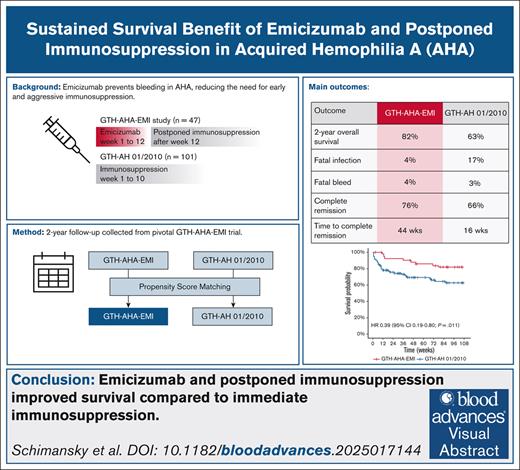
Key Points
Emicizumab and postponed immunosuppression improved survival compared to immediate immunosuppression.
Low rates of infection- and bleed-related mortality were observed at 2 years follow-up.
Visual Abstract
Acquired hemophilia A (AHA) is a severe bleeding disorder caused by neutralizing autoantibodies against coagulation factor VIII (FVIII). Standard treatment involves immunosuppressive therapy (IST), which carries a significant risk of serious infections, the leading cause of death in patients with AHA. The GTH-AHA-EMI study investigated the use of emicizumab to prevent bleeding during the first 12 weeks of management while postponing IST. We collected 2-year follow-up data from GTH-AHA-EMI patients (n = 47) and compared outcomes to a propensity score (PS)–matched cohort from the GTH-AH 01/2010 study (n = 101), in which patients received immediate IST. Outcome measures included overall survival (OS), infection- and bleed-related mortality, and time to complete remission (CR). The study cohorts were well-matched in age, sex, underlying conditions, baseline FVIII activity, inhibitor titer, and performance status. The PS-matched 2-year OS was 82% in the GTH-AHA-EMI cohort vs 63% in GTH-AH 01/2010 (hazard ratio, 0.39; 95% confidence interval, 0.19-0.80). Infection-related mortality was lower with emicizumab (4% vs 16%), whereas bleed-related mortality was similar (4% vs 3%). Spontaneous remission of AHA occurred in 15% of GTH-AHA-EMI patients. Time to CR estimated by the Kaplan-Meier method was longer with postponed IST in GTH-AHA-EMI (44 vs 16 weeks), but the estimated proportion of patients achieving CR was similar (76% vs 66%). In conclusion, emicizumab allowed for postponed IST initiation during early AHA management in the GTH-AHA-EMI study. Delayed IST was safe and effective. Compared to PS-matched historic controls receiving immediate IST but no emicizumab, GTH-AHA-EMI patients had fewer fatal infections and improved OS. This trial was registered at www.ClinicalTrials.gov as #NCT04188639.
Introduction
Acquired hemophilia A (AHA) is an autoimmune disorder caused by neutralizing antibodies against coagulation factor VIII (FVIII), leading to severe impairment of clotting.1 With an estimated incidence of 1.5 per million inhabitants per year, the disease is rare, but the incidence appears to be rising.2,3 Patients often present with serious or life-threatening bleeding. In the presence of FVIII inhibitors, bleeding is managed using either porcine-sequence recombinant FVIII,4,5 or bypassing agents such as recombinant activated FVIIa,6 or activated prothrombin complex concentrate.7,8 Although these treatments are effective for acute bleeding control,9,10 they are not suitable for long-term or outpatient management due to their intravenous administration and short half-life.
The standard of care for AHA involves immunosuppressive therapy (IST) with glucocorticoids (GC), rituximab (RTX), cyclophosphamide (CTX), mycophenolate mofetil, or combinations thereof. The goal of IST is to eradicate FVIII autoantibodies and thereby eliminate the risk of bleeding. Partial remission (PR, defined as FVIII recovered to >50% and no bleeding after withholding hemostatic medication for at least 24 hours) and complete remission (CR, defined as PR plus negative FVIII inhibitor and IST stopped) is achieved in 60% to 80% of patients over a variable period.11,12 Until PR is achieved, patients remain at risk of bleeding.2,6 A major complication of IST, particularly in the often frail AHA population, is infection.13 In fact, fatal infections have consistently been the leading cause of death in patients with AHA in Western Europe.2,12,14-17
Emicizumab, a monoclonal activated FVIII-mimetic antibody, restores hemostasis even in the presence of FVIII inhibitors.18 The AGEHA study (June 2020 to August 2022)19,20 and the GTH-AHA-EMI study (March 2021 to January 2023),21 both employed a rapid emicizumab loading protocol to protect newly diagnosed patients with AHA from bleeding. These studies demonstrated that emicizumab effectively prevented clinically relevant new bleeds (CRNB); in GTH-AHA-EMI the breakthrough bleeding rate during the first 12 weeks—while patients were not receiving IST—was just 0.04 events per patient-week,21 which was significantly lower than in historic controls.22 Patients required a median of 10 hospital days before transitioning to outpatient management with weekly subcutaneous emicizumab.
Beyond its role in bleed prevention, emicizumab has the potential to mitigate the need for early, intensive IST and its associated complications.23,24 In the GTH-AHA-EMI trial, IST was postponed during the 12-week emicizumab prophylaxis period, and rates of serious infections were notably low. However, most patients did not achieve remission within this timeframe, confirming that IST remains necessary for long-term disease control. Whether postponed IST is safe and effective remains uncertain.
Here we collected 2-year follow-up data from GTH-AHA-EMI patients, focusing on overall survival (OS), infection- and bleed-related mortality, IST administration after week 12, and CR of AHA. We compared these outcomes to propensity score (PS)–matched data from the GTH-AH 01/2010 study, which used a standardized IST regimen without emicizumab. Our findings suggest that postponed IST in GTH-AHA-EMI was effective, safe, and associated with reduced mortality.
Methods
GTH-AHA-EMI study
GTH-AHA-EMI was an open-label, nonrandomized clinical trial that used 12 weeks of emicizumab prophylaxis to prevent CRNB in patients diagnosed with AHA based on a FVIII activity <50 IU/dL and a detectable FVIII inhibitor, who had not previously received IST (ClinicalTrials.gov identifier: #NCT04188639).21 Patients received an emicizumab loading dose of 6 mg/kg on day 1 and 3 mg/kg on day 2, followed by a maintenance dose of 1.5 mg/kg per week from day 8 onwards until week 12. IST was not permitted during the first 12 weeks and was initiated at the investigator’s discretion thereafter. All patients gave written informed consent before enrollment. All study procedures were in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and were approved by the regulatory authorities in Germany and Austria and by the ethics committees of all participating centers.
GTH-AHA-EMI 2-year follow-up collection
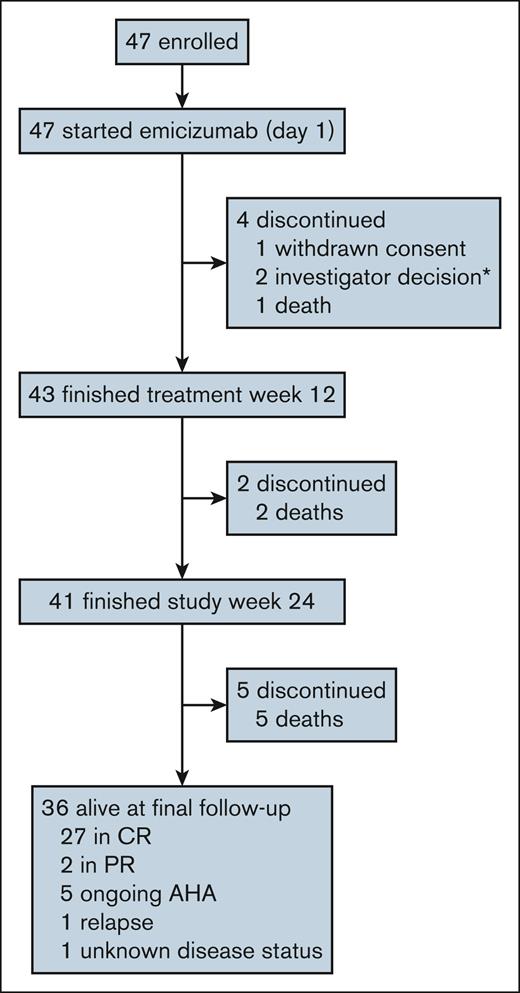
The 2-year follow-up was collected from all GTH-AHA-EMI participants between February and June 2024 (Figure 1). Investigators completed an electronic case report form providing information on the last patient contact (hospital visit or telephone contact), survival status, cause of death, disease status, bleeding and other adverse events, and the use of hemostatic medications and IST.
Patient disposition at final follow-up (2 years after enrollment). CONSORT flow diagram summarizing enrollment and follow-up in the GTH-AHA-EMI study. ∗One patient who was withdrawn by investigator decision, died in week 13; this death was included in the mortality analysis. CONSORT, Consolidated Standards of Reporting Trials.
Patient disposition at final follow-up (2 years after enrollment). CONSORT flow diagram summarizing enrollment and follow-up in the GTH-AHA-EMI study. ∗One patient who was withdrawn by investigator decision, died in week 13; this death was included in the mortality analysis. CONSORT, Consolidated Standards of Reporting Trials.
GTH-AH 01/2010 study
GTH-AH 01/2010 was a prospective study that enrolled patients with AHA, who were treated with an escalating IST regimen until achieving PR.12 The IST regimen consisted of prednisolone alone in weeks 1 to 3, followed by CTX added in weeks 4 to 6, and RTX added in weeks 7 to 10 if PR was not yet achieved. IST escalation was withheld if a steady increase in FVIII activity was observed during the 7 days preceding the planned escalation in weeks 4 and 7 and continued if no further improvement was noted during the subsequent 7-day period. Once PR was achieved, IST was discontinued, and prednisolone was tapered.
Outcome measures
Day 1 was defined as the day of first emicizumab dose in GTH-AHA-EMI and the day of first IST dose in GTH-AH 01/2010. The primary outcome of this analysis was OS. Secondary outcomes included fatal infection, fatal bleeding, number and rate of CRNB, and time to PR or CR. CRNB was defined as any bleeding that required intervention by a healthcare professional or caused pain or any other kind of disturbance of daily life. PR was defined as FVIII activity restored to >50 IU/dL (measured with a chromogenic FVIII assay using bovine reagents in the GTH-AHA-EMI study) and no active bleeding after discontinuation of hemostatic drugs for >24 hours. CR was defined as PR plus absent FVIII inhibitor, GC reduced to <15 mg prednisolone or equivalent per day (or the last dose of GC given for concomitant disorders before the diagnosis of AHA), and any other IST stopped.
Statistical analysis
The PS-matching strategy and methodology was previously reported.22 Inverse probability of treatment weights were assigned to each patient based on a PS-model incorporating factors previously identified as prognostic for bleeding and survival, including FVIII activity, World Health Organization performance status (WHO-PS), malignancy, diabetes, and renal disorder.6,12 Balance between study populations was checked.22 OS, time to PR, and time to CR were estimated using the Kaplan-Meier method and compared using weighted Cox regression, with hazard ratios (HR), 95% confidence intervals (CIs), and Wald test P values reported. The mean rate of bleeds per patient-week was estimated using a negative binomial regression model, with observation time as an offset variable. Continuous data were compared using the Wilcoxon rank-sum test. Frequency of categorical data was compared using Pearson χ2 test and Fisher exact test for cell counts of ≥5 and <5, respectively. All hypotheses were tested as 2-sided, with statistical significance defined as P < .05. Analysis was performed using R version 4.4.1.25
Results
Final follow-up of the GTH-AHA-EMI study
Of the 47 patients initially enrolled in the GTH-AHA-EMI study between March 2021 and June 2022, 41 had completed the trial at week 24.21 At the final 2-year follow-up in June 2024, 36 patients were alive, and 5 had died; 29 (81%) of 36 patients were in PR or CR, whereas 6 had ongoing AHA or relapse (Figure 1).
OS analysis
To assess the effect of the initial treatment strategy on OS, we compared individual patient data from the GTH-AHA-EMI study (emicizumab, no IST before week 12, n = 47) with the GTH-AH 01/2010 study (no emicizumab, IST immediately after diagnosis, n = 101). Baseline characteristics of the unmatched cohorts were already similar in terms of age, sex, baseline FVIII activity and inhibitor, WHO-PS, and underlying or concomitant disorders (Table 1).
Baseline characteristics of patients
| Characteristic . | Immunosuppression (GTH-AH 01/2010) N = 101∗ . | Emicizumab (GTH-AHA-EMI) N = 47∗ . | P value† . |
|---|---|---|---|
| Age in years | 74 (62-81) | 76 (66-80) | .683 |
| >74 | 49 (49%) | 26 (55%) | .441 |
| Female sex | 43 (43%) | 23 (49%) | .469 |
| FVIII activity in IU/dL | 1.4 (0.0-3.4) | 1.4 (0.0-5.6) | .319 |
| <1 | 46 (46%) | 18 (38%) | .407 |
| Inhibitor in BU/mL | 19 (8-71) | 12 (4-47) | .089 |
| >20 | 48 (48%) | 18 (38%) | .293 |
| WHO-PS >2 | 37 (37%) | 19 (40%) | .658 |
| Underlying disorders | |||
| Malignancy | 13 (13%) | 6 (13%) | .986 |
| Autoimmunity | 20 (20%) | 7 (15%) | .472 |
| Postpartum | 5 (5.0%) | 1 (2.1%) | .665 |
| Concomitant disorders | |||
| Cardiac disease | 38 (38%) | 13 (28%) | .235 |
| Renal disorders | 36 (36%) | 14 (30%) | .483 |
| Diabetes mellitus | 28 (28%) | 14 (30%) | .795 |
| Characteristic . | Immunosuppression (GTH-AH 01/2010) N = 101∗ . | Emicizumab (GTH-AHA-EMI) N = 47∗ . | P value† . |
|---|---|---|---|
| Age in years | 74 (62-81) | 76 (66-80) | .683 |
| >74 | 49 (49%) | 26 (55%) | .441 |
| Female sex | 43 (43%) | 23 (49%) | .469 |
| FVIII activity in IU/dL | 1.4 (0.0-3.4) | 1.4 (0.0-5.6) | .319 |
| <1 | 46 (46%) | 18 (38%) | .407 |
| Inhibitor in BU/mL | 19 (8-71) | 12 (4-47) | .089 |
| >20 | 48 (48%) | 18 (38%) | .293 |
| WHO-PS >2 | 37 (37%) | 19 (40%) | .658 |
| Underlying disorders | |||
| Malignancy | 13 (13%) | 6 (13%) | .986 |
| Autoimmunity | 20 (20%) | 7 (15%) | .472 |
| Postpartum | 5 (5.0%) | 1 (2.1%) | .665 |
| Concomitant disorders | |||
| Cardiac disease | 38 (38%) | 13 (28%) | .235 |
| Renal disorders | 36 (36%) | 14 (30%) | .483 |
| Diabetes mellitus | 28 (28%) | 14 (30%) | .795 |
Data are given as median (interquartile range) or n (%).
Wilcoxon rank sum test, Pearson χ2 test, or Fisher exact test.
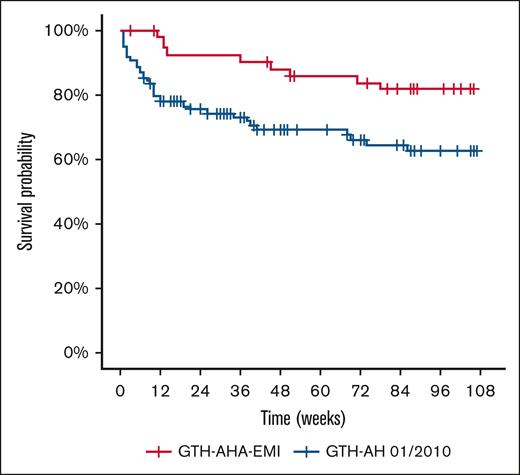
PS-matched OS is shown in Figure 2. The Kaplan-Meier estimated 1-year OS rate was 86% with emicizumab and postponed IST (GTH-AHA-EMI) and 69% with immediate IST (GTH-AH 01/2010). At 2 years, OS was 82% vs 63%, respectively, with an absolute difference of 19%. Univariate Cox regression indicated a 61% reduction in mortality (HR, 0.39; 95% CI, 0.19-0.80; P = .011).
OS of PS-matched study cohorts. Kaplan-Meier curves were drawn using weighted individual patient data. GTH-AHA-EMI (n = 47, received emicizumab prophylaxis and postponed IST). GTH-AH 01/2010 (n = 101, did not receive emicizumab prophylaxis and started IST immediately after diagnosis). The univariate Cox regression HR was 0.39 (0.19-0.80; P = .011, Wald test).
OS of PS-matched study cohorts. Kaplan-Meier curves were drawn using weighted individual patient data. GTH-AHA-EMI (n = 47, received emicizumab prophylaxis and postponed IST). GTH-AH 01/2010 (n = 101, did not receive emicizumab prophylaxis and started IST immediately after diagnosis). The univariate Cox regression HR was 0.39 (0.19-0.80; P = .011, Wald test).
Multivariate Cox regression confirmed the survival benefit associated with emicizumab and postponed IST (GTH-AHA-EMI, HR, 0.37; 95% CI, 0.16-0.85; Table 2). There was a trend toward better survival in those patients who were younger (≤74 years, HR, 0.50; 95% CI, 0.25-1.01), those with WHO-PS ≤2 (HR, 0.51; 95% CI, 0.23-1.13), and those with baseline FVIII activity ≥1 IU/dL (HR, 0.51; 95% CI, 0.24-1.09). Sex, inhibitor concentration, and concomitant disorders had no impact on survival.
Adjusted HRs from multivariate analysis of OS
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Study | |||
| Immunosuppression (GTH-AH 01/2010) | 1 | — | |
| Emicizumab (GTH-AHA-EMI) | 0.37 | 0.16-0.85 | .019∗ |
| Age, y | |||
| >74 | 1 | — | |
| ≤74 | 0.50 | 0.25-1.01 | .052 |
| Sex | |||
| Female | 1 | — | |
| Male | 1.38 | 0.67-2.81 | .382 |
| FVIII activity, IU/dL | |||
| <1 | 1 | — | |
| ≥1 | 0.51 | 0.24-1.09 | .082 |
| Inhibitor, BU/mL | |||
| >20 | 1 | — | |
| ≤20 | 0.93 | 0.39-2.20 | .866 |
| WHO-PS | |||
| Poor (>2) | 1 | — | |
| Good (≤2) | 0.51 | 0.23-1.13 | .097 |
| Malignancy | 2.17 | 0.77-6.08 | .142 |
| Renal disease | 1.80 | 0.80-4.05 | .158 |
| Diabetes mellitus | 0.81 | 0.36-1.79 | .596 |
| Characteristic . | HR . | 95% CI . | P value . |
|---|---|---|---|
| Study | |||
| Immunosuppression (GTH-AH 01/2010) | 1 | — | |
| Emicizumab (GTH-AHA-EMI) | 0.37 | 0.16-0.85 | .019∗ |
| Age, y | |||
| >74 | 1 | — | |
| ≤74 | 0.50 | 0.25-1.01 | .052 |
| Sex | |||
| Female | 1 | — | |
| Male | 1.38 | 0.67-2.81 | .382 |
| FVIII activity, IU/dL | |||
| <1 | 1 | — | |
| ≥1 | 0.51 | 0.24-1.09 | .082 |
| Inhibitor, BU/mL | |||
| >20 | 1 | — | |
| ≤20 | 0.93 | 0.39-2.20 | .866 |
| WHO-PS | |||
| Poor (>2) | 1 | — | |
| Good (≤2) | 0.51 | 0.23-1.13 | .097 |
| Malignancy | 2.17 | 0.77-6.08 | .142 |
| Renal disease | 1.80 | 0.80-4.05 | .158 |
| Diabetes mellitus | 0.81 | 0.36-1.79 | .596 |
P < .05 (Wald test).
Infection- and bleed-related mortality
Fatal infection was reported in 2 (4%) of 47 patients in GTH-AHA-EMI, which was lower to 16 (16%) of 101 patients in GTH-AH 01/2010 (Table 3). One of the 2 fatal infections in GTH-AHA-EMI was due to COVID-19 and occurred already while IST was still postponed during the first 12 weeks. The other fatal infection was pneumonia in a multimorbid 73-year-old patient in week 32. He had received RTX in weeks 15 and 16. Fatal bleeding occurred with similar frequency, in 2 (4%) of 47 GTH-AHA-EMI patients and 3 (3%) of 101 GTH-AH 01/2010.
Infection- and bleed-related mortality according to study and time
| Time (wk) . | Immunosuppression (GTH-AH 01/2010), N = 101∗ . | Emicizumab (GTH-AHA-EMI), N = 47∗ . | ||
|---|---|---|---|---|
| Fatal infections . | Fatal bleeds . | Fatal infections . | Fatal bleeds . | |
| 1-12 | 11 | 3 | – | 1 |
| 13-24 | – | – | 1 | 1 |
| 25-36 | 3 | – | 1 | – |
| 37-48 | – | – | – | – |
| 49-60 | – | – | – | – |
| 61-72 | 1 | – | – | – |
| 73-84 | – | – | – | – |
| 85-96 | 1 | – | – | – |
| Total | 16 (16) | 3 (3) | 2 (4) | 2 (4) |
| Time (wk) . | Immunosuppression (GTH-AH 01/2010), N = 101∗ . | Emicizumab (GTH-AHA-EMI), N = 47∗ . | ||
|---|---|---|---|---|
| Fatal infections . | Fatal bleeds . | Fatal infections . | Fatal bleeds . | |
| 1-12 | 11 | 3 | – | 1 |
| 13-24 | – | – | 1 | 1 |
| 25-36 | 3 | – | 1 | – |
| 37-48 | – | – | – | – |
| 49-60 | – | – | – | – |
| 61-72 | 1 | – | – | – |
| 73-84 | – | – | – | – |
| 85-96 | 1 | – | – | – |
| Total | 16 (16) | 3 (3) | 2 (4) | 2 (4) |
n (%).
Bleeding
The GTH-AHA-EMI study protocol allowed the continuation of emicizumab beyond week 12 at the investigator’s discretion. The median duration of emicizumab treatment, including the active study period, was 14 weeks (range 3-108, supplemental Figure 1).
Table 4 compares the rate of CRNB and the proportion of patients in PR between the matched study cohorts. PR was chosen for this analysis as it was shown to protect effectively from bleeding.6 During weeks 1 to 12, the CRNB rate was lower in GTH-AHA-EMI patients (0.041 events per patient-week) than in GTH-AH 01/2010 patients (0.123 events per patient-week). After week 12, the CRNB rate dropped in both cohorts but faster in GTH-AH 01/2010 as more patients were already in PR.
CRNBs according to study over time PS-matched, Kaplan-Meier estimated cumulative proportion of patients in PR, raw numbers of bleeds, and weighted negative-binomial distributed mean CRNB rates per patient-week are given
| Time (wk) . | Immunosuppression (GTH-AH 01/2010), N = 101 . | Emicizumab (GTH-AHA-EMI), N = 47 . | ||||
|---|---|---|---|---|---|---|
| PR (%) . | Bleeds (n) . | Bleeding rate . | PR (%) . | Bleeds (n) . | Bleeding rate . | |
| 1-12∗ | 78 | 127 | 0.128 | 1 | 22 | 0.042 |
| 13-24 | 82 | 9 | 0.010 | 24 | 18 | 0.034 |
| 25-36 | 84 | 1 | 0.002 | 41 | 5 | 0.009 |
| 37-48 | 84 | 1 | 0.002 | 62 | – | – |
| 49-60 | 85 | 1 | 0.003 | 62 | 1 | 0.002 |
| 61-72 | 85 | – | – | 69 | 1 | 0.002 |
| 73-84 | 85 | – | – | 73 | – | – |
| 85-96 | 85 | – | – | 81 | – | – |
| Time (wk) . | Immunosuppression (GTH-AH 01/2010), N = 101 . | Emicizumab (GTH-AHA-EMI), N = 47 . | ||||
|---|---|---|---|---|---|---|
| PR (%) . | Bleeds (n) . | Bleeding rate . | PR (%) . | Bleeds (n) . | Bleeding rate . | |
| 1-12∗ | 78 | 127 | 0.128 | 1 | 22 | 0.042 |
| 13-24 | 82 | 9 | 0.010 | 24 | 18 | 0.034 |
| 25-36 | 84 | 1 | 0.002 | 41 | 5 | 0.009 |
| 37-48 | 84 | 1 | 0.002 | 62 | – | – |
| 49-60 | 85 | 1 | 0.003 | 62 | 1 | 0.002 |
| 61-72 | 85 | – | – | 69 | 1 | 0.002 |
| 73-84 | 85 | – | – | 73 | – | – |
| 85-96 | 85 | – | – | 81 | – | – |
Results of the first 12-week period were already published.22
Characteristics of CRNBs appeared to differ between early and late periods under emicizumab treatment. As shown in supplemental Table 1, late CRNBs (after week 12, n = 25) were more frequently subcutaneous (32% vs 18%), more often associated with trauma (44% vs 27%), and more commonly remained untreated (40% vs 9%) compared to CRNBs occurring during the first 12 weeks (n = 22).
Thromboembolic events
During the first 12 weeks, 1 minor thromboembolic event was reported in GTH-AHA-EMI patients (thrombophlebitis at the site of venous catheter placement in week 1), compared to 7 events—4 of them were fatal—in GTH-AH 01/2010.22 One additional thromboembolic event occurred in the GTH-AHA-EMI cohort between weeks 13 and 24 (an ischemic stroke in the middle cerebral artery in week 20).21 At the time of final follow-up, no further thromboembolic events had been reported.
IST regimens and remission
IST was not administered during the first 12 weeks of the GTH-AHA-EMI study. Thereafter, IST initiation and regimen selection were at the investigator’s discretion. All patients completing week 12 of the study had been discharged from the hospital by week 8 and IST was given in the outpatient setting. Supplemental Figure 2 provides an overview, with additional details in supplemental Tables 2 to 5.
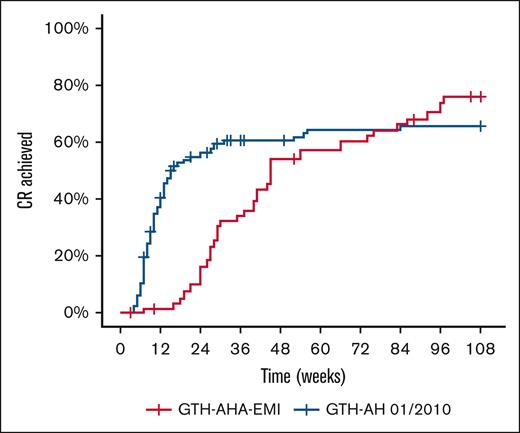
The median time to CR was 45 weeks (95% CI, 40-86) in GTH-AHA-EMI and 16 weeks (95% CI, 13-52) in GTH-AH 01/2010 (Figure 3). The Kaplan-Meier estimated proportion of patients achieving CR until end of study was 76% and 66% for GTH-AHA-EMI and GTH-AH 01/2010, respectively. As the curves were crossing towards the end of the observation period, violating the proportional hazard assumption, no formal comparison was attempted.
CR of AHA in PS-matched study cohorts. Kaplan-Meier curves were drawn using weighted individual patient data. The median time to CR was 16 weeks (95% CI, 13-52) and 45 weeks (40-86) in GTH-AH 01/2010 and GTH-AHA-EMI patients, respectively.
CR of AHA in PS-matched study cohorts. Kaplan-Meier curves were drawn using weighted individual patient data. The median time to CR was 16 weeks (95% CI, 13-52) and 45 weeks (40-86) in GTH-AH 01/2010 and GTH-AHA-EMI patients, respectively.
The most common first-line IST regimen used in GTH-AHA-EMI patients was RTX with or without GC (supplemental Figure 2; supplemental Table 2). Among the 16 patients receiving RTX first-line, 14 (88%) achieved CR after a median of 17 weeks (supplemental Table 3). GC monotherapy was used in 8 patients, and 7 (88%) of them achieved CR after a median of 25 weeks. First-line regimens including mycophenolate mofetil or CTX were used infrequently.
The immunosuppressive drug exposure differed between the study cohorts (supplemental Table 4): GC use was lower in GTH-AHA-EMI (59%) than in GTH-AH 01/2010 (100%), partly offset by a higher use of RTX (56% vs 27%). Cumulative dose per treated patient was not significantly different.
Twelve GTH-AHA-EMI patients did not receive IST before week 24, and 6 (50%) achieved CR spontaneously (supplemental Tables 3 and 4). The overall spontaneous remission rate was 15% (6/41).
Discussion
We present the final follow-up of the GTH-AHA-EMI study, 2 years after enrollment. It is demonstrated that the risk of serious infection remained low, with only 2 out of 47 patients succumbing to infection. 2-year OS was improved by an absolute 19% when comparing patients who received emicizumab and postponed IST (GTH-AHA-EMI) to PS-matched controls who received immediate IST (GTH-AH 01/2010). Time to CR was longer with postponed IST, but the proportion of patients in CR was similar at final follow-up, confirming that postponing IST did not compromise its efficacy.
The availability of emicizumab is changing clinical practice in AHA management.26,27 Over the past 30 years, despite advancements in hemostatic treatments, AHA remained a disorder with high morbidity and mortality.28,29 The risk of bleeding and the demand for expensive hemostatic drugs created pressure to achieve remission as quickly as possible. Guidelines published in 2009 recommended IST in all patients with AHA immediately at diagnosis.30 Although IST was effective in inducing remission in most patients, it carried a significant risk of serious infections. Western European registries consistently documented that the risk of fatal infection had surpassed that of fatal bleeding in AHA, highlighting the need for a paradigm shift in treatment.13
Emicizumab offers, for the first time, effective bleed prophylaxis in AHA, reducing the need for early and aggressive IST.31 This concept was introduced in the pivotal GTH-AHA-EMI study, which postponed IST in all 47 patients during the first 12 weeks of management. The AGEHA study also included 2 patients initially deemed ineligible for IST. The results of both studies suggested low rates of serious infection, but it remained unclear whether postponed IST would indeed be safer than immediate IST and whether it would maintain efficacy in the long term.
The current study closes that knowledge gap, confirming both efficacy and safety of deferring IST. IST administered after week 12 was well-tolerated in all patients and only 1 fatal infection was reported in relation to IST.
The improved safety profile of IST in GTH-AHA-EMI came at the cost of a longer time to achieve PR and CR. This also translated into a higher rate of CRNB during weeks 12 to 24. Bleeding events after week 12 were more frequently limited to subcutaneous hematomas and often managed without factor concentrates. Nevertheless, bypassing agents were still required in 10 episodes, and 1 hemorrhage in week 13 proved fatal. These findings underscore that, despite the benefits of emicizumab and deferring IST, achieving timely remission of AHA remains a critical therapeutic goal.
There appears to be a fine line between increased safety (fewer infections) and delayed efficacy (more bleeds) of postponed IST. In clinical practice, the risk of IST complications must be balanced against the risk of bleeding with delayed remission in the individual patient. It may not be necessary or advisable to postpone IST in all patients with AHA. Instead, risk factors such as age >74 years and WHO-PS >2 should be considered in the decision for or against early IST, as both were repeatedly shown to increase the risk of mortality. Baseline FVIII activity and inhibitor titer might also be considered in the decision since patients with higher FVIII were shown to achieve remission faster with short courses of IST. Since the completion of GTH-AHA-EMI, the GTH working group has suggested offering IST to patients receiving emicizumab as soon as they are deemed fit, based on an individual assessment.32
Spontaneous remission occurred in 15% of GTH-AHA-EMI patients. A prior single-center study documented 5 spontaneous remissions among 16 patients managed with minimal or no IST, demonstrating that inhibitor clearance can occur without it.33 The systematic deferral of IST under emicizumab prophylaxis made it possible in our study to observe the natural course of inhibitor resolution in a larger cohort.
During the initial 12-week deferral period applied uniformly across all patients, spontaneous remission was infrequent—seen in only 1 of 47 patients, with 2 additional individuals reaching FVIII levels around 50% by week 12.21 The final observed rate of 6 remissions among 47 patients (15%) reported here should be interpreted with caution. After week 12, the decision to initiate IST was left to the discretion of the treating investigators, potentially introducing a selection bias. Patients with lower inhibitor titers—in whom spontaneous remission was more likely anticipated—may have been more likely remained without IST. Conversely, earlier initiation of IST in most patients may have precluded the opportunity to observe spontaneous resolution, raising the possibility that the true spontaneous remission rate could be >15% observed. In current clinical practice, a careful watch-and-wait strategy considering the spontaneous course of inhibitor levels may be reasonable in frail patients protected with emicizumab.
Strengths of our study include the prospective design with comparison of PS-matched individual patient data. As it was considered infeasible to randomize emicizumab prophylaxis and IST, this appeared the best possible approach to assess the impact of our initial treatment strategy on OS. However, comparison of prospective observational studies using PS matching cannot eliminate all sources of bias and does not provide evidence equivalent to randomized clinical trials.
A limitation is that the 2 populations in the current analysis were not treated in parallel but were separated by ∼10 years. Over this time, awareness of infectious complications as a major risk of IST increased, and clinical practice may have adapted accordingly. Although IST in GTH-AH 01/2010 followed a consensus protocol, the regimen in GTH-AHA-EMI was at the investigator’s discretion. This may have resulted in more individually-tailored treatment decisions and perhaps other measures, such as antibiotic prophylaxis, better patient education, and prompt reaction to signs of infection.
Another limitation is that changes in the intensity and composition of IST regimens are difficult to assess. Compared to GTH-AH 01/2010, patients in GTH-AHA-EMI more frequently received RTX and less frequently received GC, although the average cumulative GC does was similar. Notably, previous efforts to reduce the intensity of immediate IST by giving less GC and adding RTX early on did not reduce infection-related mortality,34 whereas another study with less GC and first-line low-dose RTX and CTX reported low infection-related mortality.35 Both of these studies were single-center, retrospective studies that need prospective multicenter confirmation. A retrospective, multicenter US study reported that emicizumab was used to facilitate early outpatient management, which coincided with low reported rates of infection.36 Patients in the GTH-AHA-EMI, but not in GTH-AH 01/2010, received IST as outpatients. The outpatient setting may pose a lower risk of serious infections compared to hospitalization, a finding that has also been observed in other population groups.37,38
A final limitation of our study is the relatively small sample size, which results in wide 95% CI around the HR for mortality. Although the point estimate of 0.39 suggests a 61% relative reduction in mortality, the upper bound of the 95% CI (0.80) indicates that the true effect may have been as low as a 20% reduction. To robustly confirm such a mortality benefit, a randomized clinical trial powered to detect a 19% absolute reduction in mortality (from 37% to 18%) with 80% power and a 2-sided alpha of 0.05 would require enrollment of ∼166 patients (83 per arm, estimate based on sample size calculation for comparing 2 proportions using a normal approximation method with continuity correction).39
Further insight into the optimal timing of IST is expected from our US sister study (ClinicalTrials.gov identifier: #NCT05345197), which applies the same inclusion criteria and endpoint assessments as GTH-AHA-EMI but IST initiation at the investigator’s discretion at any time.
In conclusion, emicizumab provides early protection from bleeds in AHA but also enables IST to be postponed until patients are fit to receive it. The 2-year follow-up data from GTH-AHA-EMI suggests that a strategy of bleed protection with emicizumab and postponed IST may have contributed to improved OS compared to historic controls. This observation should be considered when deciding about individual IST-treatment strategies in patients with AHA.
Acknowledgments
This study was supported by a grant from Roche Pharma. The manufacturer of emicizumab (Hoffmann-La Roche) provided the investigational medicinal product to participating centers. A.T. applied for an unrestricted grant from Hoffman-La Roche that was provided to the study sponsor, GWT-TUD GmbH. GTH-AH 01/2010 was supported by a grant from Novo Nordisk.
No medical writer was involved in preparing the manuscript draft. All authors had access to the data and the statistical analysis plan.
Authorship
Contribution: A.T. and I.M.S. designed and supervised the data collection, had full access to raw study data and performed the analysis; I.M.S. and A.T. wrote the draft of the manuscript; and all authors contributed to data collection, the drafting of the maniscript, and reviewed and approved the final version.
Conflict-of-interest disclosure: C.D. reports honoraria for lectures or consultancy from Novo Nordisk, Roche, and Takeda. R.K. reports institutional grants for research and studies from Bayer, CSL Behring, Novo Nordisk, Octapharma, and Sobi; and honoraria for lectures or consultancy from Bayer, Biotest, Biomarin, CSL Behring, Grifols, LFB Biopharmaceuticals Ltd, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Sanofi, Sobi, and Takeda. C.H. reports honoraria for lectures or consultancy from Bristol Myers Squibb (BMS), Bayer, Sobi, Roche, Behring, Novo Nordisk, Pfizer, and Takeda. U.J.S. reports institutional grants for research and studies from Octapharma; and honoraria for lectures or consultancy from Bayer, Sobi, CSL Behring, and Pfizer. R.G. reports institutional grants for research and studies from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, and Daiichi Sankyo; and honoraria for lectures or consultancy from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, Daiichi Sankyo, and Sanofi. P.K. reports institutional grants for research and studies from Ablynx/Sanofi, Novo Nordisk, Roche, and Takeda; and honoraria for lectures or consultancy from Ablynx/Sanofi, Alexion, Biotest, CSL Behring, Novo Nordisk, Roche, Takeda, and Technoclone. J.O. reports institutional grants for research and studies from Bayer, Biotest, CSL Behring, Octapharma, Pfizer, Sobi, and Takeda; and honoraria for lectures or consultancy from Bayer, Biogen Idec, Biomarin, Biotest, CSL Behring, Chugai, Freeline, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sparks, Sobi, and Takeda. W.M. reports institutional grants for research and studies from Bayer, Biotest, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, and Takeda/Shire; and honoraria for lectures or consultancy from Bayer, Biomarin, Biotest, CSL Behring, Chugai, Freeline, LFB Biopharmaceuticals Ltd, Novo Nordisk, Octapharma, Pfizer, Regeneron, Roche, Sanofi, Takeda/Shire, and uniQure. C.P. reports institutional grants for research and studies from Chugai/Roche, Takeda, Zacros, and LeoPharma; and honoraria for lectures or consultancy from Bayer, Biomarin, Chugai/Roche, CSL Behring, Novo Nordisk, Pfizer, BMS, Sobi, and Takeda. K.T.-G. reports honoraria for lectures or consultancy from BMS, Grifols, Sanofi, Sobi, Takeda, and Roche. P.M. reports institutional grants for research and studies from Baxter Innovations, Bayer, LFB Biopharmaceuticals Ltd, Sobi, Octapharma, Pfizer, and Roche; and honoraria for lectures or consultancy from Alexion, AstraZeneca, Biotest, CSL Behring, Shire, Octapharma, Pfizer, Roche, and Takeda. K.H. reports institutional grants for research and studies from Bayer, CSL Behring, Novo Nordisk, Pfizer, and Sobi; and honoraria for lectures or consultancy from Bayer, Biotest, Chugai, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda. H.E. reports institutional grants for research and studies from Bayer Vital and CSL Behring; and honoraria for lectures or consultancy from Bayer Vital, BioMarin, CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi. S.W. reports institutional grants for research and studies from Biotest and Octapharma; and honoraria for lectures or consultancy from Biotest and Stago. A.T. reports institutional grants for research and studies from Bayer, Biotest, Chugai, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda; and honoraria for lectures or consultancy from Bayer, Biomarin, Biotest, Chugai, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda. I.M.S. declares no competing financial interests.
Correspondence: Andreas Tiede, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Carl Neuberg Str 1, 30625 Hannover, Germany; email: tiede.andreas@mh-hannover.de.
References
Author notes
Data are available on request from the corresponding author, Andreas Tiede (tiede.andreas@mh-hannover.de).
The full-text version of this article contains a data supplement.