Key Points
Frail patients as defined by the IMWG-FI have limited outcomes, with a median survival of <3 years.
Frail subgroup analyses based on geriatric impairments and comorbidities besides age revealed pronounced heterogeneity in outcomes.
Visual Abstract
Frailty, rather than age alone, is a key determinant of outcomes in older patients with multiple myeloma (MM), yet frailty assessments are often lacking in clinical trials. Consequently, data on the efficacy and tolerability of novel treatments in frail patients remain scarce. Moreover, there is substantial heterogeneity among frail patients, with some classified as frail solely due to age (>80 years) and others due to geriatric impairments and/or comorbidities. To our knowledge, the HOVON 143 trial was the first trial that was specifically designed for frail patients with newly diagnosed MM (NDMM) using the International Myeloma Working Group Frailty Index (IMWG-FI). After a median follow-up of >5 years, we report the long-term progression-free survival (PFS) and overall survival (OS) outcomes, with detailed analyses of frail subgroups. Patients who were classified as frail according to the IMWG-FI were treated with 9 induction cycles of ixazomib, daratumumab, and low-dose dexamethasone, followed by maintenance therapy until progression for a maximum of 2 years. Median PFS was 13.8 months, and median OS was 34.0 months. However, frail subgroup analyses based on geriatric impairments and comorbidities besides age revealed pronounced heterogeneity in outcomes. Both early relapse–related and nonrelapse-related mortality rates were higher in ultrafrail patients and patients who were frail due to impairments than in patients who were frail based on age alone. These findings highlight the need for a more precise frailty definition to identify patients at the highest risk of early mortality. This trial was registered at www.clinicaltrialsregister.eu as EudraCT #2016-002600-90.
Introduction
Approximately one-third of patients with multiple myeloma (MM) are aged >75 years at diagnosis, a proportion that is expected to rise in the coming years.1 Although older patients are increasingly included in clinical trials, frailty assessments are often not performed, despite evidence that age does not fully reflect frailty.2 Therefore, it is reasonable to hypothesize that in most clinical trials more fit patients are being included, irrespective of their age being comparable with those of patients in real life. This is reflected by real-world data reporting significantly higher rates of early mortality within 6 months (20%-25%) and a higher risk of disease progression as compared to those of patients treated in a clinical trial setting.3-5
To allow the extrapolation of outcome data to real-world patients, frail patients should be investigated in the context of clinical trials. In addition, the level of frailty should be defined by the International Myeloma Working Group Frailty Index (IMWG-FI),6 as it is known that when using the Simplified Frailty Index (SFI), the level of frailty is overestimated, incorrectly classifying intermediate-fit patients as frail.7,8 Finally, even when using the IMWG-FI to define frailty, there is significant heterogeneity,9 with more comorbidities or impairments being reflected in a higher IMWG frailty score ranging from 2 to 5. To better characterize this heterogeneity, it has been suggested to categorize patients with a frailty score of 3 as ultrafrail.10 However, we have previously demonstrated that a cutoff value of 3 on the IMWG frailty score more effectively distinguishes between intermediate-fit and frail patients. Accordingly, patients with a score of 2 were categorized as intermediate-fit, while those with scores ≥3 were considered frail. Given this revised classification model, we believe that a cutoff of 3 does not adequately capture the concept of ultrafrailty. Especially, as we found differences in the therapy discontinuation rates across scores ranging from 3 to 5.11 Nevertheless, clinical outcomes across frail subgroups remain insufficiently explored.12,13
To address these issues, we present the long-term progression-free survival (PFS) and overall survival (OS) outcomes of the HOVON 143 study, which was specifically designed for frail patients with MM, with detailed analyses of frail subgroups and subsequent therapies.
Methods
Patients with newly diagnosed MM who were classified as frail according to the IMWG-FI were eligible for the study. Treatment consisted of nine 28 day-induction cycles of ixazomib (4 mg on days 1, 8, and 15), daratumumab (16 mg/kg IV; cycles 1-2: days 1, 8, 15, and 22; cycles 3-6: days 1 and 15; cycles 7-9: day 1), and low-dose dexamethasone (cycles 1-2: 20 mg; subsequent cycles: 10 mg, co-administered with daratumumab), followed by maintenance therapy with 8-week cycles of ixazomib (days 1, 8, 15, 29, 36, and 43), daratumumab (day 1), and low-dose dexamethasone (day 1), until progression for a maximum of 2 years. Inclusion and exclusion criteria were described previously.9 The primary end point was overall response rate after 9 induction cycles. Secondary end points were PFS, PFS2 (defined as the time from start of treatment until the second progression or death), and OS. Frail subgroups were defined as follows: (1) patients who were aged >80 years without Activities of Daily Living (ADL) impairments or comorbidities were classified as “frail based on age alone,” (2) patients who were aged <80 years but had ADL impairments and/or comorbidities were classified as “frail based on impairments,” and (3) patients who were both aged >80 years and had ADL impairments and/or comorbidities were defined as “ultrafrail.” Comparisons of clinical outcomes across frailty subgroups were conducted as post hoc, exploratory analyses. Survival analyses were performed using the Kaplan-Meier method (R, version 4.0). P values of <.05 were considered statistically significant, and no adjustments were made for multiple comparisons. Annualized rates for adverse events (AEs) were calculated as the total number of AEs divided by the time on protocol in years. Incidence risk ratios were compared between subgroups using a negative binomial model. The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board and ethics committees before study initiation. All patients provided written informed consent.
Results
A total of 65 frail patients were included (49% with an IMWG frailty score of 2, 28% with a score of 3, 22 % with a score of 4, 1% with a score of 5). Baseline characteristics are summarized in Table 1. Median age was 81 years (range, 70-92). Twenty-five patients (45%) had ISS III, and 11 patients (20%) had high-risk cytogenetics. Thirteen (20%) patients were frail based on age >80 years alone, without any impairments in (Instrumental) ADL ([I]ADL) or comorbidities. In addition, 32 (49%) patients were frail based on impairments and/or comorbidities, and 20 (31%) were frail based on both age >80 years and having impairments in (I)ADL and/or comorbidities, defined as “ultrafrail.” Patients who were frail based on impairments less often had an IMWG frailty score of ≥4, as compared to ultrafrail patients (10% vs 55%) (Table 1).
Baseline characteristics of patients included in the HOVON 143 study
| Baseline characteristics . | Frail based on age (n = 13) . | Frail based on impairments (n = 32) . | Ultrafrail (n = 20) . | Total (N = 65) . |
|---|---|---|---|---|
| Age, median (range), y | 83.0 | 77.0 | 83.0 | 81.0 (70-92) |
| ≤75, n (%) | — | 6 (19) | — | 6 (9) |
| 76-80, n (%) | — | 26 (81) | — | 26 (40) |
| ≥80, n (%) | 13 (100) | — | 20 (100) | 33 (51) |
| Sex, n (%) | ||||
| Male | 7 (54) | 22 (69) | 13 (65) | 42 (65) |
| Female | 6 (46) | 10 (31) | 7 (35) | 23 (35) |
| ADL, n (%) | ||||
| >5 | 13 (100) | 24 (75) | 12 (60) | 49 (75) |
| <5 | — | 8 (25) | 8 (40) | 16 (25) |
| (I)ADL, n (%) | ||||
| >5 | 13 (100) | 9 (28) | 6 (30) | 28 (43) |
| <5 | — | 23 (72) | 14 (70) | 37 (57) |
| Charlson Comorbidity Index, n (%) | ||||
| 0-1 | 13 (100) | 9 (28) | 10 (50) | 32 (49) |
| ≥2 | — | 23 (72) | 10 (50) | 33 (51) |
| WHO status, n (%) | ||||
| 0-1 | 12 (92) | 16 (50) | 10 (56) | 38 (58) |
| ≥2 | 1 (8) | 16 (50) | 8 (44) | 25 (38) |
| Unknown | — | — | — | 2 (3) |
| IMWG frailty score, n (%) | ||||
| 2 | 13 (100) | 19 (59) | — | 32 (49) |
| 3 | — | 10 (31) | 9 (45) | 19 (29) |
| 4 | — | 3 (10) | 10 (50) | 13 (20) |
| 5 | — | — | 1 (5) | 1 (2) |
| ISS disease stage, n (%) | ||||
| I | 6 (46) | 2 (6) | 2 (10) | 10 (16) |
| II | 4 (31) | 13 (41) | 8 (40) | 25 (39) |
| III | 3 (23) | 16 (50) | 10 (50) | 29 (45) |
| Unknown | — | 1 (3) | — | 1 |
| Cytogenetic risk profile∗, n (%) | ||||
| Standard | 11 (85) | 19 (59) | 15 (75) | 45 (69) |
| High | 1 (8) | 7 (22) | 3 (15) | 11 (17) |
| Unknown | 1 (8) | 6 (19) | 2 (10) | 9 (14) |
| R-ISS disease stage (%) | ||||
| I | 1 (3) | 5 (38) | 2 (10) | 8 (12) |
| II | 21 (66) | 6 (46) | 11 (55) | 38 (58) |
| III | 6 (19) | 1 (8) | 5 (25) | 12 (18) |
| Unknown | 4 (13) | 1 (8) | 2 (10) | 7 (11) |
| Type of measurable disease, n (%) | ||||
| IgG | 2 (15) | 8 (25) | 5 (25) | 39 (60) |
| IgA | 9 (69) | 18 (56) | 12 (60) | 15 (23) |
| FLC | 2 (15) | 5 (16) | 2 (10) | 9 (14) |
| Other | — | 1 (3) | 1 (5) | 2 (3) |
| Median creatinine clearance, mL/min (range) | 52 | 52 | 36 | 56 (20-90) |
| Median hemoglobin, mmol/L, (range) | 6.5 | 6.1 | 6.1 | 6.2 (4.4-9.5) |
| Baseline characteristics . | Frail based on age (n = 13) . | Frail based on impairments (n = 32) . | Ultrafrail (n = 20) . | Total (N = 65) . |
|---|---|---|---|---|
| Age, median (range), y | 83.0 | 77.0 | 83.0 | 81.0 (70-92) |
| ≤75, n (%) | — | 6 (19) | — | 6 (9) |
| 76-80, n (%) | — | 26 (81) | — | 26 (40) |
| ≥80, n (%) | 13 (100) | — | 20 (100) | 33 (51) |
| Sex, n (%) | ||||
| Male | 7 (54) | 22 (69) | 13 (65) | 42 (65) |
| Female | 6 (46) | 10 (31) | 7 (35) | 23 (35) |
| ADL, n (%) | ||||
| >5 | 13 (100) | 24 (75) | 12 (60) | 49 (75) |
| <5 | — | 8 (25) | 8 (40) | 16 (25) |
| (I)ADL, n (%) | ||||
| >5 | 13 (100) | 9 (28) | 6 (30) | 28 (43) |
| <5 | — | 23 (72) | 14 (70) | 37 (57) |
| Charlson Comorbidity Index, n (%) | ||||
| 0-1 | 13 (100) | 9 (28) | 10 (50) | 32 (49) |
| ≥2 | — | 23 (72) | 10 (50) | 33 (51) |
| WHO status, n (%) | ||||
| 0-1 | 12 (92) | 16 (50) | 10 (56) | 38 (58) |
| ≥2 | 1 (8) | 16 (50) | 8 (44) | 25 (38) |
| Unknown | — | — | — | 2 (3) |
| IMWG frailty score, n (%) | ||||
| 2 | 13 (100) | 19 (59) | — | 32 (49) |
| 3 | — | 10 (31) | 9 (45) | 19 (29) |
| 4 | — | 3 (10) | 10 (50) | 13 (20) |
| 5 | — | — | 1 (5) | 1 (2) |
| ISS disease stage, n (%) | ||||
| I | 6 (46) | 2 (6) | 2 (10) | 10 (16) |
| II | 4 (31) | 13 (41) | 8 (40) | 25 (39) |
| III | 3 (23) | 16 (50) | 10 (50) | 29 (45) |
| Unknown | — | 1 (3) | — | 1 |
| Cytogenetic risk profile∗, n (%) | ||||
| Standard | 11 (85) | 19 (59) | 15 (75) | 45 (69) |
| High | 1 (8) | 7 (22) | 3 (15) | 11 (17) |
| Unknown | 1 (8) | 6 (19) | 2 (10) | 9 (14) |
| R-ISS disease stage (%) | ||||
| I | 1 (3) | 5 (38) | 2 (10) | 8 (12) |
| II | 21 (66) | 6 (46) | 11 (55) | 38 (58) |
| III | 6 (19) | 1 (8) | 5 (25) | 12 (18) |
| Unknown | 4 (13) | 1 (8) | 2 (10) | 7 (11) |
| Type of measurable disease, n (%) | ||||
| IgG | 2 (15) | 8 (25) | 5 (25) | 39 (60) |
| IgA | 9 (69) | 18 (56) | 12 (60) | 15 (23) |
| FLC | 2 (15) | 5 (16) | 2 (10) | 9 (14) |
| Other | — | 1 (3) | 1 (5) | 2 (3) |
| Median creatinine clearance, mL/min (range) | 52 | 52 | 36 | 56 (20-90) |
| Median hemoglobin, mmol/L, (range) | 6.5 | 6.1 | 6.1 | 6.2 (4.4-9.5) |
FLC, Free Light Chain; IgA, immunoglobulin A; IgG, immunoglobulin G; ISS, International Staging System; R-ISS, Revised International Staging System; WHO, World Health Organization.
Based on the presence of del17p, t(4;14) and/or t(14;16).
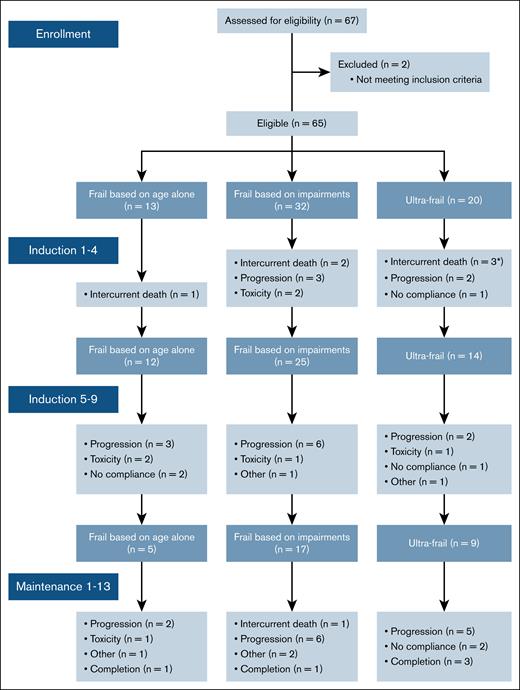
One patient discontinued the study before the start of therapy due to sepsis. Of the 64 patients who started induction therapy, 41 (64%) completed 9 cycles. Nine patients discontinued therapy between induction and maintenance, including 5 due to progressive disease, 1 due to toxicity, and 3 due to other reasons. Subsequently, 32 (50%) patients proceeded with maintenance therapy, of whom 5 (5/13 patients; 38%) were frail based on age, 18 (18/32; 56%) were frail based on impairments, and 9 (9/20; 45%) were ultrafrail. Eleven of the 32 patients (34%) completed 2 years of maintenance therapy. The reasons for treatment discontinuation are summarized in Figure 1.
Consort diagram of frail patients included in the HOVON 143 depicting timing and reason for treatment discontinuation. ∗One ultrafrail patient died due to sepsis before initiation of the first induction cycle.
Consort diagram of frail patients included in the HOVON 143 depicting timing and reason for treatment discontinuation. ∗One ultrafrail patient died due to sepsis before initiation of the first induction cycle.
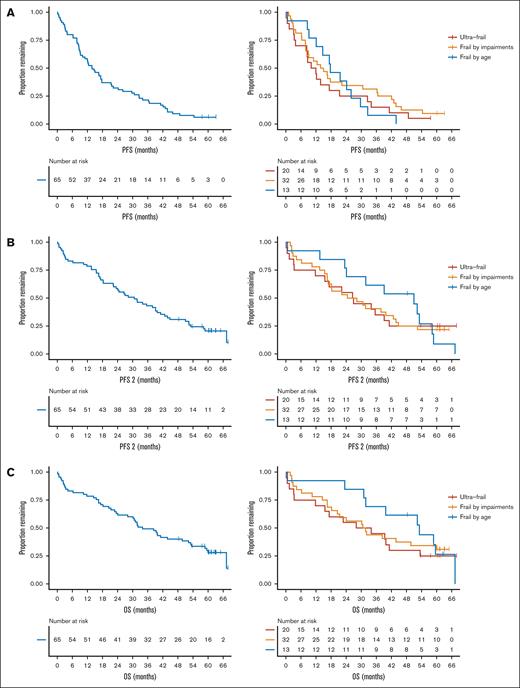
Median PFS of all patients was 13.8 months (95% confidence interval [CI], 10.1-21.4). Among patients who were frail based on age, median PFS was 17.7 months (95% CI, 12.1 to not estimable (NE)). Among patients who were frail based on impairments and those were ultrafrail, these numbers were 14.4 months (95% CI, 8.9-36.3) and 11.0 months (95% CI, 8.3-32.5), respectively (log-rank P = .58) (Figure 2A).
Survival outcomes for all frail patients and across frail subgroups. (A) PFS, (B) PFS2, and (C) OS.
Survival outcomes for all frail patients and across frail subgroups. (A) PFS, (B) PFS2, and (C) OS.
Median PFS2 of all patients was 30.7 months (95% CI, 22.3-41.2). Median PFS2 was 51.0 months (95% CI, 24.1 to NE) for patients who were frail based on age, 26.4 months (95% CI, 17.0-43.9) among those who were frail based on impairments, and 26.8 months (95% CI, 15.5 to NE) for ultrafrail patients (log-rank P = .93) (Figure 2B). Median event-free survival (EFS), as defined by Larocca et al,14 was 2.9 months (95% CI, 1.81-6.2) for all frail patients (supplemental Figure 1). EFS did not significantly differ between the frail subgroups (frail based on age: EFS = 2.9 months (95% CI, 2.5 to NE), frail based on impairments: 2.9 months (95% CI, 1.6-15.6), and ultrafrail: 2.6 months (95% CI, 0.26 to NE), log-rank P = .83).
After a median follow-up of 61.5 months, 47 (72%) patients had died. Median OS in all patients was 34.0 months (95% CI, 28.1-53.2). Patients who were frail based on age had a median OS of 53.2 months (95% CI, 31.8 to NE), whereas among patients who were frail based on impairments and those who were ultrafrail, these numbers were 31.2 months (95% CI, 20.5 to NE), and 31.0 months (95% CI, 15.5 to NE) respectively (log-rank P = .71) (Figure 2C).
Early mortality (defined as death <100 days) more often occurred in ultrafrail patients (22%) and in patients who were frail based on impairments (13%) than in patients who were frail based on age (8%) (Table 2). This was also true for non-relapse related mortality. In patients who were frail based on age alone, only 1 patient died before disease progression due to an infection. Seven of 32 (22%) patients who were frail based on impairments died due to non-relapse related causes, and among ultrafrail patients, this number was 6 of 20 (30%). These results indicate that the difference in outcome between frail subgroups was primarily driven by differences in (early) mortality between the subgroups rather than the efficacy of treatment, affecting not only PFS but also PFS2 and OS.
Incidence of relapse- and nonrelapse-related mortality across frail subgroups
| . | Frail based on age (n = 13) . | Frail based on impairments (n = 32) . | Ultrafrail (n = 20) . |
|---|---|---|---|
| Early mortality (<100 days), n (%) | 1 (8) | 4 (13) | 5 (25) |
| Relapse-related | — | — | 1 |
| Nonrelapse-related | 1 | 4 | 3 |
| Unknown | — | — | 1 |
| Nonrelapse-related mortality, n (%) | 1 (8) | 7 (22) | 6 (30) |
| Relapse-related mortality, n (%) | 4 (31) | 10 (31) | 4 (15) |
| Cause of death | 1 infection | 1 infection 4 toxicity 1 other∗ 1 unknown | 2 infection 3 other† 1 unknown |
| . | Frail based on age (n = 13) . | Frail based on impairments (n = 32) . | Ultrafrail (n = 20) . |
|---|---|---|---|
| Early mortality (<100 days), n (%) | 1 (8) | 4 (13) | 5 (25) |
| Relapse-related | — | — | 1 |
| Nonrelapse-related | 1 | 4 | 3 |
| Unknown | — | — | 1 |
| Nonrelapse-related mortality, n (%) | 1 (8) | 7 (22) | 6 (30) |
| Relapse-related mortality, n (%) | 4 (31) | 10 (31) | 4 (15) |
| Cause of death | 1 infection | 1 infection 4 toxicity 1 other∗ 1 unknown | 2 infection 3 other† 1 unknown |
Second primary malignancy.
One with heart failure, 1 with bleeding due to thrombocytopenia, and 1 with lymphoma.
To further investigate this finding, we determined the PFS during second-line treatment specifically in patients who received the treatment (“second PFS”), defined as the time from the start of second-line treatment to the time of second progression. Median second PFS was 11.3 months (95% CI, 8.5-19.9) for all patients. Median second PFS was not inferior in patients who were defined ultrafrail at diagnosis (10.1 months in patients who were frail based on age (95%, CI, 6.6 to NE), 11.3 months in patients who were frail based on impairments (95% CI, 8.2-27.5), and 16.1 months in ultrafrail patients (95% CI, 6.4 to NE) (log-rank P = 1) (supplemental Figure 2).
To assess the feasibility of second-line treatment in frail subgroups, we analyzed patients who relapsed after first-line treatment. Of the 65 included patients, 46 had an indication for second line-treatment (15 died before disease progression and 4 discontinued therapy for reasons other than progression and did not yet experience progressive disease (PD)). Thirty-seven of these 46 patients (80%) received second-line treatment, which was comparable across frailty subgroups: 9 of 12 (75%) of those who were frail based on age alone, 19 of 22 (86%) of those frail based on impairments, and 9 of 12 (75%) of those who were ultrafrail. Second-line treatment was mainly lenalidomide-based (33/37 [89%] patients), including 20 of 37 lenalidomide-dexamethasone, 4 of 37 lenalidomide-cyclophosphamide-prednisone, 4 of 37 elotuzumab-lenalidomide-dexamethasone, 2 of 37 carfilzomib-lenalidomide-dexamethasone, 2 of 37 daratumumab-lenalidomide-dexamethasone (DRd), 1 of 37 lenalidomide-prednisone.
With longer follow-up, including maintenance therapy, the most frequent AEs were comparable as described before,6 both with respect to type and incidence. Briefly, severe (grade ≥3) hematologic AEs, most often thrombocytopenia, occurred in 21 of 65 (35%) patients. Severe infections (grade ≥3) were reported in 15 of 65 (23%) patients. All-grade polyneuropathy occurred in 25 of 65 (38%) patients (grade 1: 12%, grade 2: 18%, and grade 3: 8%) and did not differ between frail subgroups (7/13 [54%] patients who were frail based on age alone, 12/31 [39%] who were frail based on impairments, 6/20 [30%] who were ultrafrail). Cumulative incidence and annualized rates of AEs (both hematologic and non-hematologic) did not differ between frail subgroups (supplemental Figure 3; Table 3). However, when corrected for time on protocol, severe infections (grade ≥3) occurred more often in patients who were frail based on impairments (0.36 infections per patient-year [py]) and in those who were ultrafrail (0.42 infections per py) than in patients who were frail based on age alone (0.07 infections per py) (P = .08) (Table 4).
Annualized rates of AEs (corrected for time on protocol in patient-years) during the HOVON 143 study
| Annualized rate of AE . | Frail based on age . | Frail based on impairments . | Ultrafrail . |
|---|---|---|---|
| Hematologic AE (all grades) | 0.45 | 0.23 | 0.48 |
| Non-hematologic AE (all grades) | 6.74 | 5.88 | 7.40 |
| Severe non-hematologic AE (grade ≥3) | 1.78 | 2.36 | 2.31 |
| Infections (all grades) | 0.46 | 0.77 | 0.74 |
| Severe infections (grade ≥3) | 0.07 | 0.36 | 0.42 |
| Annualized rate of AE . | Frail based on age . | Frail based on impairments . | Ultrafrail . |
|---|---|---|---|
| Hematologic AE (all grades) | 0.45 | 0.23 | 0.48 |
| Non-hematologic AE (all grades) | 6.74 | 5.88 | 7.40 |
| Severe non-hematologic AE (grade ≥3) | 1.78 | 2.36 | 2.31 |
| Infections (all grades) | 0.46 | 0.77 | 0.74 |
| Severe infections (grade ≥3) | 0.07 | 0.36 | 0.42 |
Rates of infections corrected for time on protocol (in patient-years) compared across subgroups using Poisson models
| Annualized rates of infections . | Frail based on age alone (reference group) . | Frail based on impairments . | Ultrafrail . | ||
|---|---|---|---|---|---|
| Infections per py (95% CI) . | Infections per py (95% CI) . | P value . | Infections per py (95% CI) . | P value . | |
| Infections (all grades) | 0.46 (0.22-0.96) | 0.77 (0.34-1.75) | .22 | 0.74 (0.30-1.80) | .30 |
| Severe infections (grade ≥3) | 0.07 (0.01-0.47) | 0.36 (0.04-2.73) | .10 | 0.42 (0.05-3.28) | .08 |
| Annualized rates of infections . | Frail based on age alone (reference group) . | Frail based on impairments . | Ultrafrail . | ||
|---|---|---|---|---|---|
| Infections per py (95% CI) . | Infections per py (95% CI) . | P value . | Infections per py (95% CI) . | P value . | |
| Infections (all grades) | 0.46 (0.22-0.96) | 0.77 (0.34-1.75) | .22 | 0.74 (0.30-1.80) | .30 |
| Severe infections (grade ≥3) | 0.07 (0.01-0.47) | 0.36 (0.04-2.73) | .10 | 0.42 (0.05-3.28) | .08 |
Discussion
The long-term analysis of the HOVON 143 study, specifically designed for frail patients as defined by the IMWG frailty score, showed that the median OS was <3 years, despite the use of a triplet regimen including a CD38 monoclonal antibody and a proteasome inhibitor. However, frail subgroup analyses based on geriatric impairments and comorbidities besides age revealed pronounced heterogeneity in outcomes. More than one-third of the ultrafrail patients died before experiencing disease progression, which largely contributed to the poor long-term outcomes observed in the whole group of frail patients. In contrast, patients who were defined as frail based on age >80 years alone appeared less vulnerable, as exemplified by a much lower non-relapse- and early relapse-related mortality rate of <8%. Although not statistically different, likely due to the small sample size, patients who were frail based on impairments or those who were ultrafrail had an inferior PFS, PFS2, and OS. These findings highlight the unmet need for more tolerable therapies for frail patients as 80% of frail patients do experience geriatric impairments and comorbidities, whereas frailty-adjusted therapy may be less required for those classified as frail based on age alone.
Notably, the UK-MRA FiTNEss trial investigates the value of frailty-adjusted treatment using the IMWG frailty score to identify the level of fitness. In this study, patients are either treated with a standard dosing of ixazomib-lenalidomide-dexamethasone or a dose-adapted approach, in which the dose of lenalidomide and dexamethasone are adapted based on the level of frailty (ie, fit, intermediate-fit, or frail). Preliminary data show that compared to the standard approach, frailty-adjusted treatment lead to superior OS in the intention to treat population, whereas this was not observed in the intermediate-fit or frail population.15 However, a definitive and comprehensive analysis, particularly regarding the groups that derive the greatest benefit and whether frail subpopulations are differentially affected, remains to be determined.
Other studies have used the SFI,16 instead of the gold standard IMWG frailty score, substituting (I)ADL assessment by the Eastern Cooperative Oncology Group (ECOG) Performance Status. The IFM-2017 study, a prospective phase 3 trial in patients classified as frail by the SFI, reported significant PFS and OS benefits for DR compared to Rd, with a median PFS of 48.5 months and a median OS that was not reached.17 However, they showed that 21% of the patients who were classified as frail by the SFI would have been classified as intermediate-fit by the IMWG-FI. This corresponds with the discordance rate of 25% that we have previously described between frailty defined by the SFI and the IMWG-FI.7 Given that a substantial proportion of frail patients in this study would have been classified as intermediate-fit by the IMWG-FI, the outcomes of this study cannot be directly compared to our study. Interestingly, the annualized infection rates reported by the authors in both the DR arm (0.07) and Rd arm (0.09) were comparable to the infection rate that we found in patients who were frail based on age alone (0.07), thus supporting a less pronounced level of frailty in their study population.
A post-hoc analysis of the MAIA trial also using the SFI, found that frail patients who were treated with DRd had a 3-year PFS rate of 61.5%.18 The superior PFS as compared to our findings may be explained by differences in treatment regimens of lenalidomide vs ixazomib. The hypothesis that combining a CD38 monoclonal antibody with a proteasome inhibitor may be less effective than combining it with an immunomodulatory drug is supported by a non–head-to-head comparison with the ALCYONE trial. In this study, patients treated with daratumumab-bortezomib-melphalan-prednisone had a substantially lower 3-year PFS rate of ∼40% in the frail subgroup compared with those treated with DRd.19 However, differences in frailty levels because of having used the SFI in both registration trials vs the IMWG-FI in our trial likely contribute as well. This is supported by comparing our trial with the ALCYONE trial, as both studies used daratumumab in combination with a proteasome inhibitor. We observed a median OS of <3 years, whereas in the ALCYONE trial, 3-year OS was ∼75%. However, this non–head-to-head comparison has limitations, and an impact by differences between bortezomib and ixazomib cannot be excluded.20
Our results indicate that there is an urgent need for more trials specifically designed for frail patients with newly diagnosed MM, including the most vulnerable with geriatric impairments and comorbidities. Especially, as we have shown that treatment also increases the general quality of life of frail patients,12 exploring tolerable and effective therapies to further improve the outcome for frail patients is warranted. To better characterize heterogeneity within the frail population, we examined the individual components of the IMWG frailty score. We found that there was a subgroup of frail patients who more often discontinued therapy, as well as had higher rates of early mortality and an inferior prognosis.21 These findings informed our definition of ultrafrail patients as patients aged >80 years and having impairments in ADL/(I)ADL and/or comorbidities. We acknowledge that this definition of ultrafrail needs to be validated in other patient cohorts.
There is scarce data on the tolerability and efficacy of T-cell redirecting therapies with bispecific antibodies and CAR T cells in patients aged >65 years. Several studies, although with limited number of patients aged >75 years, suggest that higher age or worse World Health Organization performance status did not lead to higher rates of Cytokine Release Syndrome (CRS), Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), hematologic toxicity, or infections following CAR T-cell therapy.22-25 Only 1 study investigated the impact of frailty, showing that frail patients had inferior PFS and OS compared to non-frail patients.26 In addition, T-cell–redirecting bispecific antibodies were found to be feasible and effective in patients aged >70 years.10,27,28 However, how T-cell–redirecting therapies perform in frail patients, especially in the subgroups we define here, remains to be investigated. Comparable outcomes across different frailty levels would indicate that the IMWG-FI should be redefined to predict outcome of T-cell redirecting therapies.
In conclusion, there is pronounced heterogeneity in outcomes among frail patients. A more precise definition of frail patients is essential to distinguish patients at the highest risk of early mortality from those who benefit from treatment. These findings underscore the need for future trials sufficiently powered to study frail subgroups.
Acknowledgments
The HOVON 143 trial was supported by research funding from Janssen Pharmaceuticals and Takeda Pharmaceutical Company Limited.
Authorship
Contribution: S.Z. and K.N. designed the trial. F.S., K.G., C.A.M.S., M.R.S., K.N., and S.Z. analyzed the data and interpreted the results. M.-D.L., R.v.K., E.v.d.S., Y.M.B., N.T., I.N., I.L., E.G.M.d.W., Y.S., A.K., G.-J.T., J.C.R., M.W., K.d.H., M.-C.V., N.D.-R., N.C.H.P.d.G., and P.F.Y. included and treated patients in the clinical trial. F.S., K.N., and S.Z. wrote the first draft of the manuscript. All authors helped critically review the manuscript.
Conflict-of-interest disclosure: K.G. has received payment/honoraria for presentations from Bristol Myers Squibb and BeiGene (no personal funding). C.A.M.S. has received payment/honoraria for presentations from Amgen, Celgene, Janssen Pharmaceuticals, Novartis, Regeneron, Sanofi, and Takeda; and provided consultancy for J&J and Regeneron. E.v.d.S. received honoraria for educational events from Janssen. A.K. provided consultancy for Amgen, Sanofi, and Janssen-Cilag. G.-J.T. received honoraria for consultancy and travel expenses from Novartis and Janssen. M.W. received honoraria for educational events from Janssen. M.-C.V. provided consultancy for Amgen, Janssen, Celgene, Sanofi, Pfizer, GlaxoSmithKline, and Menarini; and received honoraria for educational events from Amgen, Celgene, Janssen, and Takeda. P.F.Y. received honoraria for educational events from Janssen. N.W.C.J.v.d.D. has received research support from Janssen Pharmaceuticals, Amgen, Celgene, Novartis, Cellectis, and Bristol Myers Squibb; and serves on advisory boards for Janssen Pharmaceuticals, Amgen, Celgene, Bristol Myers Squibb, Takeda, Roche, Novartis, Bayer, Pfizer, Merck, Kite Pharma, AbbVie, Adaptive, and Servier, which are all paid to the institution. S.Z. received research funding from Takeda, Celgene, and Janssen. S.Z. serves on advisory boards for Takeda, Celgene, Janssen, Sanofi, Oncopeptides, and Amgen, all paid to the institution. The remaining authors declare no competing financial interests.
A complete list of investigators in the HOVON 143 Study Group appears in the supplemental Appendix.
Correspondence: Sonja Zweegman, Department of Hematology, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; email: s.zweegman@amsterdamumc.nl.
References
Author notes
Data are available on reasonable request from the corresponding author, Sonja Zweegman (s.zweegman@amsterdamumc.nl).
The full-text version of this article contains a data supplement.