Key Points
Ablation of Blimp-1 in anti-BCMA CAR T cells improves efficacy, representing a promising novel therapeutic product for MM.
Transcriptomic analysis revealed novel potential targets to further improve the therapeutic activity of anti-BCMA CAR T cells.
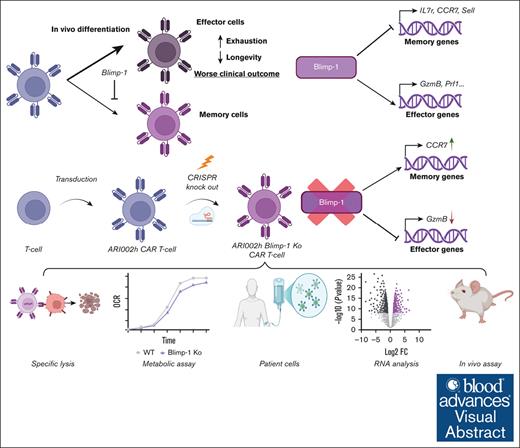
Visual Abstract
Chimeric antigen receptor (CAR) T cells directed against B-cell maturation antigen (BCMA) are an effective treatment for multiple myeloma (MM), but short persistence and frequent relapses are challenges for this immunotherapy. This lack of durability has been attributed to the premature terminal differentiation of CAR T cells, which prevents the formation of long-lived memory cells that maintain antitumor responses. To improve long-term efficacy, we used CRISPR/CRISPR-associated protein 9-mediated gene editing to ablate the expression of the transcription factor Blimp-1. Blimp-1 knockout (KO) CAR T cells displayed a memory-like phenotype compared with control (Mock) CAR T cells, but had reduced effector function, with a striking loss of granzyme B. However, in a murine model of advanced MM, Blimp-1 KO CAR T cells effectively slowed or even prevented disease progression, significantly outperforming Mock CAR T cells in improving survival (P = .006). To understand this enhanced in vivo effectiveness, Blimp-1 KO CAR T cells were characterized after being repeatedly challenged with tumor cells in vitro. In this setting, Blimp-1 KO CAR T cells maintained a highly active state with high expression of memory markers, but, crucially, demonstrated enhanced effector function and increased energetic capacity. RNA-sequencing analysis of tumor-exposed Blimp-1 KO CAR T cells confirmed the presence of a memory-like transcriptomic signature and, additionally, revealed enhanced ribosome biogenesis and repressed CAR T-cell dysfunction as mechanisms that could contribute to improved antitumor activity. Put together, our findings show that dampening Blimp-1 expression altered the phenotype and function of anti-BCMA CAR T cells, leading to augmented therapeutic efficacy in MM.
Introduction
Multiple myeloma (MM) is a hematologic neoplasm caused by the uncontrolled expansion of clonal plasma cells.1 Despite significant improvements to therapies for MM over the past 30 years, almost all patients with MM relapse until they develop an incurable form of the disease (relapsed/refractory MM).2,3
In recent years, chimeric antigen receptor (CAR) T-cell immunotherapy has demonstrated extremely promising results for patients with MM,4,5 resulting in the regulatory approval of 2 anti–B-cell maturation antigen (BCMA) CAR T-cell products.6,7 Clinical studies of these and other BCMA-directed CAR T cells have demonstrated high anti-MM activity during the first few months of administration.6-10 Unfortunately, however, durable responses are not observed in large numbers of patients, with further relapses commonplace.8
A major hindrance to long-term CAR T-cell activity is cell intrinsic deficiencies.11-14 These undesirable cell phenotypes describe a range of phenomena, including CAR T-cell dysfunction (predominantly exhaustion and senescence), premature terminal differentiation, and loss of self-renewing ability, or “stemness.”15,16 In anti-CD19 CAR T cells, there is now an abundance of evidence that these processes contribute to diminished clinical responses.17-19 Similarly, dysfunctional phenotypes have also been linked with poorer therapeutic efficacy.10,20,21 Notably, studies such as those by Yoshikawa et al22 have demonstrated that B lymphocyte-induced maturation protein-1 (Blimp-1) is a key regulator of these processes in T cells, influencing terminal differentiation and exhaustion. Various studies have demonstrated that an early differentiated memory-like phenotype in anti-BCMA CAR T cells correlates with improved responses and a lower risk of progression.10,21,23-26 However, the molecular mechanisms that control dysfunction, or a lack thereof, and effector/memory cell formation in anti-BCMA CAR T cells are yet to be elucidated.
A likely candidate for a master regulator of dysfunction, differentiation, and maintenance of stemness in CAR T cells is the transcription factor Blimp-1 (Prdm1). By both directly binding at DNA sites and recruiting factors that alter chromatin structure, Blimp-1 promotes a transcriptional profile that has a large impact on T-cell function.27-29 Indeed, Blimp-1 represses memory cell formation and the expression of genes related to stemness while driving terminal effector cell differentiation and the expression of inhibitory receptors involved in exhaustion.30-33 Furthermore, Jung et al34 have shown that Blimp-1 ablation can reduce T-cell exhaustion and improve immune checkpoint responsiveness.
As such, we hypothesized that ablation of Blimp-1 in anti-BCMA CAR T cells would favor the formation of undifferentiated memory-like cells with reduced dysfunction leading to enhanced in vivo efficacy. In this report, we used CRISPR/CRISPR-associated protein 9 (Cas9)-mediated genome editing to delete Blimp-1 in ARI0002h cells, an academic anti-BCMA CAR T-cell product that is currently in phase 1/2 of a multicenter clinical trial (NCT04309981).9,35 Although previous studies identified PRDM1 as a critical target for enhancing CAR T persistence using high-throughput knock-in engineering, our study focuses on BCMA CAR T cells and offers mechanistic insights into their long-term functional recovery.36
Methods
Primary T-cell purification and activation
T cells were obtained from buffy coats that were provided by the local blood and tissue bank (Banc de Sang i Teixits, Catalonia). Peripheral blood mononuclear cells were purified from buffy coats using Histopaque-1077 (Sigma-Aldrich) and density-gradient centrifugation. Subsequently, T cells isolated using a Pan T Cell Isolation kit (Miltenyi Biotech) were resuspended in T-cell media (47.5% Click’s media [Irving Scientific], 47.5% RPMI-1640, 5% human serum, 2 mM L-glutamine, 100 IU/mL penicillin and 100 μg/mL streptomycin) at 1 × 106/mL and plated on a 24-well plate. T cells were activated by the addition of Dynabeads Human T-Activator CD3/CD28 (Thermo Fisher Scientific) and 10 ng/mL interleukin-15 (IL-15) (Miltenyi Biotech).
CRISPR/Cas9-mediated genome editing and CAR T-cell expansion
On day 4 of the CAR T-cell production, cells were subject to CRISPR/Cas9-mediated gene disruption. Subsequently, CAR T cells were maintained in T-cell media supplemented with 10 ng/mL IL-15 every 1 to 2 days and were used for experiments on days 9 to 11. Knockout (KO) efficiency was calculated by Inference of CRISPR Edits analysis. See also supplemental Methods.
Flow cytometry
Surface and intracellular targets were stained according to the protocols outlined in the supplemental Methods. Subsequently, samples were acquired on a FACSCanto II (BD Biosciences) and dead cells were excluded based on forward and side scatter. To determine granzyme B and cytokine production, CAR T cells were first cocultured with ARP-1 cells at a 1:1 ratio for 6 hours, with GolgiPlug (BD Biosciences) added for the last 5 hours.
In vitro cytotoxicity assay
The ability of Blimp-1 KO CAR T cells to kill MM cell lines in vitro was assessed using a luminescence-based assay, as detailed previously.37 In brief, CAR T cells and luciferase-expressing MM cell lines were cocultured for 24 hours at a range of CAR T-to-MM cell ratios before analysis of surviving tumor cells by luminescence.
In vivo mouse experiments - survival
In vivo experiments to assess mouse survival were performed similarly to those described previously37,38 and as detailed in the figure legend. Briefly, irradiated 13 to 15-week-old male NOD-SCIDIL2gc−/− mice were infused with 1 × 106 ARP-1-GFP-ffLuc cells by tail vein injection on day 0. On day 14, mice were injected with untransduced T (UT) cells, Mock CAR T cells, or Blimp-1 KO CAR T cells whereas, with patient-derived CAR T cells, mice were injected with 1.5M CAR+ for UT, Mock, and Blimp-1 KO groups, 7 days after tumor injection.
Further details are provided in the supplemental Methods.
Repeated challenges of CAR T cells with tumor cells
Mock and Blimp-1 KO CAR T cells were cocultured with ARP-1-GFP-ffLuc cells at a 0.5:1 ratio for 48 hours. Thereafter, CAR T cells from the cultures were used to set up new cocultures using fresh ARP-1-GFP-ffLuc cells at the same effector-to-target ratio (0.5:1).
Further details are provided in the supplemental Methods.
RNA sequencing
After 4 challenges with tumor cells, CD8+ cells were purified from the cocultures of Mock or Blimp-1 KO CAR T cells and ARP-1-GFP-ffLuc cells using an EasySep Human CD8 Positive Selection kit (STEMCELL Technologies). Cell pellets were frozen and stored at −80°C and isolation efficiency was confirmed by flow cytometry. Total RNA was extracted from the cell pellets using an RNeasy Mini kit (Qiagen) and subject to paired-end mRNA sequencing using the NovaSeq6000 platform (Illumina) at a minimum of 30 million reads per sample.
Bradford assay
Lysates prepared for immunoblotting were diluted and mixed with Bradford reagent for 10 minutes at room temperature. Protein content was quantified by measuring A595 using a BioTek Synergy HTX Multimode Reader.
Further details are provided in the supplemental Methods.
Results
Blimp-1 KO anti-BCMA CAR T cells modulates the expression of LAG-3
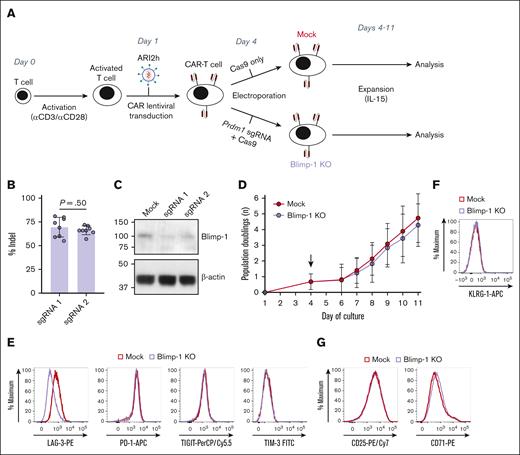
To dampen Blimp-1 expression in anti-BCMA CAR T cells, we used a CRISPR/Cas9-mediated gene editing approach using a single guide RNA (sgRNA) targeting Prdm1, the gene encoding Blimp-1 (Figure 1A). This genetic KO was introduced into ARI0002h cells with 2 different sgRNAs produced KO cells with an average editing efficiency >65%, as determined by the percentage of cell-derived DNA containing 1 or more indels (Figure 1B). Analysis of Blimp-1 loss at the protein level also demonstrated efficient KO using both sgRNAs (Figure 1C). The sgRNA that caused the highest incidence of indels and disruption of protein expression, sgRNA1, was used for subsequent analysis.
LAG-3 expression is reduced on Blimp-1 KO anti-BCMA CAR T cells. (A) Schematic of cell culture protocol for production of Mock and Blimp-1 KO CAR T cells. (B-C) Blimp-1 deletion efficiency in KO CAR T cells when electroporated with 1 of 2 different Prdm1-targeting guide RNAs (sgRNA1 or sgRNA2). Displayed as Inference of CRISPR Edits analysis to calculate percentage of cells containing Blimp-1 indels (B) and abundance of Blimp-1 protein as measured by western blotting (C). (D) CAR T-cell growth from day 1 after CD3/CD28-mediated stimulation, based on daily cell counts and displayed as the cumulative number of population doublings (n = 6). Arrow indicates day of electroporation. (E-G) Expression of inhibitory receptors lymphocyte activation gene-3 (LAG-3), programmed cell death protein-1 (PD-1), T cell immunoreceptor with Ig and ITIM domains (TIGIT) and T cell immunoglobulin and mucin-domain containing-3 (TIM-3) (E), killer cell lectin-like receptor subfamily G member-1 (KLRG-1) (F), or activation markers CD25 and CD71 (G) on CD8+ CAR+ Mock and Blimp-1 KO CAR T cells, representative of 4 (F) or 7 (E,G) experiments. Graphs show means ± standard deviation (SD).
LAG-3 expression is reduced on Blimp-1 KO anti-BCMA CAR T cells. (A) Schematic of cell culture protocol for production of Mock and Blimp-1 KO CAR T cells. (B-C) Blimp-1 deletion efficiency in KO CAR T cells when electroporated with 1 of 2 different Prdm1-targeting guide RNAs (sgRNA1 or sgRNA2). Displayed as Inference of CRISPR Edits analysis to calculate percentage of cells containing Blimp-1 indels (B) and abundance of Blimp-1 protein as measured by western blotting (C). (D) CAR T-cell growth from day 1 after CD3/CD28-mediated stimulation, based on daily cell counts and displayed as the cumulative number of population doublings (n = 6). Arrow indicates day of electroporation. (E-G) Expression of inhibitory receptors lymphocyte activation gene-3 (LAG-3), programmed cell death protein-1 (PD-1), T cell immunoreceptor with Ig and ITIM domains (TIGIT) and T cell immunoglobulin and mucin-domain containing-3 (TIM-3) (E), killer cell lectin-like receptor subfamily G member-1 (KLRG-1) (F), or activation markers CD25 and CD71 (G) on CD8+ CAR+ Mock and Blimp-1 KO CAR T cells, representative of 4 (F) or 7 (E,G) experiments. Graphs show means ± standard deviation (SD).
Owing to the previously reported negative role of Blimp-1 in T-cell proliferation,29,31 we first assessed whether ablation of Blimp-1 affected the in vitro growth of CAR T cells. We observed that the in vitro expansion of Blimp-1 KO CAR T cells was similar to control Mock transduced cells (Figure 1D), with a similar number of cell doublings and CD4:CD8 ratio at the end of the culture (supplemental Figure 1A-B). Analysis of CAR expression at this time point revealed that >70% of cells in the Blimp-1 KO culture were CAR+, demonstrating a high transduction efficiency with an albeit subtle decrease compared with Mock cells (supplemental Figure 1C). In our sequential study, we observed that lymphocyte activation gene-3 (LAG-3) expression initially increased during the early production phase, peaking on days 6 to 8 of culture. Subsequently, on days 9 to 10, its expression decreased compared with Mock cells, without affecting the expression of programmed cell death protein-1 (PD-1), killer cell lectin-like receptor subfamily G member-1 (KLRG-1), or other inhibitory receptors l (Figure 1E-F; supplemental Figures 2A and 12). Similarly, levels of the IL-2 receptor α-subunit CD25 and the iron transporter CD71, important molecules in T-cell metabolism and markers of T-cell activation, were also unchanged (Figure 1G; supplemental Figure 2B).
Genetic ablation of Blimp-1 represses effector cell differentiation in favor of a memory-like phenotype
Further phenotyping of expanded Blimp-1 KO CAR T cells confirmed increased abundance of memory cell-associated markers CD127 (IL-7 receptor α-chain) and CCR7 (Figure 2A), as anticipated given the known function of Blimp-1 in repressing memory cell formation.31,33 The transcription factor T cell factor-7 (TCF-7), a critical regulator of memory fate decisions in T cells and a Blimp-1 target gene,39 was also more highly expressed in Blimp-1 KO CAR T cells (Figure 2B).
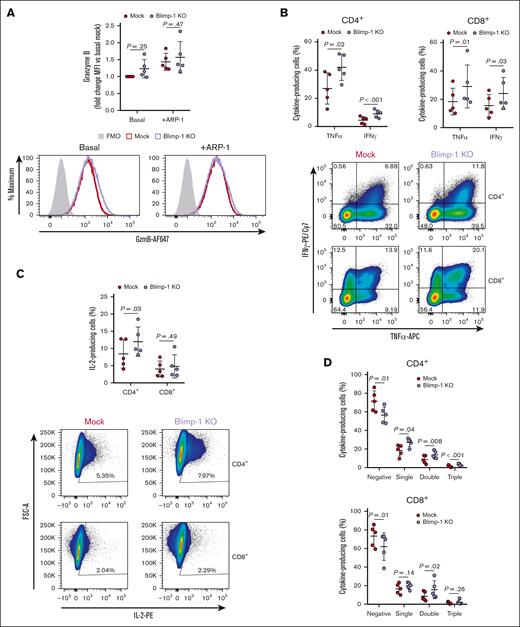
Blimp-1 KO CAR T cells display a memory-like phenotype. (A-B) Surface expression of CD127 (IL-7 receptor α-chain [IL-7Rα]) and CCR7 (A) or intracellular expression of TCF-7 (B) in CAR+ Mock and Blimp-1 KO CAR T cells. Representative histograms (left). Median fluorescence intensity (MFI) quantification (right). Fluorescence minus one (FMO) control in panel B shows Mock cells stained with all antibodies except TCF-7. (C) UT cells or Mock or Blimp-1 KO CAR T cells were cocultured with luciferase-expressing ARP-1 cells for 24 hours at effector-to-target (E:T) ratios ranging from 1:1 to 0.125:1. Displayed is the ARP-1 cell survival, as determined by bioluminescence, compared with ARP-1 cells cultured alone (n = 9). (D-G) Mock and Blimp-1 KO CAR T cells were cocultured with ARP-1 cells for 6 hours and production of effector molecules in CAR+ cells was assessed by flow cytometry. (D) Granzyme B expression in CD8+ cells, evaluated without (basal) and with (+ARP-1) ARP-1 cell stimulation. Representative histograms (left). Summary data showing fold change of MFI compared with basal Mock (right). FMO control represents Mock cells stained with all antibodies except granzyme B. (E) Production of TNF-α and IFN-γ in CD8+ cells. (F) Production of IL-2 in CD4+ and CD8+ cells. (G) Proportion of cells producing none (negative), 1 (single), 2 (double), or all 3 (triple) of the analyzed cytokines. Graphs show means ± SD for the panels A-B,D-G or mean ± standard error of the mean (SEM) for the panel C.
Blimp-1 KO CAR T cells display a memory-like phenotype. (A-B) Surface expression of CD127 (IL-7 receptor α-chain [IL-7Rα]) and CCR7 (A) or intracellular expression of TCF-7 (B) in CAR+ Mock and Blimp-1 KO CAR T cells. Representative histograms (left). Median fluorescence intensity (MFI) quantification (right). Fluorescence minus one (FMO) control in panel B shows Mock cells stained with all antibodies except TCF-7. (C) UT cells or Mock or Blimp-1 KO CAR T cells were cocultured with luciferase-expressing ARP-1 cells for 24 hours at effector-to-target (E:T) ratios ranging from 1:1 to 0.125:1. Displayed is the ARP-1 cell survival, as determined by bioluminescence, compared with ARP-1 cells cultured alone (n = 9). (D-G) Mock and Blimp-1 KO CAR T cells were cocultured with ARP-1 cells for 6 hours and production of effector molecules in CAR+ cells was assessed by flow cytometry. (D) Granzyme B expression in CD8+ cells, evaluated without (basal) and with (+ARP-1) ARP-1 cell stimulation. Representative histograms (left). Summary data showing fold change of MFI compared with basal Mock (right). FMO control represents Mock cells stained with all antibodies except granzyme B. (E) Production of TNF-α and IFN-γ in CD8+ cells. (F) Production of IL-2 in CD4+ and CD8+ cells. (G) Proportion of cells producing none (negative), 1 (single), 2 (double), or all 3 (triple) of the analyzed cytokines. Graphs show means ± SD for the panels A-B,D-G or mean ± standard error of the mean (SEM) for the panel C.
We then assessed whether this gain of memory phenotype had a detrimental impact on effector function. Interestingly, Blimp-1 KO CAR T cells retained a high capacity to eliminate MM cell lines in vitro with only a minor reduction in killing ability compared with Mock CAR T cells (Figure 2C; supplemental Figure 3A). Consistent with a loss of effector phenotype, Blimp-1 KO CAR T cells exhibited low basal expression of the cytolytic molecule granzyme B (Figure 2D). In response to tumor cells, Blimp-1 KO CAR T cells maintained the ability to upregulate granzyme B, but produced approximately half the quantity of Mock cells. Upregulation of the effector molecule interferon gamma (IFN-γ) was also impaired in CD8+ Blimp-1 KO CAR T cells, but the effector cytokine tumor necrosis factor α (TNF-α) was unchanged (Figure 2E; supplemental Figure 3B). In contrast to the regulation of IFN-γ, production of the cytokine IL-2 was increased in Blimp-1 KO CAR T cells (Figure 2F; supplemental Figure 3C). As a result of the differential regulation of IFN-γ and IL-2, the overall proportion of polyfunctional cytokine-producing CAR T cells was unchanged (Figure 2G). Put together, these data suggest that KO of Blimp-1 promotes a memory-like phenotype while retaining a sufficient, albeit dampened, effector function.
Blimp-1 KO CAR T cells demonstrate superior control of MM in a murine model
The Blimp-1 KO in anti-CD19 CAR T cells improves antitumor responses in a leukemia model,22 but whether Blimp-1 loss enhances anti-BCMA CAR T-cell function in MM remains unknown. Therefore, we sought to assess the ability of Blimp-1 KO CAR T cells to control MM using a previously established murine model35,40 (Figure 3A). To enable us to observe differences in responses to Mock and Blimp-1 KO CAR T cells, a “stress model” with a relatively low number of CAR T cells was infused such that we would expect improved survival, but not complete tumor clearance. Indeed, Mock and Blimp-1 KO CAR T cells initially reduced tumor burden compared with animals injected with UT cells, which rapidly developed terminal levels of disease (Figure 3B-D). However, although Mock-treated mice quickly relapsed, Blimp-1 KO CAR T cells demonstrated long-term efficacy, completely preventing progression in some animals and therefore dramatically increasing survival time (Figure 3E; supplemental Figure 4A). Analysis of the bone marrow and spleen of mice at the time of euthanasia showed that CAR T cells were absent or scarce, representing <1% of cells in all animals that succumbed to the disease (supplemental Figure 4B). In the 3 mice that survived from the Blimp-1 KO group, it was notable that 2 had a large presence of CAR T cells in the spleen, of which one also had a notable population in the bone marrow (supplemental Figure 4B). Intriguingly, further analysis of these CAR T cells revealed markedly different CD4:CD8 ratios (supplemental Figure 4C). Put together, these data demonstrate the enhanced potential of Blimp-1 KO anti-BCMA CAR T cells to achieve long-term tumor clearance.
Improved disease control by Blimp-1 KO CAR T cells in a myeloma mouse model. (A-E) UT cells or Mock or Blimp-1 KO CAR T cells were injected into mice previously infused with luciferase-expressing ARP-1 (ARP-1-ffLuc) cells. (A) Schematic showing experimental plan. (B-C) Disease progression, observed as bioluminescence signal from weekly images of mice. Displayed are photos (B) and quantification (C) from individual mice. (D) Quantification of bioluminescence on day 28, showing means ± SD (1-way analysis of variance [ANOVA] with Tukey multiple comparisons test). (E) Overall survival of animals from each group. Arrows in the panels C,E show days on which T cells/CAR T cells were infused. Dotted line in the panel C indicates bioluminescence threshold for euthanizing mice (3 × 108 p/s/cm2/sr).
Improved disease control by Blimp-1 KO CAR T cells in a myeloma mouse model. (A-E) UT cells or Mock or Blimp-1 KO CAR T cells were injected into mice previously infused with luciferase-expressing ARP-1 (ARP-1-ffLuc) cells. (A) Schematic showing experimental plan. (B-C) Disease progression, observed as bioluminescence signal from weekly images of mice. Displayed are photos (B) and quantification (C) from individual mice. (D) Quantification of bioluminescence on day 28, showing means ± SD (1-way analysis of variance [ANOVA] with Tukey multiple comparisons test). (E) Overall survival of animals from each group. Arrows in the panels C,E show days on which T cells/CAR T cells were infused. Dotted line in the panel C indicates bioluminescence threshold for euthanizing mice (3 × 108 p/s/cm2/sr).
To assess whether improved CAR T migration led to enhanced Blimp-1 KO tumor control, CAR T cells were quantified 1 week after infusion. No differences were found in the bone marrow or spleen between Mock and Blimp-1 KO CAR T cells. (supplemental Figure 4D). This finding suggests that the different in vivo efficacy was caused by altered antimyeloma activity and not homing.
Patient-derived Blimp-1 KO CAR T cells maintain the killing capacity in vitro and tend to better control MM in a murine model
As previously shown in experiments with CAR T cells from healthy donors, we aimed to determine whether CAR T cells derived from myeloma patients selected for CAR T-cell therapy could be successfully engineered Blimp-1 KO, while remaining viable and maintaining their killing capacity both in vitro and in vivo. Both Mock and Blimp-1 KO CAR T cells demonstrated the ability to maintain their killing capacity in vitro (supplemental Figure 9B). In addition, mice injected with Mock and Blimp-1 KO CAR T cells from patients showed reduced tumor burden compared with animals injected with UT cells, which rapidly developed the disease. Over time, Mock-treated mice began to relapse earlier than those treated with Blimp-1 KO CAR T cells; eventually, the number of relapsed mice was higher in Mock-treated mice than the Blimp-1 KO group, indicating a trend toward better disease control (supplemental Figure 13).
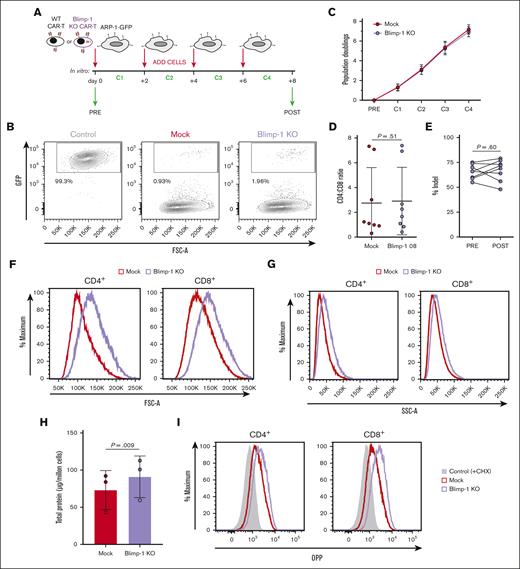
Loss of Blimp-1 enhances CAR T-cell protein synthesis and increases cell protein content
To better understand why Blimp-1 deletion improved anti-BCMA CAR T-cell antimyeloma activity, we set up an in vitro coculture model to mimic repeated antigen exposure (Figure 4A). In this system, both Mock and Blimp-1 KO CAR T cells eliminated almost all ARP-1 MM cells by the end of the challenges (Figure 4B; supplemental Figure 5A), signifying a similar cytotoxic capacity. As such, Mock and Blimp-1 KO CAR T cells rapidly expanded in response to the tumor cells, both ending with similar proportions of CD4+ and CD8+ cells (Figure 4C-D). A comparable percentage of cells displaying Prdm1 mutations before and after the challenges in the heterogenic pool of Blimp-1 KO cells also supports the conclusion that the challenges did not favor the expansion of cells with or without Blimp-1 deletion (Figure 4E).
Blimp-1 KO CAR T cells successfully eliminate tumor cells in multiple rounds of cocultures. (A) Schematic of cell culture protocol for repeated challenges of Mock and Blimp-1 KO CAR T cells with GFP-expressing ARP-1 (ARP-1-GFP) cells. (B) Survival of ARP-1-GFP cells at the end of the consecutive cocultures, assessed by flow cytometry to detect GFP+ cells and displayed as fluorescence-activated cell sorting (FACS) plots. Control cells are ARP-1-GFP cells cultured without CAR T cells. (C) CAR T-cell expansion from before the first challenge (PRE) and after each of the 4 successive challenges (C1-C4), based on cell counts and displayed as the cumulative number of population doublings (n = 10). (D) Ratio of CD4+ and CD8+ cells at the end of the fourth challenge. (E) Efficiency of Blimp-1 deletion in KO CAR T cells before (pre) and after (post) repeated challenges measured by Inference of CRISPR Edits analysis to calculate percentage of cells containing indels in the Prdm1 gene. (F-G) Forward scatter (F) and side scatter (G) analysis of CD4+ and CD8+ CAR+ Mock and Blimp-1 KO CAR T cells. Shown are representative histograms (n = 8). (H) Total protein content of repeatedly challenged Mock and Blimp-1 KO CAR T cells, measured by Bradford assay. (I) De novo protein synthesis in CD4+ and CD8+ CAR+ Mock and Blimp-1 KO CAR T cells, as measured by the incorporation of O-propargyl-puromycin (OPP). Negative control is cells treated with cycloheximide (CHX). Shown are representative histograms (n = 2). Graphs show means ± SD. GFP, green fluorescent protein.
Blimp-1 KO CAR T cells successfully eliminate tumor cells in multiple rounds of cocultures. (A) Schematic of cell culture protocol for repeated challenges of Mock and Blimp-1 KO CAR T cells with GFP-expressing ARP-1 (ARP-1-GFP) cells. (B) Survival of ARP-1-GFP cells at the end of the consecutive cocultures, assessed by flow cytometry to detect GFP+ cells and displayed as fluorescence-activated cell sorting (FACS) plots. Control cells are ARP-1-GFP cells cultured without CAR T cells. (C) CAR T-cell expansion from before the first challenge (PRE) and after each of the 4 successive challenges (C1-C4), based on cell counts and displayed as the cumulative number of population doublings (n = 10). (D) Ratio of CD4+ and CD8+ cells at the end of the fourth challenge. (E) Efficiency of Blimp-1 deletion in KO CAR T cells before (pre) and after (post) repeated challenges measured by Inference of CRISPR Edits analysis to calculate percentage of cells containing indels in the Prdm1 gene. (F-G) Forward scatter (F) and side scatter (G) analysis of CD4+ and CD8+ CAR+ Mock and Blimp-1 KO CAR T cells. Shown are representative histograms (n = 8). (H) Total protein content of repeatedly challenged Mock and Blimp-1 KO CAR T cells, measured by Bradford assay. (I) De novo protein synthesis in CD4+ and CD8+ CAR+ Mock and Blimp-1 KO CAR T cells, as measured by the incorporation of O-propargyl-puromycin (OPP). Negative control is cells treated with cycloheximide (CHX). Shown are representative histograms (n = 2). Graphs show means ± SD. GFP, green fluorescent protein.
When analyzing the CAR T-cell/tumor cell cocultures after the final challenge, we consistently observed that Blimp-1 KO CAR T cells had an increased forward and side scatter compared with Mock CAR T cells, implying larger cell size and enhanced granularity, respectively (Figure 4F-G; supplemental Figure 5B-C). In T cells, alterations to cell size are commonly associated with changes in protein mass.41,42 Therefore, we measured total protein content in postchallenge Blimp-1 KO CAR T cells. Interestingly, we found that Blimp-1 KO CAR T-cell protein content was indeed augmented compared with Mock CAR T cells (Figure 4H). This increase was accompanied by upregulated de novo protein synthesis (Figure 4I), as measured by the incorporation of a puromycin analog into nascent protein chains.
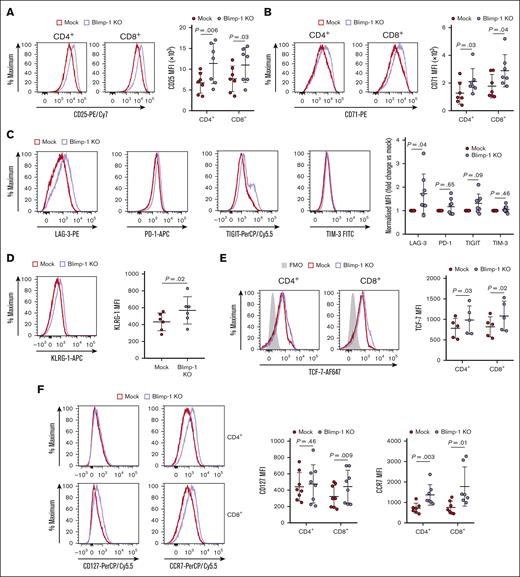
Tumor-exposed Blimp-1 KO CAR T cells display memory cell characteristics
The changes to protein metabolism in Blimp-1 KO CAR T cells were indicative of an altered cellular phenotype. The expression of CD25 and CD71, important molecules involved in T-cell activity and metabolism, was higher on tumor cell–challenged Blimp-1 KO CAR T cells than on Mock CAR T cells (Figure 5A-B). The abundance of many inhibitory receptors often correlates with cell activity, and we observed that inhibitory receptors associated with exhaustion were frequently upregulated on cells with Blimp-1 deletion (Figure 5C; supplemental Figure 6). In addition, KLRG-1 was also more highly expressed on Blimp-1 KO CAR T cells (Figure 5D).
Antigen-experienced Blimp-1 KO CAR T cells display an effector-memory phenotype. (A-F) Repeatedly challenged Mock and Blimp-1 KO CAR T cells were subject to phenotyping by flow cytometry to evaluate cell surface expression of CD25 (A), CD71 (B), LAG-3, PD-1, TIGIT and TIM-3 (C), KLRG-1 (D), and CD127 (IL-7Rα) and CCR7 (F), or intracellular expression of TCF-7 (E), in CAR+ Mock and Blimp-1 KO CAR T cells. Shown are CD4+ and CD8+ cells, except for the panels C-D where only CD8+ cells are displayed. Representative histograms (left). MFI quantification (right). FMO control in panel E shows Mock cells stained with all antibodies except TCF-7. Graphs show means ± SD.
Antigen-experienced Blimp-1 KO CAR T cells display an effector-memory phenotype. (A-F) Repeatedly challenged Mock and Blimp-1 KO CAR T cells were subject to phenotyping by flow cytometry to evaluate cell surface expression of CD25 (A), CD71 (B), LAG-3, PD-1, TIGIT and TIM-3 (C), KLRG-1 (D), and CD127 (IL-7Rα) and CCR7 (F), or intracellular expression of TCF-7 (E), in CAR+ Mock and Blimp-1 KO CAR T cells. Shown are CD4+ and CD8+ cells, except for the panels C-D where only CD8+ cells are displayed. Representative histograms (left). MFI quantification (right). FMO control in panel E shows Mock cells stained with all antibodies except TCF-7. Graphs show means ± SD.
The change in the phenotype of Blimp-1 KO CAR T cells after long-term exposure to tumor cells led us to question whether the expression of memory-like markers was also altered. We observed that the transcription factor TCF-7, a critical regulator of the stem cell memory-like phenotype, was more abundant in Blimp-1 KO CAR T cells (Figure 5E), but the difference was less dramatic than that observed before the tumor cell challenges (Figure 2B). Furthermore, CD8+ Blimp-1 KO CAR T cells maintained higher expression of both CD127 and CCR7, similar to before the tumor cell challenges (Figure 5F). Tumor cell–exposed CD4+ Blimp-1 KO CAR T cells also had higher expression of CCR7, but not CD127.
Taken together, these findings illustrate that Blimp-1 deletion causes fundamental changes to the phenotype of anti-BCMA CAR T cells in response to prolonged tumor cell exposure.
Blimp-1 deletion promotes increased effector function in CAR T cells expanded with tumor cells
The maintenance of cytotoxic function over the course of the in vitro challenges suggested that Blimp-1 KO CAR T cells overcame their initial deficiency in effector molecule function. To promote effector molecule release, CAR T cells previously cocultured with multiple rounds of MM cell lines were subject to another short 6-hour challenge with tumor cells. Interestingly, basal expression and tumor cell–induced production of granzyme B in cocultured Blimp-1 KO CAR T cells recovered to levels similar to those observed for Mock CAR T cells (Figure 6A). Production of the effector molecules IFN-γ and TNF-α in Blimp-1 KO CAR T cells was not reduced either and, remarkably, was augmented compared with Mock CAR T cells in both CD4+ and CD8+ cells (Figure 6B). A larger proportion of CD4+ cells produced IL-2 as well (Figure 6C), resulting in an increase in the percentage of cells producing 1, 2, or all 3 cytokines and a reduction in the number of cells not producing cytokines (Figure 6D). For CD8+ cells, the proportion of CAR T cells producing no cytokine was similarly decreased, and the fraction of cells producing 2 cytokines was higher than in Mock CAR T cells.
Effector function of tumor-exposed Blimp-1 KO CAR T cells is superior to Mock CAR T cells. (A-D) Mock and Blimp-1 KO CAR T cells subject to repeated challenges with ARP-1 cells were cocultured with ARP-1 cells for another 6 hours and production of granzyme B (A) and cytokines (B-D) in CAR+ cells was assessed by flow cytometry. (A) Granzyme B expression in CD8+ cells, evaluated without (basal) and with (+ARP-1) ARP-1 cell stimulation. Representative histograms (left). Summary data showing fold change of MFI compared with basal Mock (right). FMO control represents Mock cells stained with all antibodies except granzyme B. (B-C) Production of TNF-α and IFN-γ (B) and IL-2 (C) in CD4+ and CD8+ cells. Summary data (top). Representative FACS plots, with numbers showing the percentage of cells in that quadrant (bottom). (D) Proportion of cells producing none (negative), 1 (single), 2 (double), or all 3 (triple) of the analyzed cytokines. Graphs show means ± SD.
Effector function of tumor-exposed Blimp-1 KO CAR T cells is superior to Mock CAR T cells. (A-D) Mock and Blimp-1 KO CAR T cells subject to repeated challenges with ARP-1 cells were cocultured with ARP-1 cells for another 6 hours and production of granzyme B (A) and cytokines (B-D) in CAR+ cells was assessed by flow cytometry. (A) Granzyme B expression in CD8+ cells, evaluated without (basal) and with (+ARP-1) ARP-1 cell stimulation. Representative histograms (left). Summary data showing fold change of MFI compared with basal Mock (right). FMO control represents Mock cells stained with all antibodies except granzyme B. (B-C) Production of TNF-α and IFN-γ (B) and IL-2 (C) in CD4+ and CD8+ cells. Summary data (top). Representative FACS plots, with numbers showing the percentage of cells in that quadrant (bottom). (D) Proportion of cells producing none (negative), 1 (single), 2 (double), or all 3 (triple) of the analyzed cytokines. Graphs show means ± SD.
After confirming the maintenance of cytotoxic function, metabolic differences became more apparent after tumor exposure. Seahorse measurements of oxygen consumption rate (OCR), spare respiratory capacity (SRC), and adenosine triphosphate (ATP) production were conducted under stress conditions to assess metabolic activity. Blimp-1 KO CAR T cells showed increased OCR, indicating enhanced metabolic activation (supplemental Figure 11D). SRC was also higher, reflecting greater metabolic flexibility and capacity to meet energy demands (supplemental Figure 11E).
In addition, ATP production, including both mitochondrial ATP and glycolytic ATP, was elevated, suggesting improved metabolic response under stress (supplemental Figure 11F). In addition, the increased ATP levels have been shown to enhance protein synthesis capacity.43 Collectively, these results indicate that Blimp-1 KO CAR T cells become functionally more potent than Mock CAR T cells after repeated tumor challenges, effectively reversing the relatively lower effector-like capabilities observed before antigen exposure.
Blimp-1 controls the expression of key gene sets to regulate differentiation and dysfunction
To obtain a global overview of the impact caused by the genetic deletion of Prdm1, the transcriptome of tumor cell–exposed Blimp-1 KO CAR T cells was characterized by RNA sequencing (supplemental Figure 7). Although Blimp-1 is thought of as a master regulator of gene transcription, its deletion affected less than 5% of the CD8+ CAR T-cell transcriptome (fold change >1.5; P < .05), suggesting that Blimp-1–mediated control of gene expression is selective (Figure 7A). Among the subset of genes altered by the loss of Blimp-1 was those related to T-cell differentiation (Figure 7B). Increased abundance of the genes Tcf1, Ccr7, and Il7r, as observed at the protein level (Figure 5E-F), was accompanied by a memory cell-like transcriptional signature (Figure 7C). Indeed, some direct targets of Blimp-1 transcriptional repression, including Tcf1,39Myc,44 and Bcl6,45 were higher expressed in Blimp-1 KO cells, although others such as CD27 were lower (Figure 7D). In contrast, Blimp-1 KO cells had lower levels of important effector-related genes, including Id2, Eomes, and the genes encoding the effector molecules perforin, granulysin, and granzyme A (Figure 7E). Notable exceptions were Irf4, which was more abundant in Blimp-1 KO cells, and granzyme B and Tbx21 (T-bet), which were unchanged. Interestingly, we observed a decreased abundance of the granzyme B-promoting transcription factor Znf683 (Hobit) in Blimp-1 KO cells, meaning that Hobit was not compensating for the loss of Blimp-1–mediated upregulation of granzyme B (Figure 7F).
Loss of Blimp-1 selectively remodels the CAR T-cell transcriptome. (A-H) Transcriptome of CD8+ Mock and Blimp-1 KO CAR T cells subject to repeated challenges with ARP-1 cells. (A) Comparison of normalized counts of all identified genes between Mock and Blimp-1 KO cells with each point representing an individual gene. Genes that were significantly more or less abundant in Blimp-1 KO cells (fold change >1.5-fold; false discovery rate [FDR] <0.05) are highlighted in purple or blue, respectively. Dashed line shows equivalent expression in Mock and Blimp-1 KO. (B) Volcano plot showing the difference between gene expression in Blimp-1 KO and Mock cells. Genes that comprise the Gene Ontology term “GOBP_T_CELL_DIFFERENTIATION” (GO:0030217) are highlighted in red. Dashed horizontal line shows FDR = 0.05 and dashed vertical lines show fold change of 1.5. (C) Heat map showing z-score normalized expression of key genes related to T-cell memory phenotype (left). Heat map displaying FDR of genes shown in left heat map, with FDR <0.05 in green (right). (D) Normalized count data of Myc, Bcl6, and CD27 genes. (E) As panel C, but showing effector phenotype-related genes. (F) Normalized count data of Znf683 (Hobit) gene. (G) Gene set enrichment analysis (GSEA) analysis of GOBP_RIBOSOME BIOGENESIS (red: NES, 2.15; FDR, 1.50 × 10−7) and GOCC_PRERIBOSOME (blue: normalized enrichment score (NES), 2.30; FDR, 9.67 × 10−7). (H) Normalized count data of Adora2a (A2AR), Ikzf3 (Aiolos), and Sox4 genes. Graphs show means ± SD and different symbols represent different donors.
Loss of Blimp-1 selectively remodels the CAR T-cell transcriptome. (A-H) Transcriptome of CD8+ Mock and Blimp-1 KO CAR T cells subject to repeated challenges with ARP-1 cells. (A) Comparison of normalized counts of all identified genes between Mock and Blimp-1 KO cells with each point representing an individual gene. Genes that were significantly more or less abundant in Blimp-1 KO cells (fold change >1.5-fold; false discovery rate [FDR] <0.05) are highlighted in purple or blue, respectively. Dashed line shows equivalent expression in Mock and Blimp-1 KO. (B) Volcano plot showing the difference between gene expression in Blimp-1 KO and Mock cells. Genes that comprise the Gene Ontology term “GOBP_T_CELL_DIFFERENTIATION” (GO:0030217) are highlighted in red. Dashed horizontal line shows FDR = 0.05 and dashed vertical lines show fold change of 1.5. (C) Heat map showing z-score normalized expression of key genes related to T-cell memory phenotype (left). Heat map displaying FDR of genes shown in left heat map, with FDR <0.05 in green (right). (D) Normalized count data of Myc, Bcl6, and CD27 genes. (E) As panel C, but showing effector phenotype-related genes. (F) Normalized count data of Znf683 (Hobit) gene. (G) Gene set enrichment analysis (GSEA) analysis of GOBP_RIBOSOME BIOGENESIS (red: NES, 2.15; FDR, 1.50 × 10−7) and GOCC_PRERIBOSOME (blue: normalized enrichment score (NES), 2.30; FDR, 9.67 × 10−7). (H) Normalized count data of Adora2a (A2AR), Ikzf3 (Aiolos), and Sox4 genes. Graphs show means ± SD and different symbols represent different donors.
Beyond the hypothesized modification to the memory/effector signature, gene set enrichment analysis revealed that genes involved in ribosome biogenesis and RNA processing were upregulated in Blimp-1 KO cells (Figure 7G; supplemental Table 1), fitting with our finding that these cells increased protein synthesis rates (Figure 4I). In addition, we conducted RNA analysis to identify other overexpressed targets for potential new objectives, such as a double KO with NR4A3, as has been performed with other CAR T cells34; however, we did not find that the single KO affected the expression of this gene. Further exploration revealed that Adora2a (A2AR), Ikzf3 (AIOLOS), and Sox4, previously shown to enhance CAR T-cell function when downmodulated,46-49 were less abundant in Blimp-1 KO CAR T cells (Figure 7H), indicating that the impact of Blimp-1 ablation on CAR T-cell activity is multifaceted.
Discussion
As a hugely promising treatment option for relapsed/refractory MM, there is a great need to improve the effectiveness of anti-BCMA CAR T cells. In this study, we demonstrate that genetic deletion of Blimp-1, a transcription factor critical in the regulation of T-cell memory/effector fate decisions, enhances the ability of anti-BCMA CAR T cells to prevent disease progression and improve survival in a murine xenograft model. Although Blimp-1 KO CAR T cells that had not been exposed to tumor cells displayed a naïve/memory-like phenotype, with a corresponding dampening of effector function, antigen-experienced Blimp-1 KO CAR T cells recovered antimyeloma activity while retaining high expression of memory markers, a phenotype that is ideal for persistent CAR T cells.
One important finding from our study was that Blimp-1 dynamically regulates the production of effector molecules in CAR T cells. Most strikingly, granzyme B was much less abundant in Blimp-1 KO CAR T cells compared with Mock CAR T cells, corresponding with previous studies.22,30,31,50 As one of the major mechanisms of CAR T-cell–mediated killing,51 it is likely that the drop in tumor cell–mediated granzyme B release was responsible for the poorer in vitro killing ability. However, an overpopulation of effector cells that have high expression of effector molecules, including granzyme B, in the preinfusion product is associated with poorer clinical responses to anti-BCMA CAR T cells,20 and thus, lower basal levels of granzyme B may be advantageous.
After undergoing repeated cocultures with tumor cells, Blimp-1 KO CAR T cells were no longer defective in granzyme B compared with Mock CAR T cells. This apparent discrepancy could be explained by Blimp-1-independent control of granzyme B expression by T-bet (Tbx21),52 which is expressed normally in Blimp-1 KO cells (Figure 7E). Hobit, another transcriptional promoter of granzyme B,53 was less abundant in Blimp-1 KO cells and therefore is not likely to be critical. Regardless of the exact mechanism, the recovery of granzyme B levels after tumor exposure is potentially critical to the cytolytic capacity of Blimp-1 KO CAR T cells and could provide an explanation as to why Blimp-1 KO CAR T cells were able to maintain persistent tumor cell killing and better long-term control disease in our murine xenograft model. Metabolic studies provide new insights into the enhanced in vivo functionality of Blimp-1 KO cells. These cells demonstrate no significant alterations in OCR, SRC, or ATP production during the resting state. However, upon exposure to elevated stress conditions, Blimp-1 KO cells exhibited notable differences. They display an increased oxygen consumption, enhanced mitochondrial and glycolytic ATP production, and a higher SRC. These findings suggest that Blimp-1 KO cells possess a more active phenotype, characterized by greater reactivity and improved metabolic flexibility. Therefore, the increased activity and efficiency of these cells may contribute to a dampening of exhaustion and facilitate prolonged immune responses.
This research additionally provides new insights into the ability to generate genetic alterations in patient-derived cells. In cells derived from patients with refractory MM, we achieved more than 60% KO efficiency, allowing in vitro expansion while maintaining their killing capacity. Furthermore, an in vivo study demonstrated a clear trend toward improved disease control with these modified cells.
Cytokine production was also affected by Blimp-1 ablation, with a similarly notable difference between before and after the MM cell cocultures. After the challenges, Blimp-1 KO CAR T cells produced increased amounts of TNF-α and IFN-γ, as predicted owing to the known role of Blimp-1 to directly bind and suppress the transcription of their respective genes.54 Expression of the gene encoding IL-2 is also inhibited by Blimp-1,28,31 and consistent with this knowledge, postchallenge Blimp-1 KO CD4+ CAR T cells upregulated IL-2 production. However, these cytokines were not secreted at higher levels before the tumor cell challenges. Put together with the granzyme B data, it seems that the role of Blimp-1 in controlling CAR T-cell effector function changes after prolonged exposure to tumor cells. Indeed, the superior ability of Blimp-1 KO CAR T cells to increase the production of effector molecules after long-term exposure to tumor cells is a clear indication of reduced disease-induced dysfunction and is a potential advantage in prolonging functional persistence.
Our findings build on previous research, particularly the work of Yoshikawa et al.22 Although their study showed Blimp-1 deletion reduces T-cell exhaustion in CAR T-cell and viral models, we focus on BCMA CAR T cells in MM. Blimp-1 KO CAR T cells uniquely recover effector function after repeated antigen exposure, suggesting long-term efficacy in MM patients. Another key conclusion from our study was that Blimp-1 controls the expression of LAG-3. LAG-3 expression levels were higher in Blimp-1 KO CAR T cells during days 6 to 8; however, they decreased on days 9 and 10. The relative surface expression of LAG-3 in Mock and Blimp-1 KO CAR T cells was completely reversed after tumor cell cocultures. Previously, we observed that in vivo efficacy of anti-BCMA CAR T cells was ameliorated in the group that had lower LAG-3 expression,38 suggesting that the increased abundance of this checkpoint molecule on Blimp-1 KO CAR T cells could be deleterious to efficacy. Interestingly, it seems that the regulation of LAG-3 by Blimp-1 is largely dependent on context. Although Shin et al demonstrated that Blimp-1 promotes the expression of LAG-3 on CD8+ T cells during chronic viral infection,32 recent studies showed that Blimp-1 deletion has no effect on LAG-3 expression in anti-CD19 CAR T cells22 and actually increases LAG-3 levels in Waldenström’s macroglobulinemia cells.55 In a similar vein, Jung et al34 showed that Blimp-1 modulation could improve responses to immune checkpoint inhibitors, further underscoring the role of Blimp-1 in regulating exhaustion markers such as LAG-3. Our study extends these findings by showing that Blimp-1 KO BCMA CAR T cells also have altered LAG-3 expression after tumor challenge, suggesting new avenues for combinatorial approaches with checkpoint blockade therapies.
Put together, our findings demonstrate a critical role for Blimp-1 in restraining anti-BCMA CAR T-cell efficacy. We believe that we provide substantial evidence that the implementation of Blimp-1 KO anti-BCMA CAR T cells as a new therapy for MM could lead to improved patient responses compared with conventional anti-BCMA CAR T cells. Reproduction of the effects of Blimp-1 KO observed in vitro using a second Prdm1-targeting gRNA validated our findings (supplemental Figure 8). However, the role of Blimp-1 in CAR T cells seems to be dependent on the specific type of malignancy: for prostate cancer, a stress test in vivo model revealed limited, if any, benefit to Blimp-1 suppression,34 whereas, for anti-CD19 CAR T cells and anti-BCMA CAR T cells in hematologic malignancies, a single Blimp-1 KO is sufficient to improve antitumor efficacy.22
Although Dai et al36 used a high-throughput CRISPR-based screening approach to identify Blimp-1 as a key target for improving CAR T-cell persistence across multiple malignancies, their study lacked the detailed mechanistic analysis that we provide here. Our work not only validates Blimp-1 as a critical modulator of CAR T-cell function but also reveals how Blimp-1 KO affects the long-term recovery of effector function, particularly after prolonged antigen exposure in MM.
Overall, we provide novel insights into the regulation of anti-BCMA CAR T-cell efficacy and demonstrate that targeting Blimp-1 improves anti-MM activity after prolonged exposure to tumor cells. Compared with previous works, our study uniquely contributes to the growing body of literature on Blimp-1 modulation by showing that Blimp-1 deletion not only promotes memory-like CAR T phenotypes but also enhances functional recovery after repeated challenges, which is crucial for achieving durable responses for patients with MM.
Acknowledgments
The authors thank the Multiple Myeloma Research Center (Little Rock, AR) for providing the ARP-1 cell line, Sergi Vaqué Salsench and Miquel Bosch i Crespo for excellent technical support, and the Centro Nacional de Análisis Genómico (Barcelona, Spain) for assistance with RNA sequencing.
This study was supported by research funding from the Spanish Institute of Health Carlos III (PI19/00669, PI22/00647, and ICI19/00025), the Asociación Española Contra el Cáncer (LABAE21971FERN), Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR, Generalitat de Catalunya; 2021SGR01292), and the International Myeloma Society and Paula and Rodger Riney Foundation (INT_IMS_TRG_22).
Authorship
Contribution: A.M.B. conceived and designed the study and wrote the original draft of the manuscript; A.M.B., J.M.P., and D.F.M. were responsible for data curation, visualization, and performing data analysis; C.F.d.L. acquired funding; A.M.B., J.M.P., A.O.-C., J.C., and O.C. performed research; A.M.B. and C.F.d.L. oversaw project administration and supervision; A.U.-I. and C.F.d.L. contributed resources; and A.M.B., A.O.-C., D.F.M., L.G.R.-L., A.U.-I., and C.F.d.L. reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anthony M. Battram, Institut d'Investigacions Biomèdiques August Pi i Sunyer, C/Rosselló 149-153, 08036 Barcelona, Spain; email: battram@recerca.clinic.cat; and Carlos Fernández de Larrea, Amyloidosis and Myeloma Unit, Department of Hematology, Hospital Clínic of Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer, University of Barcelona, C/Rosselló 149-153, 08036 Barcelona, Spain; email: cfernan1@clinic.cat.
References
Author notes
A.M.B. and J.M.P. contributed equally to this study.
RNA-sequencing data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (accession number GSE255977).
Other data generated in this study are available upon reasonable request from the corresponding authors, Anthony M. Battram (battram@recerca.clinic.cat) and Carlos Fernández de Larrea (cfernan1@clinic.cat).
The full-text version of this article contains a data supplement.

![Blimp-1 KO CAR T cells display a memory-like phenotype. (A-B) Surface expression of CD127 (IL-7 receptor α-chain [IL-7Rα]) and CCR7 (A) or intracellular expression of TCF-7 (B) in CAR+ Mock and Blimp-1 KO CAR T cells. Representative histograms (left). Median fluorescence intensity (MFI) quantification (right). Fluorescence minus one (FMO) control in panel B shows Mock cells stained with all antibodies except TCF-7. (C) UT cells or Mock or Blimp-1 KO CAR T cells were cocultured with luciferase-expressing ARP-1 cells for 24 hours at effector-to-target (E:T) ratios ranging from 1:1 to 0.125:1. Displayed is the ARP-1 cell survival, as determined by bioluminescence, compared with ARP-1 cells cultured alone (n = 9). (D-G) Mock and Blimp-1 KO CAR T cells were cocultured with ARP-1 cells for 6 hours and production of effector molecules in CAR+ cells was assessed by flow cytometry. (D) Granzyme B expression in CD8+ cells, evaluated without (basal) and with (+ARP-1) ARP-1 cell stimulation. Representative histograms (left). Summary data showing fold change of MFI compared with basal Mock (right). FMO control represents Mock cells stained with all antibodies except granzyme B. (E) Production of TNF-α and IFN-γ in CD8+ cells. (F) Production of IL-2 in CD4+ and CD8+ cells. (G) Proportion of cells producing none (negative), 1 (single), 2 (double), or all 3 (triple) of the analyzed cytokines. Graphs show means ± SD for the panels A-B,D-G or mean ± standard error of the mean (SEM) for the panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024013209/4/m_blooda_adv-2024-013209-gr2.jpeg?Expires=1769141284&Signature=FsTOGEkoGe2C6jP5C9dZowRXcvvENyC6X~YBdnx96upaOhp-93-DpEWPi1OVKr5KgmrrfS8EYq4aOsGpG9FAZliWr2J15detCVhy~JNm4pqOjF96EVbdGF3BSJes-aii~L6TBoAD2p6vfHbPikygV~tDd4tC6hi0Njw2U~HLd8VV5a8sM4qyJcwnYlpQ8BQYC1LvhWD3aSNRmlwNuUjZUqICfwvJyBgfY3wSWjooSMnnLsdnP5elUHHhQzT9H3dzOf5irTeJMq56hZVGJFjDPeXL8FYXHLyXu5nXSQhpj79cUhNI75FpdX37M04mNLLT1p8jq~VdcoC77-p-jIK53w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Improved disease control by Blimp-1 KO CAR T cells in a myeloma mouse model. (A-E) UT cells or Mock or Blimp-1 KO CAR T cells were injected into mice previously infused with luciferase-expressing ARP-1 (ARP-1-ffLuc) cells. (A) Schematic showing experimental plan. (B-C) Disease progression, observed as bioluminescence signal from weekly images of mice. Displayed are photos (B) and quantification (C) from individual mice. (D) Quantification of bioluminescence on day 28, showing means ± SD (1-way analysis of variance [ANOVA] with Tukey multiple comparisons test). (E) Overall survival of animals from each group. Arrows in the panels C,E show days on which T cells/CAR T cells were infused. Dotted line in the panel C indicates bioluminescence threshold for euthanizing mice (3 × 108 p/s/cm2/sr).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024013209/4/m_blooda_adv-2024-013209-gr3.jpeg?Expires=1769141284&Signature=eftCUs9Q0dJulqhS0mrf8bPSraUz2Cupo-2r6clcI8p-MwDFO5-NT5BMI8uUDJoKWHsnSzLMg1pSTXvf1TCn6B2hesy02-jGqRIOxWg6-BA9seCqmvcvRRD4EdEDK5wV~fyAfQwRy~mem6eGHS9wk6pOpNXRvk~LzNxKlXcPoWvgau7rAuQqRDC82Knrc2TKVwaqxjs6m5lRbsrjZC~scV8k8W8lEGM8XJNRLOrfi6vVpISgIPSrD1vloZb3qB7xFfkeZ0kiRVtLIf996hu15BceRPDQM0zHds4GnkbdmVUNbDQ3RAyUd9wPIRn0c5fARlJIVjEw0i2~IObihlAmrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Loss of Blimp-1 selectively remodels the CAR T-cell transcriptome. (A-H) Transcriptome of CD8+ Mock and Blimp-1 KO CAR T cells subject to repeated challenges with ARP-1 cells. (A) Comparison of normalized counts of all identified genes between Mock and Blimp-1 KO cells with each point representing an individual gene. Genes that were significantly more or less abundant in Blimp-1 KO cells (fold change >1.5-fold; false discovery rate [FDR] <0.05) are highlighted in purple or blue, respectively. Dashed line shows equivalent expression in Mock and Blimp-1 KO. (B) Volcano plot showing the difference between gene expression in Blimp-1 KO and Mock cells. Genes that comprise the Gene Ontology term “GOBP_T_CELL_DIFFERENTIATION” (GO:0030217) are highlighted in red. Dashed horizontal line shows FDR = 0.05 and dashed vertical lines show fold change of 1.5. (C) Heat map showing z-score normalized expression of key genes related to T-cell memory phenotype (left). Heat map displaying FDR of genes shown in left heat map, with FDR <0.05 in green (right). (D) Normalized count data of Myc, Bcl6, and CD27 genes. (E) As panel C, but showing effector phenotype-related genes. (F) Normalized count data of Znf683 (Hobit) gene. (G) Gene set enrichment analysis (GSEA) analysis of GOBP_RIBOSOME BIOGENESIS (red: NES, 2.15; FDR, 1.50 × 10−7) and GOCC_PRERIBOSOME (blue: normalized enrichment score (NES), 2.30; FDR, 9.67 × 10−7). (H) Normalized count data of Adora2a (A2AR), Ikzf3 (Aiolos), and Sox4 genes. Graphs show means ± SD and different symbols represent different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/3/10.1182_bloodadvances.2024013209/4/m_blooda_adv-2024-013209-gr7.jpeg?Expires=1769141284&Signature=ps-cxL5FXVIhV08cEzPBPPUx3Ic04-JSCJSw57Hh~dzUJzqMKeSXodZE7NZKZXeNtfjL~jujYCixrO4a1ZHC~guTXlucV0JMMjZVZ9gtpHBprrkcqX5eQfOSIK1otD1DcWuFldOH6UQjjIDHx8ox-n7CTNbh2fUEUoQWy6E4amIK1-x9qU2yyWJyEjmk-LQHlt8xqUDmsgtI1mI~g4x-w-yr3UtPUdISrdAwAXe1xmtFNjg3EC4vxXfxFs8etKIWWsdjzzWHbDn3xw8BR1Dlt6OXDT8INbmXxCuk-Xx8sodhpekd8OwsqvHqBIbESaySPvGNWaq-yaAGjMf10MUWqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)