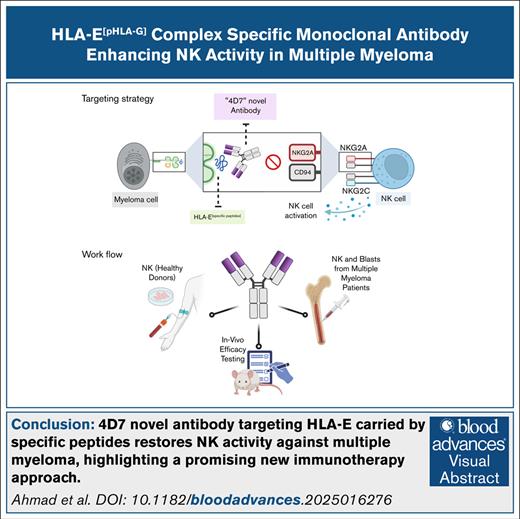
Key Points
A novel antibody selectively blocks the HLA-E[pHLA-G]:NKG2A IC, enhancing NK cell attack on MM while sparing healthy cells.
This targeted approach reduces tumor growth in vivo, and may minimize toxicity compared with current NKG2A inhibitors.
Visual Abstract
HLA-E presenting the HLA-G leader peptide VMAPRTLFL (HLA-E[pHLA-G]) on tumor cells plays a crucial role in suppressing natural killer (NK) and cytotoxic CD8+ T cells through NKG2A interaction. While blocking HLA-E:NKG2A is a promising immune checkpoint (IC) approach in cancer therapy, toxicity remains a major clinical concern. We developed a novel IC inhibitor that selectively prevents HLA-E:NKG2A interaction, a monoclonal antibody that selectively targets the HLA-E[pHLA-G] complex, distinguishing cancerous from noncancerous cells. In clinical bone marrow samples from patients with multiple myeloma (MM), 4D7 specifically recognized tumor-associated HLA-E–peptide complexes. Using NK cells from healthy donors, 4D7 effectively blocked the HLA-E:NKG2A interaction, and enhanced NKG2A-positive NK cell activity in autologous MM cell cocultures. Importantly, 4D7 did not inhibit NKG2C-positive NK cells, preserving their activity, even though NKG2C also interacts with HLA-E. In MM-bearing mice treated with human NK cells, 4D7 significantly reduced tumor growth. This targeted approach activates NK cells only against tumor cells presenting HLA-E–peptide complexes, potentially minimizing toxicity compared with current NKG2A inhibitors. The development of 4D7 highlights a promising advancement in immunotherapy for hematologic malignancies, offering improved outcomes for patients with MM, and a foundation for broader application across cancer types.
Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by the uncontrolled proliferation of malignant plasma cells in the bone marrow (BM).1 Despite significant advancements in therapy, MM remains largely incurable, with most patients eventually experiencing relapse and developing treatment resistance.2 This underscores the urgent need for novel therapeutic strategies. Natural killer (NK) cells have emerged as a promising approach due to their inherent ability to eliminate tumor cells without prior sensitization.3 However, the efficacy of NK cell-based therapies in MM is often hampered by tumor-mediated immune escape mechanisms. It is important to note that the significance of the HLA-E:HLA-G axis in inhibiting NK activity through NKG2A, which is crucial to the work described here, also plays a vital role in pregnancy by modulating NK cell activity and contributing to maternal-fetal tolerance.4,5
One such mechanism involves the HLA-E:NKG2A immune checkpoint (IC). HLA-E, a nonclassical major histocompatibility complex class Ib molecule, is frequently upregulated by MM cells.6-10 This upregulation enables MM cells to interact with NKG2A, an inhibitory receptor expressed in NK cells, leading to NK cell suppression and immune evasion.8 NKG2A is expressed on NK cells and a subset of CD8+ T cells, recognizing HLA-E loaded with peptides derived from the leader sequences of other HLA molecules.6,11,12 The peptide VMAPRTLFL, derived from HLA-G, forms a complex with HLA-E (HLA-E[pHLA-G]) that is considered the strongest natural ligand for the inhibitory NKG2A/CD94 receptor.12-14
Several tumors exploit the HLA-E:NKG2A IC to evade immune surveillance and promote progression.15,16 In patients with MM, overexpression of HLA-E correlates with unfavorable treatment outcomes.17-19 Recent clinical trials have shown promise for the therapeutic anti-NKG2A antibody (monalizumab) in head and neck squamous cell carcinoma and non–small cell lung cancer, highlighting the potential of targeting this axis.20,21
While monoclonal antibodies (mAbs) specific to major histocompatibility complex class I and explicit peptides have been reported, no research has focused on developing antibodies that can bind to the human HLA-E molecule in complex with a specific peptide.22 Currently, there are no mAbs that specifically target HLA-E–peptide complexes interacting with NKG2A.23 Existing antibodies, such as 3D12 and 4D12, block the HLA-E molecule, but are not designed to specifically inhibit the HLA-E:NKG2A interaction.23,24
In this study, we hypothesized that a mAb specifically targeting the HLA-E:VMAPRTLFL complex could effectively block the HLA-E:NKG2A inhibitory axis and enhance NK cell activity against MM cells. We developed a novel mAb that specifically blocks HLA-E in complex with VMAPRTLFL, and evaluated its efficacy in promoting the activation of NK cells isolated from patients with MM. This strategy aims to enhance the clearance of circulating tumor cells that evade NK cell recognition via the HLA-E:NKG2A IC, offering potential applications in treating various NK cell-sensitive cancers. Our findings may pave the way for a new class of IC inhibitors, providing a promising avenue for improving outcomes in MM and potentially other hematologic malignancies. This version integrates our objectives smoothly into the narrative while maintaining clarity and focus on our research’s significance.
Materials and methods
Cell lines and culture conditions
Wild-type 721.221-(ATCC CVCL_6263), 721.221 transfectants expressing HLA-E and HLA-G, RPMI 8226-(ATCC CRM-CCL-155), U266-(ATCC TIB-196), and U937-(ATCC CRL-1593.2) were cultured in RPMI-1640 (Gibco). SP2/0-Ag14-(ATCC CRL-1581) was cultured in Dulbecco’s modified Eagle medium (Gibco). Media were supplemented with 10% fetal bovine serum, 1% L-glutamine, Pen-Strep, minimum essential medium-Eagle, sodium pyruvate, and HEPES (N-2-hydroxyethylpiperazine-Nʹ-2-ethanesulfonic acid).
Generation of hybridoma cells and mAb purification
Four-week-old BALB/c mice were immunized subcutaneously with 15 μg of SCT-8343 in Complete Freund's Adjuvant, followed by boosts in incomplete Freund adjuvant on days 14, 35, 52, and 101. On day 112, an incomplete Freund adjuvant booster was administered, and sera were tested by enzyme-linked immunosorbent assay (ELISA). Splenocytes from high-titer mice were fused with SP2/0-Ag14 cells, cultured in hypoxanthine-aminopterin-thymidine medium, and screened by ELISA. The 4D7-B6 hybridoma was selected, expanded in hypoxanthine-thymidin medium, and purified using HiTrap Protein G columns.
ELISA assay for mAb 4D7 antigen binding
Ninety-six–well plates were coated with 2 μg/mL of SCTs (SCT-8341, SCT-8343, SCT-8345) in Na2HPO4 buffer, pH 9. After blocking, plates were incubated with 2 μg/mL 4D7 or immunoglobulin G (IgG)1κ control, followed by peroxidase-conjugated anti-mouse IgG and 3,3',5,5'-tetramethylbenzidine detection.
Flow cytometry
Cells were detached with Versene, counted, and incubated on ice with 4D7 or control antibodies, followed by allophycocyanin (APC) goat anti-mouse IgG. Bone marrow–derived mast cells (BMMCs) were stained with APC-4D7, BV785 anti-CD138, and fluorescent dye R-phycoerythrin anti-CD38. Data were acquired on a Beckman Coulter CytoFLEX.
HLA-E peptide stripping
Cells (0.5 × 106) were washed, incubated in citric acid buffer (pH 5) for 1.5 minutes on ice, washed, and treated with peptides (20 μg/mL) in RPMI-1640 with 10% dimethyl sulfoxide for 1 hour at 37°C. Recognition by 4D7 was tested by flow cytometry.
Molecular docking
Erep and Eatt represent the repulsive and attractive van der Waals energies, Eelec is the electrostatic term, and EDARS is a structure-based potential derived from the decoys as reference state (DARS) method.
Kinetic analysis of the mAb by surface plasmon resonance
SCT-8343 was immobilized on a general layer compact chip (Bio-Rad) using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide coupling. 4D7 kinetics were analyzed on a ProteOn XPR36 with 5 concentrations (78-4.875 μM), followed by a 600 second dissociation step. Data were analyzed using ProteOn Manager 3.01.
Isolation and culture of primary human NK cells
Primary NK cells were enriched using RosetteSep and cultured in CellGenix SCGM with 10% AB plasma and 300 IU/mL interleukin-2. NK cells were cocultured with target cells (150 000 per well) and 10 μg/mL 4D7 for 4 hours. Degranulation was assessed by CD107a staining and fluorescence-activated cell sorting.
Isolation of BMMCs from patients with MM
Mononuclear cells from the BM of patients with MM, containing primary myeloma cells, were separated using a Ficoll-Hypaque density gradient as follows: heparinized BM aspirates, obtained as part of routine diagnosis, were mixed in a 1:1 (v/v) ratio with complete 2% EDTA RPMI-1640 washing medium. The mixture was filtered through a 70 μm nylon tissue strainer to eliminate potential excess tissues and clumps. Single-cell suspensions were isolated using Ficoll (Lymphocyte Separation Medium, MPBio) by continuous centrifugation at 400g for 30 minutes at 25°C. The collected BMMCs were then washed twice with a washing medium.
Cell stimulation and IFN-γ assays
Target and effector cells (50 000 per well) were cocultured with 10 μg/mL 4D7 for 4 hours. Interferon-gamma (IFN-γ) levels were measured using ELISA MAX (BioLegend).
In vivo efficacy of mAb 4D7 in U266-bearing mice
NOD scid gamma (NSG) mice were housed at Ben Gurion University of the Negev in accordance with Institutional Animal Care and Use Committee approved protocols (IL56-06-2023E). Each mouse was implanted subcutaneously in the flank with 5 × 106 U266 MM cells. Seven days after implantation, mice received an IV injection of 1 × 107 human NK cells, and were assigned to 1 of 3 treatment groups. The vehicle group received phosphate-buffered saline (PBS)+human interleukin-15 (hIL-15; 2 μg per mouse, intraperitoneally [IP]) 3 times per week. The IgG control group received an control antibody (15 mg/kg, IP) and recombinant hIL-15, 3 times weekly, along with NK cell inoculation. The 4D7 treatment group received the 4D7 (15 mg/kg, IP) and IL-15 on the same schedule. Tumor growth was monitored by serial caliper measurements until day 26 after implantation, when all mice were euthanized for final analysis.
Ki67 immunohistochemistry
Tumors were fixed, paraffin-embedded, sectioned, and stained for Ki67 (AbCam, ab16667, 1:200). Slides were analyzed using Qupath-0.2.3 software, with staining thresholds validated against matched negative controls.
Ethics
The study protocol was approved by the Institutional Review Board at Soroka University Medical Center, Be’er Sheva, Israel (approval number: 0054-23-SOR). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Results
Generation and initial characterization of mAb 4D7 to HLA-E[pHLAG] complex
We generated a T-cell receptor-like mAb targeting HLA-E[pHLA-G] through murine immunization campaigns and hybridoma screening. Among hundreds of clones, only 4D7 (IgG-κ) specifically bound recombinant HLA-E[pHLA-G] (disulfide-trapped HLA-E/β2m-SCT-VMAPRTLFL12). Direct ELISA showed minimal reactivity with HLA-E[pHSP60] (QMRPVSRVL-conjugated control; P < .0001). Unlike commercial 3D12 antibody, 4D7 required HLA-E for pHLA-G recognition (Figure 1A), and showed no cross-reactivity with HLA-A/B/C variants (supplemental Figure 1). Surface plasmon resonance confirmed the high affinity of 4D7 for HLA-E[pHLA-G] (KD = χ2/Rmax < 10%), with no detectable binding to HLA-E[pHSP60] (Figure 1B). Computational modeling (ClusPro2,25 ABodyBuilder226) revealed the Fv region of 4D7 binds HLA-E[pHLA-G] with lower free energy (–547.4 kcal/mol) vs HLA-E[pHSP60] (–476.5 kcal/mol) or peptide-empty HLA-E (–383.5 kcal/mol; Table 1; Figure 1C-D). The generated structure was validated using a Ramachandran plot (supplemental Figure 2). Structural modeling revealed 4D7 binds HLA-E via 10 hydrogen bonds and salt bridges targeting residues (supplemental Table 1), R65, D69, H155, and D162, the critical sites for NKG2A/CD94.27 We focused on D69 and H155 as it was reported that mutagenesis of these 2 amino acids to alanine did not affect the binding of HLA-E to NKG2C/CD94, while mutagenesis to alanine of the other 6 amino acids of HLA-E affected the binding of HLA-E to both NKG2A/CD94 and NKG2C/CD94 (R65A, Q72A, R75A, R79A, D162A, and E166A).27 We thus investigated whether the reported D69A and H155A mutations in the HLA-E backbone would have any significant impact on the model-predicted binding of 4D7 to the HLA-E[pHLA-G] complex.
Recognition of recombinant HLA-E–peptide complexes by the mAb 4D7. (A) ELISA demonstrating the binding specificity of 4D7 or 3D12 to wells coated with 2 μg/mL of recombinant HLA-E[pHLAG] (blue bar), HLA-E [pHSP60] (light gray bar), or pHLA-G (dark gray bar). Murine IgG1κ isotype (2 μg/mL) served as the control. Absorption was measured at O.D. 650 nm. Error bars represent ± standard deviation (SD). Significance was tested by a 2-way analysis of variance (ANOVA). ∗∗∗∗P < .0001. (B) A ProteOn array showed the affinity of 4D7 at concentrations ranging from 0 to 40 nmol/L to 5 μg of HLA-E[pHLA-G]. Data were analyzed using the equilibrium model. (C-D) Binding model comparison of the variable chain of mAb 4D7 using ClusPro2 web server, depicted in blue and green (PDB ID: 3CDG), represented in shades of gray for the HLA-E alpha chain, and purple for the β2 microtubulin, in the presence (C) and absence (D) of the VMAPRTLFL peptide shown in red. O.D., optical density; W/o, without.
Recognition of recombinant HLA-E–peptide complexes by the mAb 4D7. (A) ELISA demonstrating the binding specificity of 4D7 or 3D12 to wells coated with 2 μg/mL of recombinant HLA-E[pHLAG] (blue bar), HLA-E [pHSP60] (light gray bar), or pHLA-G (dark gray bar). Murine IgG1κ isotype (2 μg/mL) served as the control. Absorption was measured at O.D. 650 nm. Error bars represent ± standard deviation (SD). Significance was tested by a 2-way analysis of variance (ANOVA). ∗∗∗∗P < .0001. (B) A ProteOn array showed the affinity of 4D7 at concentrations ranging from 0 to 40 nmol/L to 5 μg of HLA-E[pHLA-G]. Data were analyzed using the equilibrium model. (C-D) Binding model comparison of the variable chain of mAb 4D7 using ClusPro2 web server, depicted in blue and green (PDB ID: 3CDG), represented in shades of gray for the HLA-E alpha chain, and purple for the β2 microtubulin, in the presence (C) and absence (D) of the VMAPRTLFL peptide shown in red. O.D., optical density; W/o, without.
Summary of HLA-E complexes: cluster size and lowest free binding energy
| Complex . | Cluster size . | Lowest free binding energy . |
|---|---|---|
| HLA-EVMAPRTLFL complex | 156 | –547.4 |
| HLA-EQMRPVSRVL complex | 91 | –476.5 |
| HLA-E w/o peptide complex | 79 | –383.5 |
| Complex . | Cluster size . | Lowest free binding energy . |
|---|---|---|
| HLA-EVMAPRTLFL complex | 156 | –547.4 |
| HLA-EQMRPVSRVL complex | 91 | –476.5 |
| HLA-E w/o peptide complex | 79 | –383.5 |
This table presents the results of binding energy analysis for 3 HLA-E complexes. The “Complex” column lists each HLA-E complex analyzed, including those bound to the VMAPRTLFL and QMRPVSRVL peptides, as well as the HLA-E molecule without peptide. “Cluster size” indicates the number of conformational clusters identified for each complex during the analysis. “Lowest free binding energy” (in kcal/mol) refers to the most favorable binding energy observed for each complex, reflecting the stability of peptide binding.
w/o, without.
We then docked 4D7 to D69A, H155A, and D69A+H155A mutant HLA-E[pHLA-G] complexes. The free binding energy in all 3 cases was found to be considerably reduced vs that to wild-type HLA-E[pHLA-G] (D69A: –521 kcal/mol, H155A: –514 kcal/mol, and D69A+H155A: –501.4 kcal/mol). Thus, mutation to D69A, H155A, or D69A+H155A disrupted the hydrogen bond formation and binding of mAb 4D7 to the HLA-E residues mentioned above. For wild-type HLA-E[pHLA-G], the predicted binding of D69 and H155 of HLA-E was to T66 of the 4D7 light chain and K66 of the 4D7 heavy chain, respectively (supplemental Figure 3).
Amino acid mutations impacting 4D7 binding to HLA-E and peptide specificity in tumor cells
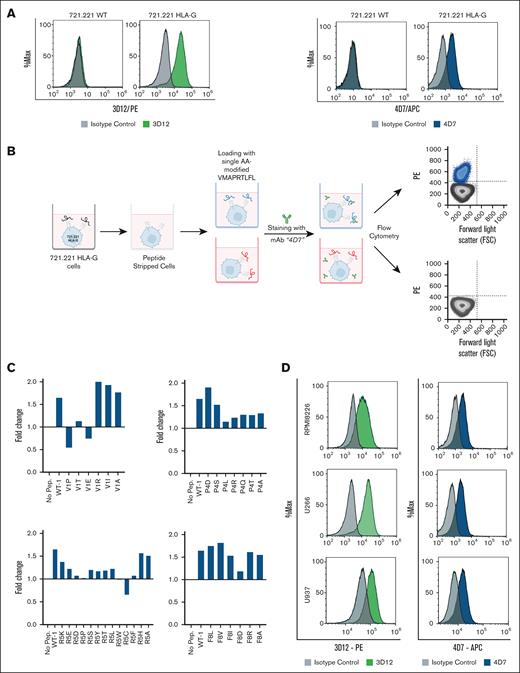
We evaluated the ability of 4D7 to bind HLA-E–peptide complexes on cell membranes. The 721.221 leukemia cell line, which lacks classical HLA class I and expresses nondetectable endogenous HLA-E, upregulates HLA-E when transfected with HLA-G due to stabilization by the HLA-G leader peptide.28 Flow cytometry showed that both 4D7 and the commercial anti-HLA-E mAb 3D12 (recognizing the alpha3 domain of the HLA-E heavy chain29) stained HLA-G-transfected 721.221 cells, but not wild-type 721.221 cells (Figure 2A). Additionally, 4D7 stained the JeG-3 cell line, confirming its recognition of HLA-E[pHLA-G] complexes on trophoblast-derived cells (supplemental Figure 4).
Staining of human leukemic cell lines for the HLA-E receptor and the acid-washing protocol for loading various peptides. (A) Staining of wild-type (WT) 721.221 and 721.221 HLA-G with mAb 3D12 (green panel) and 4D7 (blue panel). 0.2 × 106 cells per well were incubated with the mAb PE-conjugated mouse anti-human HLA-E (3D12) and APC-conjugated mouse anti-human HLA-E (4D7) or a matched isotype control. (B) Schematic representation of the acid wash protocol, followed by flow cytometry determination of surface expression levels of HLA-E loaded with different nonapeptides. (C) HLA-E staining of acid-washed and peptide-loaded 721.221 HLA-G cells. 721.221 HLA-G cells were treated briefly with an acid buffer (citric acid in Na2HPO4 buffer, pH 5). Thirty-two different exogenous peptides, 20 μg/mL, with single amino acid mutations and the WT peptide were loaded on peptide-stripped 721.221 HLA-G cells. The treated cells were then stained with mAb 3D12 and mAb 4D7 or matched isotype control. The results were normalized to staining with commercial antibody to HLA-E. (D) Staining of U266, U937, and RPMI8226 cell lines with mAb 3D12 (green panel) and mAb 4D7 (blue panel). Murine IgG1k was used as an isotype control. For staining, all the antibodies were used at a concentration of 5 μg/mL. Samples were acquired by a CytoFLEX flow cytometer, and histograms were plotted using Kaluza software. PE, fluorescent dye R-phycoerythrin.
Staining of human leukemic cell lines for the HLA-E receptor and the acid-washing protocol for loading various peptides. (A) Staining of wild-type (WT) 721.221 and 721.221 HLA-G with mAb 3D12 (green panel) and 4D7 (blue panel). 0.2 × 106 cells per well were incubated with the mAb PE-conjugated mouse anti-human HLA-E (3D12) and APC-conjugated mouse anti-human HLA-E (4D7) or a matched isotype control. (B) Schematic representation of the acid wash protocol, followed by flow cytometry determination of surface expression levels of HLA-E loaded with different nonapeptides. (C) HLA-E staining of acid-washed and peptide-loaded 721.221 HLA-G cells. 721.221 HLA-G cells were treated briefly with an acid buffer (citric acid in Na2HPO4 buffer, pH 5). Thirty-two different exogenous peptides, 20 μg/mL, with single amino acid mutations and the WT peptide were loaded on peptide-stripped 721.221 HLA-G cells. The treated cells were then stained with mAb 3D12 and mAb 4D7 or matched isotype control. The results were normalized to staining with commercial antibody to HLA-E. (D) Staining of U266, U937, and RPMI8226 cell lines with mAb 3D12 (green panel) and mAb 4D7 (blue panel). Murine IgG1k was used as an isotype control. For staining, all the antibodies were used at a concentration of 5 μg/mL. Samples were acquired by a CytoFLEX flow cytometer, and histograms were plotted using Kaluza software. PE, fluorescent dye R-phycoerythrin.
To assess how specific amino acids in pHLA-G affect 4D7 binding, we generated 32 single-residue mutants of the VMAPRTLFL sequence, targeting positions 1, 4, 5, and 8, residues known to interact with NKG2A/NKG2C.30 These mutant peptides were loaded onto acid-washed 721.221 HLA-G cells, which predominantly present exogenously supplied peptides on endogenous HLA-E (Figure 2B). Acid washing removed endogenous peptides, allowing precise evaluation of 4D7 binding to each mutant HLA-E–peptide complex.
We examined the expression levels of HLA-E loaded with the different exogenous nonapeptides, and revealed several binding principles regarding 4D7 recognition of HLA-E–peptide complexes. Positions 1 (3/6 mutations), 4 (5/7), and 5 (11/13) significantly reduced 4D7 binding, while position 8 showed minimal impact (1 mutation affected; Figure 2C). This demonstrated that mAb 4D7 recognition of HLA-E–peptide is substantially affected by changes to positions 1, 4, and 5 of the original VMAPRTLFL.
Given prior evidence that positions 2/3/6/7/9 anchor VMAPRTLFL to HLA-E,31 we focused mutagenesis on positions 1/4/5/8. Computational modeling revealed 4D7 engages positions 4/5 via hydrogen/van der Waals interactions (supplemental Table 2), while position 1 mutations induced conformational changes affecting a critical hydrogen bond between 4D7 and the peptide’s 2 to 3 bond (Figure 2C; supplemental Figure 5). This explains why V1P (but not V1R) significantly reduced binding, aligning with experimental data. We next assessed 4D7 binding to cancer cell lines with high HLA-E expression. MM and acute myeloid leukemia (AML) are known to display strong HLA-E membrane expression,31,32 while HLA-G overexpression is less frequent. Using HLA-G–negative MM (RPMI8226, U266) and AML (U937) cell lines33 (supplemental Figure 6), we found that 4D7 robustly stained all 3 (Figure 2D). This indicates that 4D7 can recognize HLA-E–peptide complexes on tumor cells, even in the absence of HLA-G, likely by binding a subset of peptides presented by HLA-E.
4D7 stains primary neoplastic plasma cells derived from BM of patients with MM who were treatment naïve
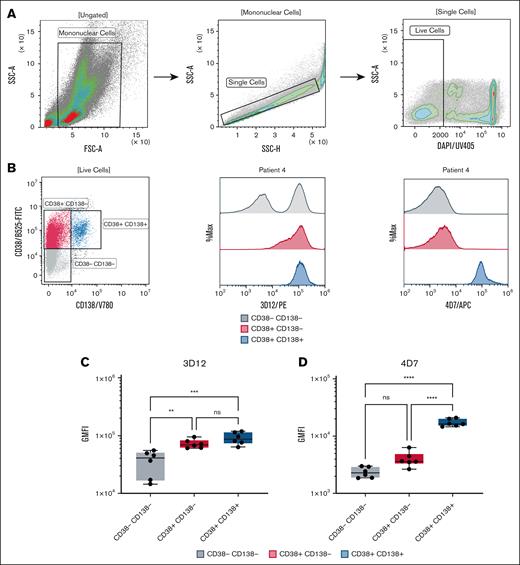
To investigate the effect of HLA-E–peptide complexes on primary human cancer cells, we stained freshly isolated primary BMMCs from newly diagnosed patients with MM who were treatment naïve with various markers, specifically, markers for immunophenotyping neoplastic plasma cell populations34 (CD38+CD138+) and with either the 4D7 mAb or the 3D12 mAb that recognizes the alpha3 domain of the HLA-E heavy chain (gating strategy shown in Figure 3A). Neoplastic plasma cells35 (CD38+CD138+) showed positive staining with both 4D7 and 3D12 (Figure 3B). However, staining patterns differed among the other cell populations. mAb 3D12 did not differentiate between double-positive and single-positive cells, but could distinguish these cells from the double-negative population (Figure 3C; P < .01). Moreover, for a few patients, the double-negative CD38–CD138– BMMCs also contained a subgroup that manifested weaker staining by 3D12 (Figure 3B). In contrast, mAb 4D7 successfully differentiated the double-positive population from both single-positive and double-negative populations (Figure 3D; P < .0001). However, it could not distinguish between single-positive and double-negative populations.
HLA-E expression in BMMCs from patients with MM. BMMCs were isolated from the BM of 6 patients with active MM who were treatment naïve. (A) Representative gating strategy for BM cell populations from patient 4. (B) Representative dot plot showing CD38 and CD138 subpopulations (left), and overlay histograms of HLA-E staining using mAb 3D12 (PE-conjugated, middle) and mAb 4D7 (APC-conjugated, right) in CD38–CD138– (gray), CD38+CD138– (red), and CD38+CD138+ (blue) subpopulations. (C-D) Box plots summarizing HLA-E staining GMFI across the subpopulations for all 6 patients with MM using 3D12 (C) and 4D7 (D) antibodies. Cells (5 × 105) were stained with BV780-conjugated anti-CD38 and fluorescein isothiocyanate-conjugated anti-CD138 antibodies (5 μg/mL each). Data were acquired using a CytoFLEX flow cytometer and analyzed with Kaluza software. FSC-A, forward scatter area; GMFI, geometric mean fluorescence intensity; ns, not significant; PE, fluorescent dye R-phycoerythrin; SSC-A, side scatter area.
HLA-E expression in BMMCs from patients with MM. BMMCs were isolated from the BM of 6 patients with active MM who were treatment naïve. (A) Representative gating strategy for BM cell populations from patient 4. (B) Representative dot plot showing CD38 and CD138 subpopulations (left), and overlay histograms of HLA-E staining using mAb 3D12 (PE-conjugated, middle) and mAb 4D7 (APC-conjugated, right) in CD38–CD138– (gray), CD38+CD138– (red), and CD38+CD138+ (blue) subpopulations. (C-D) Box plots summarizing HLA-E staining GMFI across the subpopulations for all 6 patients with MM using 3D12 (C) and 4D7 (D) antibodies. Cells (5 × 105) were stained with BV780-conjugated anti-CD38 and fluorescein isothiocyanate-conjugated anti-CD138 antibodies (5 μg/mL each). Data were acquired using a CytoFLEX flow cytometer and analyzed with Kaluza software. FSC-A, forward scatter area; GMFI, geometric mean fluorescence intensity; ns, not significant; PE, fluorescent dye R-phycoerythrin; SSC-A, side scatter area.
As shown in supplemental Figure 7, the proportion of CD38+CD138+ plasma cells within the BMMC samples varied among patients, ranging from ∼4% to 13%.
These findings demonstrate that mAb 4D7 can effectively differentiate between neoplastic plasma cells and other BM-derived cells. This highlights the potential therapeutic applications of 4D7.
Functional impact of the 4D7 mAb on NK cells derived from healthy donors cocultured with target cell lines
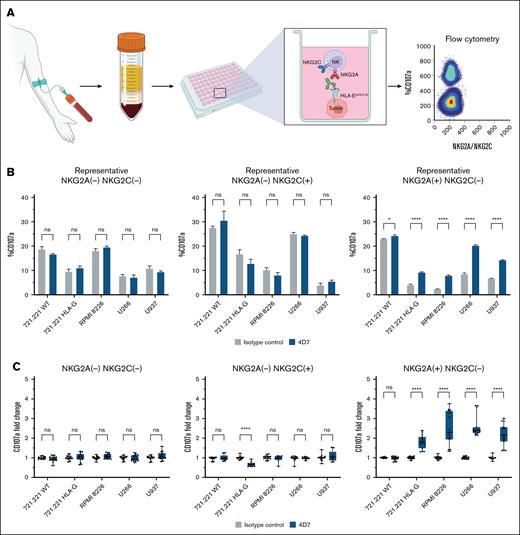
Both NKG2A and NKG2C receptors recognize HLA-E–peptide complexes, but with different specificities. While NKG2A is an inhibitory receptor, NKG2C functions as an activating receptor. Given these distinct roles, we sought to investigate the impact of the 4D7 on 3 subsets of primary human NK cells: NKG2A–NKG2C–, NKG2A+NKG2C–, and NKG2A–NKG2C+ (Figure 4A). It is worth noting that our primary NK cell culture protocol, applied to most healthy donors we tested, yielded a negligible fraction of NKG2A+NKG2C+ double-positive NK cells.
Impact of mAb 4D7 on primary human NK cell activity from healthy donors. (A) Schematic of the experimental design. Primary NK cells (5 × 104) from healthy donors were cocultured with target cells (1.5 × 105) for 4 hours in the presence of mAb 4D7 or matched isotype control (murine IgG1, 10 μg/mL). NK cell CD107a expression was then assessed by flow cytometry. (B) Representative CD107a degranulation percentage for donor 5. (C) Normalized mean results from 5 healthy donors. Panels show NK subsets: NKG2A–NKG2C– (top), NKG2A–NKG2C+ (middle), and NKG2A+NKG2C– (bottom). After incubation, NK cells were stained for subset markers (2 μg/mL). Data were acquired using a CytoFLEX flow cytometer and analyzed with GraphPad Prism v10. Isotype control degranulation was normalized to 1, with 4D7 mAb results adjusted accordingly. Experiments were performed in triplicate for each donor. Error bars represent ± SD. ∗P < .05; ∗∗∗∗P < .0001 (2-way ANOVA).
Impact of mAb 4D7 on primary human NK cell activity from healthy donors. (A) Schematic of the experimental design. Primary NK cells (5 × 104) from healthy donors were cocultured with target cells (1.5 × 105) for 4 hours in the presence of mAb 4D7 or matched isotype control (murine IgG1, 10 μg/mL). NK cell CD107a expression was then assessed by flow cytometry. (B) Representative CD107a degranulation percentage for donor 5. (C) Normalized mean results from 5 healthy donors. Panels show NK subsets: NKG2A–NKG2C– (top), NKG2A–NKG2C+ (middle), and NKG2A+NKG2C– (bottom). After incubation, NK cells were stained for subset markers (2 μg/mL). Data were acquired using a CytoFLEX flow cytometer and analyzed with GraphPad Prism v10. Isotype control degranulation was normalized to 1, with 4D7 mAb results adjusted accordingly. Experiments were performed in triplicate for each donor. Error bars represent ± SD. ∗P < .05; ∗∗∗∗P < .0001 (2-way ANOVA).
To assess NK cell function across these subsets, we used the CD107a degranulation assay. As targets, we utilized 5 cell lines to evaluate NK cell function: HLA-E positive RPMI8226, U266, U937, 721.221 HLA-G, or wild-type 721.221 (Figure 2). As shown in supplemental Figure 8, we compared the binding patterns of 4D7 and 3D12 antibodies in day 0 peripheral blood mononuclear cells from 3 healthy donors, demonstrating that while 3D12 stained a distinct population compared with its isotype control, 4D7 did not show specific binding to resting peripheral blood mononuclear cells. The results for a representative donor are presented in Figure 4B, while Figure 4C displays the averaged, normalized results for all 5 healthy donors. A notable increase in NK cell activity was observed for NKG2A+NKG2C– NK cells when 4D7 was introduced to cocultures of NK cells and HLA-E-positive target cells (Figure 4B-C). This enhancement was particularly evident across all HLA-E expressing cell lines tested (P < .0001). In contrast, wild-type 721.221 cells, which express dull to null levels of HLA-E, did not activate NKG2A+NKG2C– NK upon 4D7 introduction. The activity of NKG2A–NKG2C– (double negative) and NKG2A–NKG2C+ NK cells remained unaffected by 4D7 when cocultured with HLA-E-positive MM or AML cell lines (Figure 4B-C). Interestingly, mAb 4D7 was able to reduce the activation of NKG2A–NKG2C+ NK cells in HLA-G 721.221 cells (P < .0001).
Overall, these findings suggest that the mAb 4D7 enhanced NK activity by blocking the interaction between NKG2A and the HLA-E–peptide complex, thus highlighting the potential role of mAb 4D7 as a specific IC inhibitor that can overcome the NKG2A:HLA-E inhibitory axis.
Impact of 4D7 on the activity of NK cell subsets in patients with MM who were treatment naïve
We further tested the effect of mAb 4D7 on NK cell activity using BMMCs isolated from 6 patients with MM who were treatment naïve. NK cells were cocultured autologously with BMMCs containing neoplastic plasma cells from the same patients, as well as with 2 established MM cell lines: U266 and RPMI8226 (Figure 5A). Degranulation of NK cells derived from the BMMCs of 6 patients with MM was assessed using CD107a (Figure 5B). Patients 2, 3, and 5 exhibited a significant increase in CD107a expression levels following the addition of mAb 4D7 to the autologous culture, while the remaining patients showed only marginal improvements under the same conditions (Figure 5B, bottom panel). Interestingly, this effect was primarily observed in the NKG2A+ single-positive NK cell fraction.
Impact of mAb 4D7 on MM-derived primary human NK cell activity. (A) Schematic of the experimental design. Primary NK cells were cocultured with target cell lines for 4 hours in the presence of 4D7 mAb or isotype control (murine IgG1, 10 μg/mL). (B) Normalized CD107a degranulation results for autologous (top), U266 (middle), and RPMI8226 (bottom) target cells. (C) Normalized IFN-γ production results for autologous (top), U266 (middle), and RPMI8226 (bottom) target cells. NK cells were cocultured with target cells (E:T ratio 1:3), and subsequently stained for CD107a, CD16, CD56, NKG2A, and NKG2C (2 μg/mL each). Supernatants were collected for IFN-γ measurements. Data were acquired using a CytoFLEX flow cytometer and analyzed with GraphPad Prism v10. CD107a-positive NK cell percentages were calculated and normalized as described in Figure 4. Results represent the average of 6 patients with MM, with experiments performed in triplicate for each donor. Error bars represent ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (2-way ANOVA). ns, not significant.
Impact of mAb 4D7 on MM-derived primary human NK cell activity. (A) Schematic of the experimental design. Primary NK cells were cocultured with target cell lines for 4 hours in the presence of 4D7 mAb or isotype control (murine IgG1, 10 μg/mL). (B) Normalized CD107a degranulation results for autologous (top), U266 (middle), and RPMI8226 (bottom) target cells. (C) Normalized IFN-γ production results for autologous (top), U266 (middle), and RPMI8226 (bottom) target cells. NK cells were cocultured with target cells (E:T ratio 1:3), and subsequently stained for CD107a, CD16, CD56, NKG2A, and NKG2C (2 μg/mL each). Supernatants were collected for IFN-γ measurements. Data were acquired using a CytoFLEX flow cytometer and analyzed with GraphPad Prism v10. CD107a-positive NK cell percentages were calculated and normalized as described in Figure 4. Results represent the average of 6 patients with MM, with experiments performed in triplicate for each donor. Error bars represent ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (2-way ANOVA). ns, not significant.
We then cultured the NK cells with MM cell lines: U266 and RPMI8226, and observed that the NKG2A single-positive NK cell fraction was significantly enhanced by the 4D7 mAb for all patients. Nonetheless, NKG2C single-positive and double-negative NK cell fractions were not affected by the 4D7 mAb, further validating the specificity of the antibody’s effect on the NKG2A positive NK cell subpopulation. Activation levels of the NK cells were further assessed using IFN-γ levels. Incubation of NK cells with autologous MM revealed partial similarity to degranulation assay, with patients 3 and 5 showing significant elevation in both IFN-γ and CD107a levels. In addition, patients 1 and 4 showed only elevation on IFN-γ levels, while displaying only modest elevation in CD107, while patient 2 displayed an opposite trend. Conversely, patient 6 exhibited no changes in both CD107a and IFN-γ levels after mAb 4D7 treatment (Figure 5C).
These findings suggest that mAb 4D7 has an effective impact on enhancing the activity of NK cells from patients with MM, potentially improving their ability to target and overcome NKG2A:HLA-E inhibition.
In vivo efficacy of mAb 4D7
The efficacy of mAb 4D7 was evaluated in a U266 CDX model implanted in NSG mice. Tumor growth was monitored 26 days after implantation, and tumor volumes were measured regularly. The experimental timeline, as illustrated in Figure 6A, outlines U266 tumor implantation, primary NK cell inoculation, hIL-15 supplementation, and mAb 4D7 or control treatments, as well as the tumor volume measurement schedule. As shown in Figure 6B, by the end point of the study (day 26 after implantation), the mAb 4D7 treatment group showed statistically significant inhibition of tumor growth compared with PBS or isotype control treatment groups (2-way analysis of variance, P < .01). This growth suppression effect of the mAb 4D7 treatment was corroborated by the results of Ki67 staining performed on termination day to evaluate the proliferation status of tumor cells across the treatment groups. Quantification of Ki67 staining is depicted in the box and whiskers plot (Figure 6C), and representative images are shown in Figure 6D. The PBS vehicle and IgG1 isotype control groups exhibited high levels of Ki67-positive cells, reflecting robust tumor cell proliferation. In contrast, treatment with mAb 4D7 resulted in a significant reduction in Ki67-positive cells, indicating decreased tumor cell proliferation compared with the vehicle group (Welch analysis of variance, P < .0001). Overall, these findings suggest that mAb effectively enhanced NK function to reduce tumor cell growth and proliferation, as indicated by tumor volume measurements and Ki67 expression.
Evaluation of mAb 4D7 IC efficacy in a U266 CDX model implanted in NSG mice. (A) Experimental timeline illustrating U266 tumor implantation (day 0), pNK cell inoculation (day 7), hIL-15 supplementation and 4D7/control treatments from day 9, and tumor volume measurement schedule. (B) Tumor volume measurements of the different treatment groups, including vehicle, hIgG1, and mAb 4D7. (C) Ki67 staining quantification is presented as a boxplot. (D) Representative images of Ki67 staining in tumor sections from each treatment group. ∗∗P < .01; ∗∗∗∗P < .0001 (ANOVA). ns, not significant; pNK, primary NK cells.
Evaluation of mAb 4D7 IC efficacy in a U266 CDX model implanted in NSG mice. (A) Experimental timeline illustrating U266 tumor implantation (day 0), pNK cell inoculation (day 7), hIL-15 supplementation and 4D7/control treatments from day 9, and tumor volume measurement schedule. (B) Tumor volume measurements of the different treatment groups, including vehicle, hIgG1, and mAb 4D7. (C) Ki67 staining quantification is presented as a boxplot. (D) Representative images of Ki67 staining in tumor sections from each treatment group. ∗∗P < .01; ∗∗∗∗P < .0001 (ANOVA). ns, not significant; pNK, primary NK cells.
Discussion
Many cancers evade immune detection by exploiting ICs.36,37 One such mechanism involves the interaction between HLA-E and the inhibitory receptor NKG2A, which is expressed on NK cells. In this study, we developed a mAb called 4D7, specifically targeting the HLA-E[pHLA-G] complex, a unique ligand complex presented by cancer cells. Our results show that mAb 4D7 effectively blocks the HLA-E:NKG2A IC axis, enhancing NK cell activity against hematologic cancer cell lines without broadly inhibiting HLA-E interactions with other receptors, such as NKG2C or T-cell receptors.38,39
By focusing on the HLA-E[pHLA-G] complex rather than HLA-E alone, we aimed to develop a more targeted approach with potentially reduced toxicity compared with anti-NKG2A (monalizumab) or broad anti-HLA-E antibodies. Our mAb 4D7 specifically targets the dual ligand complex involved in immune suppression, which could offer a safer alternative by minimizing undesirable activation of the immune system often associated with less specific approaches. Moreover, this specificity makes mAb 4D7 a promising candidate for future clinical applications, potentially in combination therapies like antibody-drug conjugates to provide both checkpoint blockade and direct tumor cell elimination.40 No previous studies have successfully developed mAbs targeting specific HLA-E with peptide complexes, making this a novel approach in the field of IC inhibition.36,41
Importantly, the HLA-E:HLA-G axis also plays a critical role in pregnancy by modulating NK cell activity and contributing to maternal-fetal immune tolerance. Dysregulation of this pathway has been implicated in pregnancy disorders such as preeclampsia and recurrent miscarriage.4,5 The specificity of mAb 4D7 toward the HLA-E[pHLA-G] complex suggests potential utility in studying these disorders and exploring therapeutic interventions targeting this pathway in reproductive immunology. However, attempts to generate mAbs against other specific HLA-ligand complexes have been explored, albeit with limited success. These efforts highlight the challenges of achieving selectivity and safety in targeting ICs.
We showed that the 4D7 mAb exhibits high specificity towards the HLA-E[pHLA-G] complex, but not towards the complex formed with the HLA-E[pHSP60] (Figure 1A). Expression of HLA-G in cell lines capable of expressing HLA-E significantly enhanced the binding of the 4D7 mAb to HLA-E–peptide complexes. To further explore the specificity and applicability of mAb 4D7, we studied MM and AML cell lines that lack HLA-G expression. Interestingly, we found that mAb 4D7 can also bind to other HLA-E complexes presenting a certain subset of peptides. This observation underscores the influence of peptide composition on mAb 4D7 binding, with key amino acids at positions 1, 4, 5, and 8 within the VMAPRTLFL HLA-G leader-derived peptide playing a crucial role in mediating interaction with mAb 4D7, similarly to their role in NKG2A receptor binding. Additionally, in silico mutations at 2 specific positions within the HLA-E molecule, D69, and H155, significantly disrupted the binding of mAb 4D7 to the HLA-E[pHLA-G] complex, regardless of the peptide presented (supplemental Figure 3). Notably, these positions are also critical for HLA-E binding to the NKG2A receptor but not the NKG2C receptor, providing further evidence of the selective interference of 4D7 with the NKG2A-mediated inhibitory pathway on NK cells, and not with the NKG2C-mediated activation pathway. To our knowledge, no other studies have successfully developed mAbs that selectively target the HLA-E[pHLA-G] complex, although there have been efforts to create mAbs specific to other HLA-ligand complexes, with varying degrees of success. Our work highlights mAb 4D7 as a novel mAb with a distinct binding profile that enables selective blockade of the HLA-E:NKG2A interaction while sparing HLA-E interactions with the NKG2C receptor.
To further evaluate the anticancer potential of the 4D7 mAb, we investigated its ability to activate NK cells against primary human MM target cells.9,19 Given that MM overexpress high levels of HLA-E, and NKG2A plays a significant role in suppressing NK activity in this cancer, we aimed to determine whether mAb 4D7 could reverse this suppression. Our results demonstrated a significant increase in the activation of autologous NKG2A+ NK cells against primary MM cells upon the addition of mAb 4D7. This enhanced activation was closely tied to both HLA-E expression on MM cells and NKG2A receptor levels on NK cells. Moreover, mAb 4D7 exhibited remarkable selectivity, distinguishing between normal (CD38+CD138–) and cancerous (CD38+CD138+) BM cells in patients with MM who were untreated,35 unlike the commercial anti-HLA-E antibody, 3D12, which was unable to differentiate between healthy and cancerous cells. We further showed the potency of 4D7 in vivo; 4D7 treatment of MM-bearing and NK-inoculated mice significantly reduced MM tumor growth. Interestingly, our analysis revealed that the magnitude of 4D7-mediated NK cell activation did not fully correlate with the proportion of CD38+CD138+ plasma cells in the BM samples, as shown in supplemental Figure 7. This suggests that factors beyond the abundance of neoplastic plasma cells, such as heterogeneity in the expression of NK cell ligands or other components of the tumor microenvironment, may influence the responsiveness to mAb 4D7.
However, our study has some limitations. First, the antibody’s recognition of similar peptides with mutations at specific positions could potentially lead to off-target binding, affecting the specificity of our results. Second, we observed a weak stain of blasts in the sample of 1 patient with MM, raising concerns about possible overstaining of certain BM cells. This finding underscores the need for a more comprehensive sample collection to identify additional negative controls and refine our understanding of the staining specificity of 4D7. Furthermore, we acknowledge the necessity of expanding our cohort of patients with MM to enhance the statistical power of our study. A larger sample size would allow us to draw more robust conclusions about the performance of 4D7 in this patient population, and potentially uncover any rare or subtle staining patterns that may have been missed in our current analysis.
In summary, our study introduces the 4D7 mAb as a novel therapeutic candidate that selectively blocks the HLA-E:NKG2A axis without interfering with the activating NKG2C pathway.13 By enhancing NK cell activity specifically against MM cells while sparing normal cells, mAb 4D7 has the potential to improve therapeutic outcomes in patients with MM, reducing common side-effects associated with traditional treatments. These findings provide a new avenue for targeting NK cell ICs, shifting our understanding of how selective inhibition can be achieved in cancer immunotherapy.
Acknowledgments
The authors thank the Oncology Department at Soroka University Medical Center and the Deutsches Krebsforschungszentrum (DKFZ). The authors also thank the members of their laboratories for their comments, suggestions, and support during this work.
This work was funded by the Cooperation Program in Cancer Research of the DKFZ and Israel’s Ministry of Science, Technology and Space (MOST; DKFZ-MOST no. CA194; A.P. and F.M.); United States-Israel Binational Science Foundation (2023087 and 2019377; A.P. and K.S.C.); Israel Science Foundation (ISF) grant (2484/19); ISF-National Research Foundation (3127/19); and Israel Cancer Research Fund acceleration (81071411).
Authorship
Contribution: M.A.A., F.M., and A.P. were responsible for the conception and design of the study; M.A.A., O. Rouvio, M.Z., D.B.M., N.O., R.G., and A.P. were responsible for the provision of study materials or patients; M.A.A., O. Radinsky, and A.P. were involved in the collection and assembly of data; M.A.A., B.K., S.D., K.W., E.G., M.E., and A.P. were responsible for data analysis and interpretation; M.A.A., O. Radinsky, B.K., F.M., D.J., M.M., K.S.C., and A.P. were responsible for writing the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angel Porgador, The Shraga Segal Department of Microbiology, Immunology and Genetics, Faculty of Health Sciences, Ben-Gurion University of the Negev, David Ben-Gurion Blvd 1, Be’er-Sheva 84105, Israel; email: angel@bgu.ac.il.
References
Author notes
All data supporting the findings of this study are available within the article and its supplemental material.
Data are available on request from the corresponding author, Angel Porgador (angel@bgu.ac.il).
The full-text version of this article contains a data supplement.

![Recognition of recombinant HLA-E–peptide complexes by the mAb 4D7. (A) ELISA demonstrating the binding specificity of 4D7 or 3D12 to wells coated with 2 μg/mL of recombinant HLA-E[pHLAG] (blue bar), HLA-E [pHSP60] (light gray bar), or pHLA-G (dark gray bar). Murine IgG1κ isotype (2 μg/mL) served as the control. Absorption was measured at O.D. 650 nm. Error bars represent ± standard deviation (SD). Significance was tested by a 2-way analysis of variance (ANOVA). ∗∗∗∗P < .0001. (B) A ProteOn array showed the affinity of 4D7 at concentrations ranging from 0 to 40 nmol/L to 5 μg of HLA-E[pHLA-G]. Data were analyzed using the equilibrium model. (C-D) Binding model comparison of the variable chain of mAb 4D7 using ClusPro2 web server, depicted in blue and green (PDB ID: 3CDG), represented in shades of gray for the HLA-E alpha chain, and purple for the β2 microtubulin, in the presence (C) and absence (D) of the VMAPRTLFL peptide shown in red. O.D., optical density; W/o, without.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/21/10.1182_bloodadvances.2025016276/2/m_blooda_adv-2025-016276-gr1.jpeg?Expires=1768006330&Signature=rIKXTsKct01nE250dqNxb2NCx8rYrrQuF0j2-bDqcH7W3-ckLSPiGBNcF4i3RMBSy-I7DsbmKP5E52DDPoMXcwCImSjOexCU08hBKEhwk-iaaoiE1rP6AIlQT4HwOL2mup-SY6rubCAQKN8cktroVMQxKv7IlHfWzhhEL-ckW8em7RZJvMEZ-gRsZpzz-ciqQFCot4FZVO71kkRSoMHw1IHAhil-dG7CSHFIrMFDDo3yXl5VN3bkhJWF06gW~7hbkmagyloNguWf68r6S2Duevq4b4J0IpJW2aM8gTKO6m~~PVB9TAERxhOaAawKGmtKIAtItY04abWtujdHvMGcjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)