, using intensified early baseline screening and close monitoring during the initial phase after diagnosis. Although clinical risk scores have limitations in predicting CNS involvement, further studies are needed to explore the biological markers linked to CNS dissemination.46 Additionally, research should investigate alternative therapeutic strategies, including intensified CNS-directed therapies or novel agents that target the genetic vulnerabilities observed in high-risk patients.
Our study has limitations due to the retrospective nature, although the strength of the study was the large, unselected population of patients with high coverage of the national registers and a high completeness rate of data. In contrast to some previous studies, positron emission tomography-computed tomography was routinely used for primary staging, outcome assessment, and diagnosis of relapse in the period of the present study, increasing the probability of detecting the involvement of EN sites. The estimation of the cumulative incidence of SCNSL and mortality is conducted using competing risks to reduce any overestimation using the conventional KM method. The lack of molecular data is a limitation as biological subclassifications have shown a major impact on the risk of SCNSL. Furthermore, one-third of SCNSL diagnoses were based solely on neuroimaging and not cytologically or histologically proven. The last update of the DNPR database implies a lack of information on MRIs performed in the latter 2.5 years of the total study duration of 7.5 years. Given that a complete review of the chart was not conducted for all 2451 patients with DLBCL, patients with potential SCNSL not covered by the registries may have been omitted. Finally, we emphasize that our retrospective study was not designed to evaluate the efficacy of CNS prophylaxis in the prevention of SCNSL.
In conclusion, we provide novel information from a large, unselected study population using an analytic approach to account for competing events. Our real-world data demonstrate a lower cumulative incidence of SCNSL in the clinical setting than previously reported, with the number and location of EN sites being the strongest predictors of SCNSL. Distinct clinical subgroups of SCNSL, including a group of long-term survivors, were identified and the high-risk designation by the CNS-IPI was validated.
Acknowledgments
This research was supported by data facilitated by the Danish Lymphoid Cancer Research data resource, the Danish Lymphoma registry, and the Danish National Patient Registry.
Authorship
Contribution: L.M.P. conceptualized the study; E.R.T., T.H.N., and L.M.P. designed the study; D.S.H., S.Ø., M.R., M.R.C., A.L.A.-M., K.M.E., and L.M.H. collected and assembled the data; C.B. and C.U.N. provided the data; E.R.T., T.H.N., L.M.P., T.C.E.-G., C.B., and C.U.N. performed data analysis and interpretation; E.R.T. wrote the first draft of the manuscript; and all authors revised the manuscript and approved the final version.
Conflict-of-interest disclosure: M.R.C. reports consultancy for AbbVie, AstraZeneca, Genmab, Gilead, Incyte, Janssen, and Roche, and has received funding for travel expenses from AbbVie, AstraZeneca, Genmab, Janssen, Pfizer, and Roche. A.L.A.-M. has received funding from Genentech Inc. C.B. has received honoraria from Octapharma and AstraZeneca. C.U.N. has received research grants and/or consultancy fees from AbbVie, AstraZeneca, Janssen, Lilly, Genmab, BeiGene, Octapharma, CSL Behring, MSD, and Takeda. L.M.P. received a research grant from AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Lars Møller Pedersen, Department of Hematology, Zealand University Hospital, Vestermarksvej 15-17, DK-4000 Roskilde, Denmark; email: lmpn@regionsjaelland.dk.
References
Author notes
The data that support the findings of this study are available on reasonable request from the corresponding author, Lars Møller Pedersen (lmpn@regionsjaelland.dk), and according to Danish legislation. Data can be shared on collaborative basis on the Danish Lymphoid Cancer Research data resource.
The full-text version of this article contains a data supplement.