Key Points
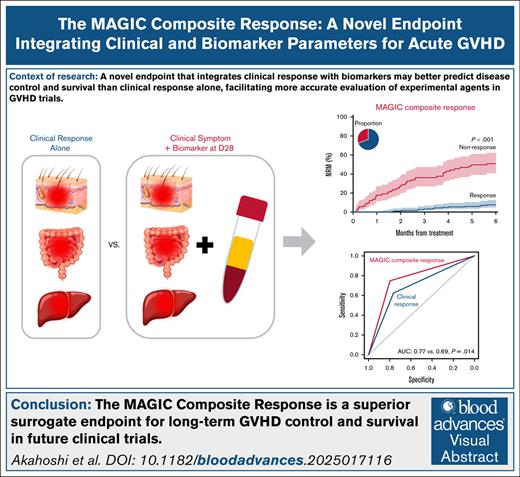
The MCR integrates clinical and biomarker data to predict long-term outcomes more accurately.
When biomarkers are unavailable at onset, MCR remains superior to changes in clinical severity and can serve as a better trial end point.
Visual Abstract
Changes in the clinical symptoms of acute graft-versus-host disease (GVHD) are currently used to assess treatment responses. The Mount Sinai Acute GVHD International Consortium (MAGIC) consortium has recently revealed that the integration of serum biomarkers with clinical symptoms at the onset of treatment in a MAGIC composite score (MCS) more accurately predicts treatment response and 6-month nonrelapse mortality (NRM) than clinical symptoms alone. In this study, we evaluated whether the integration of serum biomarkers and clinical symptoms on day 28 (D28) would also better predict NRM than clinical response only (CRO). We analyzed data from 1135 patients receiving systemic treatment for acute GVHD and created a fourth MCS category for patients with complete resolution of symptoms and low-risk clinical biomarkers on D28. Using a classification and regression tree model with 6-month NRM as the end point, we identified status of MCS 0 or MCS 1 at D28 as responses, which we termed the MAGIC composite response (MCR). In the validation cohort (n = 309), MCR more accurately predicted 6-month NRM than CRO (area under the curve: 0.77 vs 0.69; P = .014) and demonstrated higher negative and positive predictive values. MCR correctly reclassified both clinical nonresponders and responders: 28 of 213 clinical responders (13%) became nonresponders with fivefold higher NRM (34.3% vs 6.8%, P < .001) and a larger group (29/96, 30%) of clinical nonresponders became responders with sixfold lower NRM (7.6% vs 50.7%, P < .001). These findings support the use of MCR as a superior surrogate end point for long-term GVHD control and survival in future clinical trials.
Introduction
Acute graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT).1,2 Reduction in the severity of day 28 (D28) clinical symptoms after treatment initiation serves as the gold standard for response in clinical trials.3-7 These clinical response criteria have notable limitations including modest prediction of nonrelapse mortality (NRM) because all target organs are treated equally despite clear evidence that gastrointestinal (GI) tract involvement disproportionately influences the risk of NRM.8,9
Several studies have highlighted the potential of serum biomarkers to enhance the precision of response assessments afforded by clinical manifestations alone.10-19 The biomarkers ST2 and REG3α that measure damage to the GI tract crypts are combined in a single value termed the Mount Sinai Acute GVHD International Consortium (MAGIC) algorithm probability (MAP), which has emerged as a reliable indicator of disease activity and prognosis in acute GVHD.17,18,20 Recently, we developed the MAGIC composite score (MCS), which integrates biomarker and clinical data to stratify the risk of GVHD at symptom onset and more accurately predicts response to therapy and NRM compared with clinical assessments alone (supplemental Table 1).14 The MCS grading system used data from a single time point when GVHD was first treated. We undertook the current study to explore whether the combination of biomarkers and clinical symptoms in MCS would also be valuable in assessing treatment response. We used a similar approach to that used in developing MCS to define the MAGIC composite response (MCR). We again chose 6-month NRM as the key criterion and hypothesized that MCR criteria would demonstrate superior predictive accuracy compared with current clinical response only (CRO) approach.
Methods
Patient selection
Clinical data and serum samples were obtained from the MAGIC database and biorepository, which encompasses 26 HCT centers in North America, Europe, and Asia, using a prospective-specimen-collection, retrospective-blinded-evaluation (PRoBE) study design.21,22
We included both pediatric and adult patients who received their first HCT between 2014 and 2023, all of whom received systemic treatment for acute GVHD. We excluded patients who developed primary relapse of malignancy or who received donor lymphocyte infusion or second HCT before systemic GVHD treatment. Acute GVHD was diagnosed and staged according to the published criteria.22 Minnesota risk, Manhattan risk, HCT-specific comorbidity index scores, intensity of conditioning regimens, and disease risk were classified as previously reported.9,14,23,24 A complete response (CR) was defined as complete resolution of acute GVHD manifestations, and a partial response (PR) was defined as a decrease in at least 1 organ stage without worsening of other organs, provided that the improvement was less than a CR.5 CRO included D28 CR or PR after systemic treatment, and overall response was defined by D28 CR or PR after systemic treatment without intervening secondary treatment. The training set excluded patients who died or relapsed before D28 of treatment (n = 6) for clarity in developing the MCR, but the validation set included them as nonresponders (deaths = 13, relapses = 7).
Serum samples
Serial serum samples were collected prospectively, cryopreserved, and shipped to a central laboratory. Serum concentrations of ST2 and REG3α were analyzed by enzyme-linked immunosorbent assays, as previously reported.25,26 The MAP was calculated as a single value between 0.001 and 0.999 according to the following formula: log[–log(1 – MAP)] = −11.263 + 1.844(log10ST2) + 0.577(log10REG3α).12,27 We classified Ann Arbor (AA) scores using previously validated thresholds (AA1 < 0.141; 0.141 ≤ AA2 < 0.291; AA3 ≥ 0.291).11 D28 AA3 was considered high risk because previous works had revealed this threshold to create a risk separation of groups.28,29
Statistical analysis
The initiation of systemic treatment served as the starting point for all analyses. The primary end point was 6-month NRM, and outcomes were censored at 6 months. The cumulative incidence of 6-month NRM was estimated according to the Gray method, and relapse and second allogeneic HCT were considered as competing risks. We used the Kaplan-Meier method and the log-rank test to estimate and compare overall survival probabilities. We compared categorical variables using the Fisher exact test and continuous variables using the Mann-Whitney U test. We used the area under the receiver operating characteristic curve (AUROC) analysis and the DeLong test to compare the prognostic value of the different models. The ΔAUROC and its corresponding 95% confidence intervals (CIs) were calculated using a 1000 bootstrap method.30 AUROC and negative predictive value (NPV) were used as the primary performance metric for comparisons. P values for NPV were calculated using the permutation test.31 Time-dependent ROC analysis was performed as previously described.32
We analyzed the data as follows. First, we temporally divided patients into a training cohort that underwent transplant from 2014 to 2020 and a validation cohort that underwent transplant from 2021 to 2023. We deliberately chose a more recent validation set to reflect the current practices of GVHD prophylaxis, such as increased use of posttransplant cyclophosphamide.33 We, then, categorized patients on D28 of treatment according to MCS criteria.14 We created a new category, MCS 0, for patients with a complete resolution of symptoms on D28 and low-risk biomarkers (AA1). Patients without symptoms but higher biomarker scores were assigned an MCS according to their 6-month NRM. Next, we used a classification and regression tree algorithm to create responder and nonresponder groups according to the incidence of 6-month NRM after treatment onset.34 The criteria to separate groups included a maximum depth of 2 levels with a complexity parameter of 0.1.34
All P values were two-tailed, with a threshold of <.05 indicating statistical significance. All statistical analyses were performed with R version 4.3.2 (R Foundation) and EZR version 1.63.35
Patients were prospectively monitored for acute GVHD symptoms for 100 days according to institutional review board–approved protocols. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Results
Patient characteristics
We divided 1135 patients who fulfilled all the inclusion criteria into a training cohort (n = 826) that underwent transplant from 2014 to 2020 and a validation cohort (n = 309) that underwent transplant from 2021 to 2023 (Table 1). Patients who died or relapsed before D28 of treatment (n = 6) were excluded from the training set used to develop the MCR but were included as nonresponders (n = 20) in the validation set. The population of the training set differed significantly from those in the validation set in that they were older, had a higher HCT comorbidity index, included more haploidentical and fewer cord blood donors, and used less ex vivo T-cell depletion, all of which reflect evolving trends in HCT. The median initial dose of prednisolone was approximately 1.2 mg/kg per day, and approximately 20% of patients received second-line treatment before D28 of treatment. Approximately 28% of patients with grade 1 GVHD received systemic treatment with at least 0.25 mg/kg prednisone in both cohorts. The median follow-up of survivors after treatment initiation was 23 and 17 months in the training and validation cohorts, respectively.
Patient characteristics (N = 1135)
| Recipient age . | Training (2014-2020) . | Validation (2021-2023) . | P values . |
|---|---|---|---|
| n = 826 . | n = 309 . | ||
| Median age at HCT (range), y | 54 (0-79) | 57 (0-77) | .021 |
| Recipient age, category (%) | |||
| <18 | 111 (13.4) | 44 (14.2) | .018 |
| 18-54 | 314 (38.0) | 90 (29.1) | |
| ≥55 | 401 (48.5) | 175 (56.6) | |
| Recipient sex (%) | |||
| Female | 346 (41.9) | 134 (43.4) | .703 |
| Male | 480 (58.1) | 175 (56.6) | |
| Sex mismatch (%) | |||
| Female to male | 129 (15.6) | 49 (15.9) | 1 |
| Other | 696 (84.4) | 260 (84.1) | |
| Race (%) | |||
| White | 691 (83.7) | 204 (66.0) | <.001 |
| Asian | 38 (4.6) | 13 (4.2) | |
| Black | 44 (5.3) | 11 (3.6) | |
| Others | 4 (0.5) | 4 (1.3) | |
| Unknown | 49 (5.9) | 77 (24.9) | |
| Primary disease (%) | |||
| Acute leukemia | 430 (52.1) | 156 (50.5) | .829 |
| MDS/MPN | 231 (28.0) | 95 (30.7) | |
| Malignant lymphoma | 73 (8.8) | 26 (8.4) | |
| Other | 92 (11.1) | 32 (10.4) | |
| HCT-CI (%) | |||
| 0-2 | 580 (70.2) | 183 (59.2) | .001 |
| ≥3 | 246 (29.8) | 126 (40.8) | |
| Conditioning (%) | |||
| MAC (TBI < 8 Gy) | 359 (43.5) | 132 (42.7) | .794 |
| MAC (TBI ≥ 8 Gy) | 144 (17.4) | 50 (16.2) | |
| RIC | 323 (39.1) | 127 (41.1) | |
| Donor type (%) | |||
| HLA matched related | 156 (18.9) | 48 (15.5) | .004 |
| HLA matched unrelated | 447 (54.1) | 188 (60.8) | |
| HLA mismatched related | 5 (0.6) | 1 (0.3) | |
| HLA mismatched unrelated donor | 94 (11.4) | 30 (9.7) | |
| Haploidentical | 82 (9.9) | 40 (12.9) | |
| Umbilical cord blood | 42 (5.1) | 2 (0.6) | |
| Donor source (%) | |||
| Bone marrow | 176 (21.3) | 35 (11.3) | <.001 |
| Peripheral blood | 608 (73.6) | 272 (88.0) | |
| Umbilical cord blood | 42 (5.1) | 2 (0.6) | |
| In vivo T-cell depletion (%) | |||
| No | 431 (52.2) | 165 (53.4) | .765 |
| Yes | 395 (47.8) | 144 (46.6) | |
| PTCy (%) | |||
| No | 691 (83.7) | 248 (80.3) | .208 |
| Yes | 135 (16.3) | 61 (19.7) | |
| Ex vivo T-cell depletion (%) | |||
| No | 803 (97.2) | 292 (94.5) | .042 |
| Yes | 23 (2.8) | 17 (5.5) | |
| GVHD grade at Tx | |||
| I | 229 (27.7) | 91 (29.4) | .384 |
| II | 414 (50.1) | 143 (46.3) | |
| III/IV | 153 (18.5) | 75 (24.3) | |
| MCS at Tx | |||
| MCS 1 | 487 (59.0) | 172 (55.7) | .059 |
| MCS 2 | 286 (34.6) | 104 (33.7) | |
| MCS 3 | 53 (6.4) | 33 (10.7) | |
| Second-line treatment before D28 | 159 (19.3) | 81 (26.2) | .011 |
| Initial corticosteroid dose, median prednisolone (range), mg/kg | 1.20 (0.5-4.0) | 1.27 (0.5-3.74) | .332 |
| Recipient age . | Training (2014-2020) . | Validation (2021-2023) . | P values . |
|---|---|---|---|
| n = 826 . | n = 309 . | ||
| Median age at HCT (range), y | 54 (0-79) | 57 (0-77) | .021 |
| Recipient age, category (%) | |||
| <18 | 111 (13.4) | 44 (14.2) | .018 |
| 18-54 | 314 (38.0) | 90 (29.1) | |
| ≥55 | 401 (48.5) | 175 (56.6) | |
| Recipient sex (%) | |||
| Female | 346 (41.9) | 134 (43.4) | .703 |
| Male | 480 (58.1) | 175 (56.6) | |
| Sex mismatch (%) | |||
| Female to male | 129 (15.6) | 49 (15.9) | 1 |
| Other | 696 (84.4) | 260 (84.1) | |
| Race (%) | |||
| White | 691 (83.7) | 204 (66.0) | <.001 |
| Asian | 38 (4.6) | 13 (4.2) | |
| Black | 44 (5.3) | 11 (3.6) | |
| Others | 4 (0.5) | 4 (1.3) | |
| Unknown | 49 (5.9) | 77 (24.9) | |
| Primary disease (%) | |||
| Acute leukemia | 430 (52.1) | 156 (50.5) | .829 |
| MDS/MPN | 231 (28.0) | 95 (30.7) | |
| Malignant lymphoma | 73 (8.8) | 26 (8.4) | |
| Other | 92 (11.1) | 32 (10.4) | |
| HCT-CI (%) | |||
| 0-2 | 580 (70.2) | 183 (59.2) | .001 |
| ≥3 | 246 (29.8) | 126 (40.8) | |
| Conditioning (%) | |||
| MAC (TBI < 8 Gy) | 359 (43.5) | 132 (42.7) | .794 |
| MAC (TBI ≥ 8 Gy) | 144 (17.4) | 50 (16.2) | |
| RIC | 323 (39.1) | 127 (41.1) | |
| Donor type (%) | |||
| HLA matched related | 156 (18.9) | 48 (15.5) | .004 |
| HLA matched unrelated | 447 (54.1) | 188 (60.8) | |
| HLA mismatched related | 5 (0.6) | 1 (0.3) | |
| HLA mismatched unrelated donor | 94 (11.4) | 30 (9.7) | |
| Haploidentical | 82 (9.9) | 40 (12.9) | |
| Umbilical cord blood | 42 (5.1) | 2 (0.6) | |
| Donor source (%) | |||
| Bone marrow | 176 (21.3) | 35 (11.3) | <.001 |
| Peripheral blood | 608 (73.6) | 272 (88.0) | |
| Umbilical cord blood | 42 (5.1) | 2 (0.6) | |
| In vivo T-cell depletion (%) | |||
| No | 431 (52.2) | 165 (53.4) | .765 |
| Yes | 395 (47.8) | 144 (46.6) | |
| PTCy (%) | |||
| No | 691 (83.7) | 248 (80.3) | .208 |
| Yes | 135 (16.3) | 61 (19.7) | |
| Ex vivo T-cell depletion (%) | |||
| No | 803 (97.2) | 292 (94.5) | .042 |
| Yes | 23 (2.8) | 17 (5.5) | |
| GVHD grade at Tx | |||
| I | 229 (27.7) | 91 (29.4) | .384 |
| II | 414 (50.1) | 143 (46.3) | |
| III/IV | 153 (18.5) | 75 (24.3) | |
| MCS at Tx | |||
| MCS 1 | 487 (59.0) | 172 (55.7) | .059 |
| MCS 2 | 286 (34.6) | 104 (33.7) | |
| MCS 3 | 53 (6.4) | 33 (10.7) | |
| Second-line treatment before D28 | 159 (19.3) | 81 (26.2) | .011 |
| Initial corticosteroid dose, median prednisolone (range), mg/kg | 1.20 (0.5-4.0) | 1.27 (0.5-3.74) | .332 |
HCT-CI, hematopoietic cell transplantation-specific comorbidity index; MAC, myeloablative conditioning; MDS/MPN, myelodysplastic syndromes/myeloproliferative neoplasms; PTCy, posttransplant cyclophosphamide; RIC, reduced intensity conditioning; TBI, total body irradiation; Tx, treatment.
MAGIC composite scores at D28
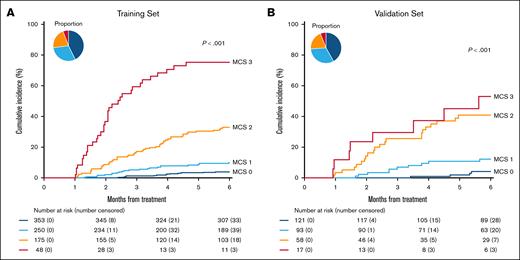
We categorized patients at D28 of treatment in the training set by both clinical severity and biomarker scores according to previous MCS criteria.14 Patients with complete resolution of their symptoms on D28 and who thus were not included in the original MCS groups were categorized as clinical grade 0 (n = 525, 63.6%) and divided into subgroups by D28 serum biomarker AA scores. We classified patients with clinical grade 0 and low-risk biomarkers, AA1, as MCS 0 to identify patients who have both resolved all clinical symptoms and possess low biomarker scores at the time of response assessment. MCS 0 patients experienced very low 6-month NRM (3.7%). Patients with clinical grade 0 and intermediate-risk biomarkers, AA2, had higher NRM (13.3%) and were classified as MCS 1; patients with clinical grade 0 and high-risk biomarkers, AA3, experienced yet higher NRM (28.8%) and were classified as MCS 2 (Table 2). The cumulative incidences of NRM of these 4 groups are found in Figure 1. Active GVHD caused a very small percentage of deaths in patients with MCS 0 and MCS 1 but most deaths in patients with MCS 2 and MCS 3 (supplemental Table 2).
Development of MCR
| Training set . | |||
|---|---|---|---|
| At D28 . | 6-Month NRM (95% CI) . | MCR∗ . | n (%) . |
| Grade 0 + AA1 | 3.7% (2.1-6.0) | Response | 353 (42.7) |
| Grade 0 + AA2 | 13.3% (8.0-20.1) | Response | 120 (14.5) |
| Grade 0 + AA3 | 28.8% (17.2-41.6) | Nonresponse | 52 (6.3) |
| MCS 1 | 4.6% (1.9-9.2) | Response | 130 (15.7) |
| MCS 2 | 31.7% (23.7-40.0) | Nonresponse | 123 (14.9) |
| MCS 3 | 70.8% (55.4-81.8) | Nonresponse | 48 (5.8) |
| Validation set | |||
| At D28 | 6-Month NRM (95% CI) | MCR∗ | n (%) |
| Grade 0 + AA1 | 3.6% (1.2-8.3) | Response | 121 (39.2) |
| Grade 0 + AA2 | 18.5% (7.3-33.6) | Response | 34 (11.0) |
| Grade 0 + AA3 | 35.4% (12.0-60.1) | Nonresponse | 15 (4.9) |
| MCS 1 | 7.2% (2.3-16.0) | Response | 59 (19.1) |
| MCS 2 | 41.8% (26.3-56.5) | Nonresponse | 43 (14.9) |
| MCS 3 | 49.0% (22.6-71.1) | Nonresponse | 17 (5.9) |
| Training set . | |||
|---|---|---|---|
| At D28 . | 6-Month NRM (95% CI) . | MCR∗ . | n (%) . |
| Grade 0 + AA1 | 3.7% (2.1-6.0) | Response | 353 (42.7) |
| Grade 0 + AA2 | 13.3% (8.0-20.1) | Response | 120 (14.5) |
| Grade 0 + AA3 | 28.8% (17.2-41.6) | Nonresponse | 52 (6.3) |
| MCS 1 | 4.6% (1.9-9.2) | Response | 130 (15.7) |
| MCS 2 | 31.7% (23.7-40.0) | Nonresponse | 123 (14.9) |
| MCS 3 | 70.8% (55.4-81.8) | Nonresponse | 48 (5.8) |
| Validation set | |||
| At D28 | 6-Month NRM (95% CI) | MCR∗ | n (%) |
| Grade 0 + AA1 | 3.6% (1.2-8.3) | Response | 121 (39.2) |
| Grade 0 + AA2 | 18.5% (7.3-33.6) | Response | 34 (11.0) |
| Grade 0 + AA3 | 35.4% (12.0-60.1) | Nonresponse | 15 (4.9) |
| MCS 1 | 7.2% (2.3-16.0) | Response | 59 (19.1) |
| MCS 2 | 41.8% (26.3-56.5) | Nonresponse | 43 (14.9) |
| MCS 3 | 49.0% (22.6-71.1) | Nonresponse | 17 (5.9) |
Categorized by classification and regression tree analyses based on 6-month NRM.
Cumulative incidence of NRM at 6 months by MCS at D28. (A) Training set: MCS 0 (dark blue): 3.7% (95% CI, 2.1%-6.0%), MCS 1 (light blue): 8.8% (95% CI, 5.7%-12.7%), MCS 2 (orange): 30.9% (95% CI, 24.2%-37.8%), MCS 3 (red): 70.8% (95% CI, 55.4%-81.8%). (B) Validation set: MCS 0: 3.6% (95% CI, 1.2%-8.3%), MCS 1: 11.3% (95% CI, 5.7%-18.9%), MCS 2: 40.1% (95% CI, 27.0%-52.9%), MCS 3: 49.0% (95% CI, 22.6%-71.1%).
Cumulative incidence of NRM at 6 months by MCS at D28. (A) Training set: MCS 0 (dark blue): 3.7% (95% CI, 2.1%-6.0%), MCS 1 (light blue): 8.8% (95% CI, 5.7%-12.7%), MCS 2 (orange): 30.9% (95% CI, 24.2%-37.8%), MCS 3 (red): 70.8% (95% CI, 55.4%-81.8%). (B) Validation set: MCS 0: 3.6% (95% CI, 1.2%-8.3%), MCS 1: 11.3% (95% CI, 5.7%-18.9%), MCS 2: 40.1% (95% CI, 27.0%-52.9%), MCS 3: 49.0% (95% CI, 22.6%-71.1%).
MCR
When we applied the classification and regression tree algorithm to these 4 groups to generate MCR, it categorized them as responders or nonresponders according to 6-month NRM (Table 2). Both MCS 0 (highly favorable) and MCS 1 (favorable) groups were categorized as responders in the training and validation sets with NRM of ∼5% to 15%, whereas MCS 2 and MCS 3 (unfavorable) experienced high NRM of >30% (Table 2). MCS 2 and MCS 3 patients were combined because of their poor outcomes and the very small number of patients in the MCS 3 group.
The MCR algorithm identified 2 important subgroups whose 6-month NRM did not completely align with a change in clinical symptoms. In the first group, patients with complete resolution of their symptoms (grade 0) and high biomarkers (AA3) at D28 of treatment, classified as MCS 2, experienced a 6-month NRM of 28.8% and were classified as nonresponders. In the second group, patients with persistence of mild symptoms (see subsequent discussion) and who were categorized as MCS 1 after D28 of treatment experienced low 6-month NRM (5%-7%) and were therefore classified as responders (Table 2).
Comparison of MCR to clinical response only
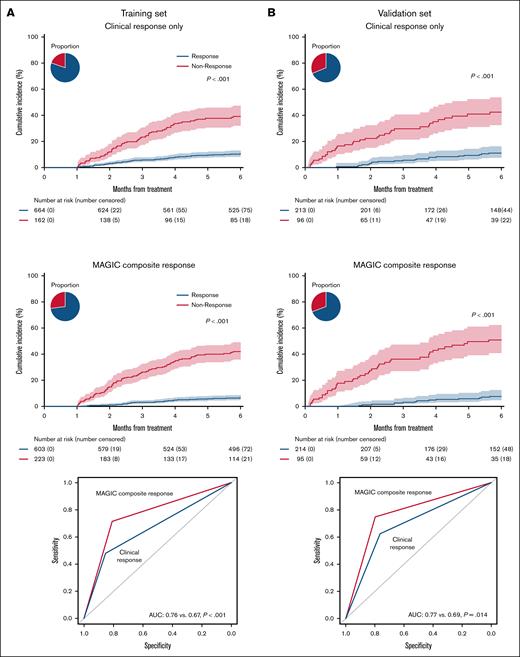
We, then, compared the 6-month NRM of responders and nonresponders predicted by the MCR algorithm to that predicted by the CRO system. The proportion of patients categorized as responders in both systems was similar (68.9% vs 69.3%). Compared with CRO, MCR produced a greater separation in 6-month NRM between groups in both the training set (33.7% vs 26.7%) and the validation set (38.9% vs 27.5%) (Figure 2, left and middle panels). This improvement resulted in a significantly greater AUROC in both the training set (0.76 vs 0.67, P < .001) and the validation set (0.77 vs 0.69, P = .014) (Figure 2, right panels). Importantly, MCR also significantly increased the NPV, which in these systems corresponds to survival (Table 3). We did not include initiation of second-line treatment in the initial calculation of either CRO or MCR because subjective considerations unrelated to treatment response are common in observational studies where treatment parameters are not clearly prespecified. In the training set, 144 of 159 patients (91%) had at least grade 2 GVHD when they received second-line therapy before D28. When initiation of second-line therapy was considered a nonresponse, as is customary in clinical trials, MCR remained superior to CRO in predicting long-term outcomes (Table 4). There was no difference in relapse between response groups according to either system, and thus MCR produced a larger separation in overall survival between groups (supplemental Figures 1 and 2).
Cumulative incidence of NRM at 6 months based on clinical and MAGIC response criteria in the training and validation sets. (A) Training set. Left: clinical responders: 9.7% (95% CI, 7.6%-12.0%), clinical nonresponders: 36.4% (95% CI, 29.0%-43.8%). Middle: MAGIC composite responders: 5.8% (95% CI, 4.1%-7.9%), MAGIC composite nonresponders: 39.5% (95% CI, 33.0%-45.8%). Right: area under the curve (AUC) by receiver operating characteristic for 6-month NRM. (B) Validation set. Left: clinical responders: 10.3% (95% CI, 6.6%-15.0%), clinical nonresponders: 37.7% (95% CI, 27.8%-47.6%). Middle: MAGIC composite responders: 6.9% (95% CI, 4.0%-11.0%), MAGIC composite nonresponders: 45.8% (95% CI, 35.3%-55.8%). Right: AUC by receiver operating characteristic for 6-month NRM. Pie charts depict percentage of responders/nonresponders.
Cumulative incidence of NRM at 6 months based on clinical and MAGIC response criteria in the training and validation sets. (A) Training set. Left: clinical responders: 9.7% (95% CI, 7.6%-12.0%), clinical nonresponders: 36.4% (95% CI, 29.0%-43.8%). Middle: MAGIC composite responders: 5.8% (95% CI, 4.1%-7.9%), MAGIC composite nonresponders: 39.5% (95% CI, 33.0%-45.8%). Right: area under the curve (AUC) by receiver operating characteristic for 6-month NRM. (B) Validation set. Left: clinical responders: 10.3% (95% CI, 6.6%-15.0%), clinical nonresponders: 37.7% (95% CI, 27.8%-47.6%). Middle: MAGIC composite responders: 6.9% (95% CI, 4.0%-11.0%), MAGIC composite nonresponders: 45.8% (95% CI, 35.3%-55.8%). Right: AUC by receiver operating characteristic for 6-month NRM. Pie charts depict percentage of responders/nonresponders.
Predictive performances for 6-month NRM
| Response criteria . | Response rates . | Sensitivity . | Specificity . | Balanced accuracy∗ . | PPV . | NPV . | P values for NPV . | AUC . | P values for AUC . | ΔAUC (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Training set | ||||||||||
| Clinical response | 80.4% | 48.0% | 85.3% | 66.7% | 36.4% | 90.3% | <.001 | 0.67 | <.001 | 0.096 (0.053-0.138) |
| MCR | 73.1% | 71.5% | 80.8% | 76.2% | 39.5% | 94.2% | 0.76 | |||
| Validation set | ||||||||||
| Clinical response | 68.9% | 61.9% | 76.4% | 69.2% | 37.7% | 89.7% | .017 | 0.69 | .014 | 0.078 (0.014-0.143) |
| MCR | 69.3% | 74.4% | 79.5% | 77.0% | 45.8% | 93.1% | 0.77 |
| Response criteria . | Response rates . | Sensitivity . | Specificity . | Balanced accuracy∗ . | PPV . | NPV . | P values for NPV . | AUC . | P values for AUC . | ΔAUC (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Training set | ||||||||||
| Clinical response | 80.4% | 48.0% | 85.3% | 66.7% | 36.4% | 90.3% | <.001 | 0.67 | <.001 | 0.096 (0.053-0.138) |
| MCR | 73.1% | 71.5% | 80.8% | 76.2% | 39.5% | 94.2% | 0.76 | |||
| Validation set | ||||||||||
| Clinical response | 68.9% | 61.9% | 76.4% | 69.2% | 37.7% | 89.7% | .017 | 0.69 | .014 | 0.078 (0.014-0.143) |
| MCR | 69.3% | 74.4% | 79.5% | 77.0% | 45.8% | 93.1% | 0.77 |
AUC, area under the curve; PPV, positive predictive value.
Balanced accuracy is the average of sensitivity and specificity, calculated as their sum divided by 2.
Predictive performances for 6-month NRM treating second line as a nonresponse
| Response criteria . | Response rates . | Sensitivity . | Specificity . | Balanced accuracy∗ . | PPV . | NPV . | P values for NPV . | AUC . | P values for AUC . | ΔAUC (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Training set† | ||||||||||
| Overall response | 73.2% | 57.7% | 78.6% | 68.2% | 32.1% | 91.4% | .001 | 0.68 | <.001 | 0.065 (0.028-0.105) |
| MCR | 68.0% | 74.0% | 75.4% | 74.7% | 34.5% | 94.3% | 0.74 | |||
| Validation set‡ | ||||||||||
| Overall response | 58.3% | 71.0% | 62.8% | 66.9% | 31.8% | 90.6% | .095 | 0.69 | .056 | 0.054 (−0.001 to 0.102) |
| MCR | 59.2% | 77.9% | 69.1% | 73.5% | 36.1% | 93.0% | 0.74 |
| Response criteria . | Response rates . | Sensitivity . | Specificity . | Balanced accuracy∗ . | PPV . | NPV . | P values for NPV . | AUC . | P values for AUC . | ΔAUC (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Training set† | ||||||||||
| Overall response | 73.2% | 57.7% | 78.6% | 68.2% | 32.1% | 91.4% | .001 | 0.68 | <.001 | 0.065 (0.028-0.105) |
| MCR | 68.0% | 74.0% | 75.4% | 74.7% | 34.5% | 94.3% | 0.74 | |||
| Validation set‡ | ||||||||||
| Overall response | 58.3% | 71.0% | 62.8% | 66.9% | 31.8% | 90.6% | .095 | 0.69 | .056 | 0.054 (−0.001 to 0.102) |
| MCR | 59.2% | 77.9% | 69.1% | 73.5% | 36.1% | 93.0% | 0.74 |
AUC, area under the curve; PPV, positive predictive value.
Balanced accuracy is the average of sensitivity and specificity, calculated as their sum divided by 2.
In the training set, 59 (7.1%) and 41 (5.0%) were reclassified by the second-line treatment when using clinical response and MCR, respectively.
In the validation set, 33 (10.7%) and 31 (10.0%) were reclassified by the second-line treatment when using clinical response and MCR, respectively.
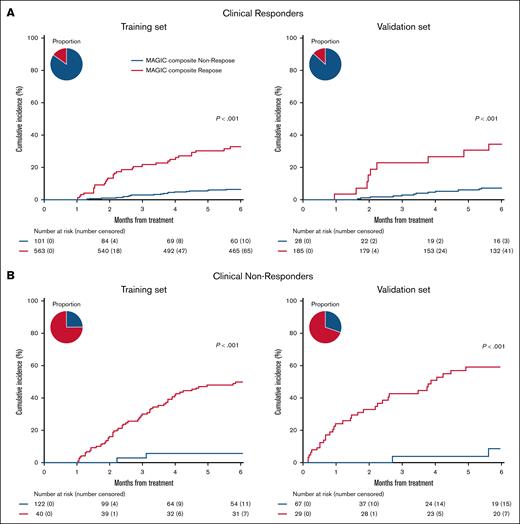
To understand why the MCR criteria predicted long-term NRM better than the CRO criteria, we evaluated the reclassification of CRO groups by MCR. In both data sets, the MCR criteria identified 15% of CRO responders with a fivefold greater incidence of NRM compared with nonresponders and who were therefore classified as MCR nonresponders (training: 30.7% vs 5.9%, P < .001; validation: 34.3% vs 6.8%, P < .001) (Figure 3A). In the validation set, 26 of these 28 reclassified patients (93%) had a high MAP at D28 of treatment (supplemental Table 3). Conversely, MCR identified nearly one-third of CRO nonresponders with sevenfold less NRM and who were therefore classified as MCR responders (training: 5.0% vs 46.7%, P < .001; validation: 7.6% vs 50.7%, P < .001). All 29 of these reclassified patients in the validation set had a low MAP at D28 of treatment (supplemental Table 4). Further analyses revealed that most MCS 1 patients (80%-85%) who were nonresponders by clinical criteria had unresolved mild skin and/or upper GI symptoms (supplemental Table 5). These observations help to explain how MCR improves sensitivity with minimal loss of specificity in predicting 6-month NRM (Figure 2) and highlight the utility of biomarkers in the improvement of long-term GVHD control. A time-dependent analysis of AUROC for NRM at time points between 2 and 12 months confirmed the consistent superiority of MCR criteria at all time points (supplemental Figure 3). The difference between groups of failure-free survival, defined as survival without subsequent lines of systemic therapy, recurrent malignancy, or death, was also greater based on MCR than CRO (supplemental Figure 4).
NRM of MCR reclassification of clinical responders and nonresponders. The 6-month cumulative incidence of NRM by clinical response reclassified by MCR criteria. (A) Clinical responders reclassified by MCR. Left: training set MAGIC composite responders: 5.9% (95% CI, 4.1%-8.0%), MAGIC composite nonresponders: 30.7% (CI, 22.0%-39.8%). Right: validation set MAGIC composite responders: 6.8% (95% CI, 3.7%-11.2%), MAGIC composite nonresponders: 34.3% (95% CI, 16.9%-52.6%). (B) Clinical nonresponders reclassified by MCR. Left: training set MAGIC composite responders: 5.0% (95% CI, 0.9%-15.0%), MAGIC composite nonresponders: 46.7% (95% CI, 37.6%-55.3%). Right: validation set MAGIC composite responders: 7.6% (95% CI, 1.3%-22.0%), MAGIC composite nonresponders: 50.7% (95% CI, 37.8%-62.2%). Pie charts depict percentage of responders/nonresponders.
NRM of MCR reclassification of clinical responders and nonresponders. The 6-month cumulative incidence of NRM by clinical response reclassified by MCR criteria. (A) Clinical responders reclassified by MCR. Left: training set MAGIC composite responders: 5.9% (95% CI, 4.1%-8.0%), MAGIC composite nonresponders: 30.7% (CI, 22.0%-39.8%). Right: validation set MAGIC composite responders: 6.8% (95% CI, 3.7%-11.2%), MAGIC composite nonresponders: 34.3% (95% CI, 16.9%-52.6%). (B) Clinical nonresponders reclassified by MCR. Left: training set MAGIC composite responders: 5.0% (95% CI, 0.9%-15.0%), MAGIC composite nonresponders: 46.7% (95% CI, 37.6%-55.3%). Right: validation set MAGIC composite responders: 7.6% (95% CI, 1.3%-22.0%), MAGIC composite nonresponders: 50.7% (95% CI, 37.8%-62.2%). Pie charts depict percentage of responders/nonresponders.
We used the entire data set to also compare MCR with CRO in several subsets that mirror potential clinical trial designs for clinically significant GVHD where a strong correlation between early response and long-term outcomes is crucial to the assessment of the intervention. Because biomarker values are not always known at the onset of treatment, we analyzed 3 common treatment scenarios in which biomarkers were not included as inclusion criteria at the initiation of treatment: grades 2 to 4 GVHD, grade 3 of 4 GVHD, and Minnesota high-risk GVHD. MCR correctly identified more nonresponders compared with CRO in all 3 scenarios resulting in significantly improved correlation between response and survival as evidenced by significantly higher NPVs (supplemental Table 6). Thus, inclusion of biomarkers at D28 significantly improved the ability of the response metric to predict long-term outcomes of GVHD even when biomarkers were not available or known at the onset of treatment. As expected, analysis of patients with MCS 2/3 GVHD also revealed statistically significant improvement in AUROC and NPV (supplemental Table 6).
Previously, we have revealed that MAP biomarkers on D28 better predicted 6-month NRM than CRO.28 Comparison of MCR status to the MAP at D28 revealed that MCR was superior in both key metrics of NPV and area under the curve (supplemental Table 7). This finding confirmed the superiority of a composite metric that includes both clinical and biomarker values in predicting long-term outcomes.
Discussion
The current standard for response in clinical trials of acute GVHD, the reduction of GVHD clinical symptoms on D28 of treatment, modestly correlates with long-term outcomes such as 6-month NRM. We evaluated whether MCR, which integrates serum biomarkers with clinical symptoms on D28, could redefine responses to improve predictive accuracy as has recently been found with MCS at the initiation of treatment.14 The MCR system was superior to the change in clinical symptoms alone in predicting 6-month outcomes as determined by key metrics such as AUROC and NPV even when biomarkers were available only on D28 and not at the beginning of treatment. Importantly, the MCR system includes patients as responders with low biomarkers and mild, but stable symptoms, who have favorable outcomes, even though they would be classified as nonresponders by clinical response criteria alone. Thus, a composite status integrating clinical symptoms and biomarkers after D28 of treatment better predicts long-term outcomes than conventional criteria that rely only on changes in clinical symptom severity.
This study suggests that the end points of clinical trials of GVHD treatment can be improved. Severe acute GVHD can be lethal, and the severity of clinical symptoms at onset does not correlate well with treatment response or eventual mortality.13,14 Recent advances that include the use of serum biomarkers can define groups at the initiation of treatment with very different risks of treatment response and NRM.14 But, currently, we use the same definition of response as the end point for both high-risk and low-risk GVHD, despite the differing treatment goals for each group. In high-risk trials,36 the primary objective is to increase the response rate, with the expectation that this will lead to reduced mortality. Thus, the end point for trials treating high-risk disease should measure a response that more accurately correlates with survival. However, the current definitions of response include changes in clinical symptoms that do not differentiate survival outcomes from nonresponding patients (eg, patients whose lower GI GVHD improves from stage IV to II have similarly high NRM as patients whose lower GI GVHD remains in stage IV).37 The MCR with its integration of symptoms and serum biomarkers predicts survival of responders (as measured by NPV) better than CRO and could be useful in such trials. In contrast, treatment of patients with low-risk disease, who have low mortality and high survival rates, should minimize treatment toxicity but maintain a high response rate. The response end point for low-risk trials38 could include reduction in toxicities such as severe infections39,40 or repeat hospitalizations provided these responses are associated with favorable long-term outcomes (eg, survival). Favorable biomarkers in patients with mild, stable disease could support their inclusion as “responders” if they meet the other salient end point criteria.
This retrospective study has several strengths. First, the analysis was performed using a large data set that enabled the development of the system in a training cohort and its validation in a more recent cohort, which better reflects current practice. Second, the observational data derive from 2 dozen HCT centers, which represent real-world evidence and increase the likelihood of their broad applicability. Third, the data and samples were collected prospectively and evaluated using a rigorous PRoBE study design.21,22 Fourth, serum biomarkers were evaluated in a single central laboratory with rigorous quality controls, ensuring accuracy and consistency.
This study also has limitations. First, although the data were prospectively collected using a PRoBE design, such real-world evidence admits to greater heterogeneity of the patient populations than when they are subjected to inclusion and exclusion criteria. Second, the MCR model was based on observational data that do not specify which changes in therapy qualify as a nonresponse to treatment. Third, the availability of newly approved second-line therapies could also influence treatment decisions in clinical trials. Thus, to be useful as a trial end point, MCR status should be formally validated with data from clinical trial patients who were subject to clear inclusion and exclusion criteria and prespecified treatment plans. Third, the existence of differences in baseline characteristics between training and validation sets might have biased the generation of the model, again underscoring the need to validate it using clinical trial data.
This study demonstrates that the combined clinical/biomarker status of a patient after D28 of treatment better predicts long-term outcomes than a change in clinical symptoms. This superiority depends in large part on the reclassification of patients as responders whose initially mild symptoms persisted for 4 weeks but whose biomarkers were low at D28. This change addresses a longstanding anomaly in GVHD clinical trials regarding nonresponding patients who nevertheless have very favorable long-term outcomes. But it must be acknowledged that a more accurate prediction of long-term outcomes at D28 is not especially useful in guiding therapeutic decisions which usually occur much earlier. Future studies should determine whether such a composite of clinical symptoms and biomarkers at earlier time points, such as after 1 or 2 weeks of treatment, can also robustly predict long-term outcomes41; if so, clinical trials of GVHD could reduce the length of time when patients are subjected to experimental therapies and allow for earlier initiation of next-line therapies.
Acknowledgments
The authors greatly appreciate the patients, their families, medical staff, and data managers in the MAGIC centers.
Y.A. is a recipient of the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad program. This work was supported by National Institutes of Health, National Cancer Institute grants P01 CA039542 and P30 CA196521, the Pediatric Cancer Foundation, the Gillespie and Solomon-Gillespie GVHD Research Fund, and German Jose Carreras Leukemia Foundation grants DJCLS 01 GVHD 2016 and DJCLS 01 GVHD 2020.
Authorship
Contribution: Y.A. designed the study, collected the clinical data, conducted the statistical analysis, and wrote the manuscript; J.P. collected the clinical data and performed statistical analysis; N.K., I.E.L., and N.S. collected the clinical data, advised statistical methods, and reviewed and revised the manuscript; P.A.-H., F.A., C.C., H.K.C., M.E., A.M.E., E.O.H., C.L.K., S. Kraus, P.M., T.O., I.P., M.Q., R.R., T.S., J.M., E.U., I.V., D.W., M.W., R.Z., Z.D., W.J.H., and R.N. collected the clinical data and reviewed and revised the manuscript; J.B., G.E., S.G., and R.Y. collected and reviewed the clinical data; S. Kowalyk and G.M. performed the laboratory analysis; J.E.L. and J.L.M.F. designed the study, interpreted the data, advised the methods, reviewed and revised the manuscript, and organized this project; and all authors contributed to the writing of the report and approved the final version of the article.
Conflict of interest disclosure: Y.A. reports honoraria from Novartis, AstraZeneca, and Takeda. P.A.-H. reports consulting or advisory fees from Incyte. F.A. reports honoraria from Kite/Gilead, Novartis, Bristol Myers Squibb, Takeda, Medac, Miltenyi Biomedicine, AbbVie, Mallinckrodt/Therakos, and Janssen; and research funding from Mallinckrodt/Therakos and Neovii. H.K.C. reports consulting or advisory fees from Actinium, Incyte, Ironwood, Orca Bio, RegImmune, and Sanofi; research funding from GlaxoSmithKline and Incyte; and honoraria from MJH Holding, Physician Education Resource, and Plexus Communications. E.O.H. reports advisory, committee, or consulting roles for AbbVie, Disc Medicine, PharmaEssentia, Blueprint Medicines, Cabaletta Bio, and the American Board of Internal Medicine; and research funding from AbbVie, Blueprint Medicines, Disc Medicine, Kartos Therapeutics, and Kite Pharma. C.L.K. reports honoraria from Sanofi, Incyte, and Mesoblast; and consulting fees from CSL Behring, Alexion, and Physician Education Resource. P.M. reports consulting fee from Miltenyi, Amgen, and Pierre Fabre. T.O. reports consulting agreements with bluebird bio, Pfizer, Medexus, Alexion, and Elixirgen Therapeutics. R.R. reports consulting or advisory role with Allogene, Gilead Sciences, Bayer, Incyte, TScan, Orca Bio, Pierre Fabre Pharmaceuticals, CareDx, Quell Biotherapeutics, Sana Biotechnology, Sail Biomedicines, and Autolus; and research funding from Atara Biotherapeutics, Incyte, Sanofi, Immatics, AbbVie, Takeda, Gilead Sciences, CareDx, TScan, Cabaletta, Synthekine, Bristol Myers Squibb, Johnson & Johnson, Allogene, Genentech, Vittoria Therapeutics, AstraZeneca, and Imugene. Z.D. reports research support from Incyte, Corp, RegImmune, Corp, Taiho Oncology, Inc, and Kura Oncology, Inc; and consulting fees from Sanofi, Incyte, Corp, Inhibrx, RegImmune, Corp, MaaT Pharma, Forte Biosciences Inc, Medexus Pharmaceuticals, Inc, and Mesoblast Ltd. R.N. reports consultant feeds from Sanofi, MaaT Pharma, and Ono Pharmaceutical; and research support from Miyarisan. J.E.L. reports research support from Equillium, Incyte, MaaT Pharma, and Mesoblast; and consulting fees from Editas, Equillium, Kamada, Mesoblast, Sanofi, bluebird bio, Inhibrx, and X4 Pharmaceuticals. J.L.M.F. reports research support from Equillium, Incyte, MaaT Pharma, and Mesoblast; and consulting fees from Editas, Equillium, Kamada, Mesoblast, Alexion, Realta, Medpace, Viracor, AlloVir, and Physician Education Resource. The remaining authors declare no competing financial interests.
Correspondence: John E. Levine, The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, Box 1410, New York, NY 10029; email: john.levine@mssm.edu; and James L. M. Ferrara, The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, New York, NY 10029; email: james.ferrara@mssm.edu.
References
Author notes
J.E.L. and J.L.M.F. contributed equally to this study.
Data are available upon reasonable request from the corresponding authors, John E. Levine (john.levine@mssm.edu) and James L. M. Ferrara (james.ferrara@mssm.edu), or the author, Yu Akahoshi (akahoshiu@gmail.com).
The full-text version of this article contains a data supplement.