Key Points
APS platelets have a reduced ability to produce extracellular adenosine, disrupting purinergic signaling and likely favoring thrombosis.
A2AR activation or cAMP elevation reduces antiphospholipid antibody–induced platelet activation, potentially mitigating APS thrombosis.
Visual Abstract
Platelet activation is a hallmark of antiphospholipid syndrome (APS). However, the mechanisms through which antiphospholipid antibodies activate platelets and the optimal way to inhibit this pathophysiology are still not fully understood. Extracellular adenosine, produced by the ectoenzyme CD73, activates cell-surface receptors to increase intracellular cyclic adenosine monophosphate (cAMP), raising the threshold for platelet activation. Here, we found that platelets from patients with APS exhibited lower CD73 activity and cAMP content than healthy controls. Platelet activation, as measured by the surface expression of CD62P and activated αIIbβ3, was negatively correlated with CD73 activity and cAMP content. Exposing healthy platelets to APS patient immunoglobulin G (IgG) in vitro significantly reduced CD73 activity and intracellular cAMP while simultaneously increasing activation markers. We identified critical roles for FcγRIIa, phospholipase C, and the Akt signaling pathway in platelet activation by APS IgG. We also tested whether various agents that boost intracellular cAMP could blunt APS IgG–induced platelet activation. Agonism of the adenosine 2A receptor (A2AR) and inhibition of phosphodiesterase 3 (PDE3) were both highly effective in this regard. In summary, a dysregulated adenosinergic axis appears to potentiate APS platelets for prothrombotic activation by antiphospholipid antibodies. A2AR agonists and PDE3 inhibitors may be useful strategies for restoring platelet homeostasis in APS.
Introduction
Antiphospholipid syndrome (APS) is an understudied thromboinflammatory disorder propelled by circulating antiphospholipid antibodies (aPL) that cause macrovascular thrombosis, microvascular damage, and obstetrical complications.1-5 Platelets play an indispensable role in thrombosis, including that associated with APS.6 Nevertheless, there is a lack of consensus about the role antiplatelet agents should play in managing this condition. Low-dose aspirin is sometimes recommended for primary prevention in individuals with high-risk aPL laboratory profiles or for secondary prevention (in combination with vitamin K antagonists) in patients with arterial manifestations.7 Additionally, aspirin is commonly deployed as an add-on therapy in patients who experience recurrent arterial or venous thrombosis despite systemic anticoagulation.8,9 Still, clinicians face a significant challenge in balancing the risk of recurrent thrombosis against the risk of bleeding in the management of APS.10
Patients who carry the highest risk of difficult-to-control APS typically have a so-called triple-positive laboratory profile, characterized by testing positive for all 3 of anticardiolipin immunoglobulin G (IgG), anti-beta-2 glycoprotein I (aβ2GPI) IgG, and lupus anticoagulant.4 The latter test is a functional assay turned positive by high titers of APS-associated aβ2GPI or antiphosphatidylserine/prothrombin (anti-PS/PT) antibodies. Past research has shown that the interaction of β2GPI (an abundant plasma protein) with glycoprotein Ib-IX and/or ApoER2 on the platelet surface enables aβ2GPI IgG to trigger platelet activation.11-14 P38, a MAPK, plays a significant signaling role in such activation, leading to downstream events like granule release and aggregation.15-17 Indeed, in models of APS, blocking ApoER2, GPIbα, or FcγRIIa prevents aβ2GPI-mediated p38 MAPK phosphorylation.12 Anti-PT (aPT) antibodies detected by the anti-PS/PT assay also activate platelets through FcγRIIa, with relatively less understanding of potential co-receptors.18
By engaging surface P2X and P2Y receptors, extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP) induce platelet activation and aggregation.19 A recent study found that platelets from patients with thrombotic APS exhibited higher levels of P2Y12 receptor expression, resulting in more efficient ADP-mediated aggregation.20 As a counterpoint to ATP- and ADP-mediated signaling, the ectoenzyme known as ectonucleotide triphosphate diphosphohydrolase-1 (CD39) tunes purinergic signaling by catalyzing the stepwise conversion of ATP and ADP into adenosine monophosphate (AMP).21 We have previously found that a deficiency in CD39 activity on both neutrophils and platelets enhances the formation of neutrophil-platelet aggregates in the blood of patients with APS.21 Working in concert with CD39, ecto-5'-nucleotidase (CD73) removes the final phosphate group from AMP, releasing homeostatic adenosine.22 This locally produced adenosine can counteract thromboinflammatory signaling by activating G-protein–coupled adenosine receptors. Human platelets express 2 G-protein–coupled adenosine receptors: (A2AR and A2BR). Although both receptors are Gs-coupled, A2AR is more abundant on the platelet surface and has a higher affinity for adenosine. Although we previously identified a key role for neutrophil A2AR activation in restraining venous thrombosis in models of APS,23 this homeostatic receptor has not been studied in the context of APS platelets.
Here, we explored the mechanisms by which aPL, particularly aβ2GPI and aPT IgG, activate platelets and how the adenosinergic axis may limit this activation. We identified that platelets from APS patients exhibited lower CD73 activity and reduced cAMP levels, both of which were associated with increased platelet activation. Additionally, we found that activating adenosine receptors or using other cAMP-boosting agents, such as phosphodiesterase inhibitors, limited aPL-induced platelet activation. Taken together, our findings highlight the potential of targeting purinergic signaling pathways in APS platelets as an innovative strategy to reduce thrombosis.
Methods
For more detailed information, please refer to the supplemental Methods.
Human participants and study approval
This study complied with all relevant ethical regulations and was approved by the University of Michigan Institutional Review Board(IRB). All participants provided informed consent before blood donation.
Human samples and clinical data capture
Blood was collected into sodium citrate tubes from 38 patients with persistent clinically relevant aPL according to American College of Rheumatology (ACR)/the European Alliance of Associations for Rheumatology (EULAR) Classification Criteria,24 8 patients with venous thromboembolic disease without aPL (VTE [aPL–]), and 20 healthy controls (supplemental Table 1). No patients had a concomitant diagnosis of lupus. To purify platelet-poor plasma, sodium citrate blood tubes were centrifuged at 2000 × g for 15 minutes.
Isolation of human platelets
Venous blood was collected in tubes containing sodium citrate as an anticoagulant. Platelets were purified through centrifugation at 200 × g for 15 minutes. For in vitro experiments, isolated platelet-rich plasma was treated with EDTA (0.5 mM) and prostaglandin E1 (PGE1) (1 μM), followed by centrifugation at 650 × g for 5 minutes. The resulting platelet pellets were washed with Tyrode buffer, which contains HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (20 mM), NaCl (135 mM), Na2HPO4 (0.34 mM), KCl (2.9 mM), NaHCO3 (12 mM), MgCl2 (1 mM), and glucose (5 mM), all adjusted to a pH of 6.5, along with the added EDTA and PGE1. Alternatively, platelet-rich plasma and Tyrode’s buffer were supplemented with EDTA (0.5 mM) and apyrase (25 mU/mL) when isolating platelets for cAMP quantification, as PGE1 has the potential to boost basal levels of cAMP. Finally, the obtained platelet pellets were resuspended in Tyrode buffer at a pH of 7.35.
Flow cytometry
Fresh whole blood (100 μL) containing citrate was placed in fluorescence-activated cell sorter mini tubes (Corning, catalog no.352054) and incubated with an Fc receptor blocker (human TruStain FcX) for 15 minutes before staining (1:50 dilution) with fluorescently labeled anti-human antibodies against CD62P (Alexa Fluor 647), CD41 (APC cyanine7 or Alexa Fluor 488), and activated integrin αIIbβ3 (PAC1, FITC) for 20 minutes at room temperature. Erythrocytes were lysed, and the remaining cells were fixed using the red blood cell lysis/fixation solution (BioLegend, catalog no. 422401). After centrifugation, cells were washed and analyzed with a BioRad-ZE5 flow cytometer. FlowJo software was used to determine platelet activation by assessing P-selectin-positive platelets (CD41–AF488+ and CD62P–AF647+) and activated integrin αIIbβ3 (CD41–APC/cy7+ and PAC1–FITC+). For in vitro experiments, 200 μL of isolated platelets (2 × 105/μL) were placed in 96-well plates (Corning, catalog no. 3363) and treated with the following receptor agonists, blockers, and inhibitors for 15 to 20 minutes at 37°C: anti-FcγRIIa (clone IV.3, 10 μg/mL), U73122 (phospholipase C [PLC] inhibitor, 1 μM), CGS21680 (A2AR agonist, 5 μM) or BAY 60-6583 (A2BR agonist, 5 μM), SCH-442416 (A2AR inhibitor, 5 μM), dibutyryl-cAMP (0.5 mM), 8-Br-cAMP (1 mM), forskolin (1 μM), cilostazol (PDE3 inhibitor, 10 μM), and BAY 60-7550 (PDE2 inhibitor, 10 μM). Then, the samples were stimulated for 15 minutes with standard platelet agonists thrombin (0.05 U/mL), convulxin (20 ng/mL), and U46619 (5 μM). In some experiments, samples were treated for 1 hour with different concentrations (50-100 μg/mL) of control IgG or APS IgG in the presence of β2GPI protein (10 μg/mL); affinity-purified aβ2GPI IgG (20 μg/mL) in the presence of β2GPI protein (10 μg/mL); or affinity-purified aPT IgG (10 μg/mL) in the presence of PT (10 μg/mL) and 2 mM calcium chloride. After treatment, platelets were stained with anti-CD62P (AF647, 1:100). Samples were then fixed with 2% paraformaldehyde, and fluorescence intensity was measured using flow cytometry (BioRad-ZE5).
Results
Platelet cAMP content is inversely associated with platelet activation in APS
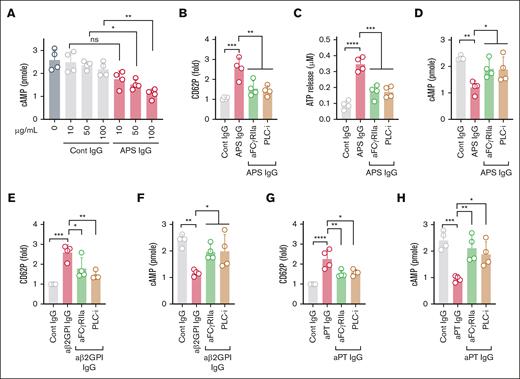
We studied 38 patients who were durably aPL-positive (aPL+), 29 of whom met the classification criteria for APS24; none of the patients were diagnosed with concomitant lupus. They were compared to 8 patients with aPL-negative unprovoked VTE [VTE (aPL–)] and 20 healthy controls (supplemental Table 1). Compared with the healthy controls, cAMP content was significantly lower in aPL+ patients (Figure 1A). In the same patients, platelet activation markers, such as surface P-selectin (CD62P) and activated glycoprotein IIb/IIIa (integrin αIIbβ3), were increased (Figure 1B-C). Additionally, the plasma of aPL+ patients had increased levels of soluble platelet factor 4 (PF4) (Figure 1D).
Relationship between platelet activation and low cAMP levels in patients with aPL+. (A) Estimation of cAMP in platelets (2 × 105/μL) purified from the fresh blood of healthy controls (n = 20), patients who are aPL+ (n = 38), and patients with aPL-negative VTE [VTE (aPL−), n = 8]. (B) Flow cytometric evaluation of platelet surface P selectin (CD62P+ events within the CD41+ population) and (C) activated αIIbβ3 receptor (PAC-1+ events within the CD41+ population) in fresh blood. (D) ELISA-based estimation of plasma PF4. Spearman correlation for platelet cAMP of aPL+ patients with surface CD62P (E), activated αIIbβ3 receptor (F), plasma PF4 (G), and anti-PS/PT IgG (H). Data represent mean ± standard deviation (SD). ∗P < .05, ∗∗P < .01, ∗∗∗∗P < .0001, and nonsignificant (ns) by 1-way analysis of variance (ANOVA) with the Tukey multiple comparisons correction. MFI, Mean Fluorescence Intensity.
Relationship between platelet activation and low cAMP levels in patients with aPL+. (A) Estimation of cAMP in platelets (2 × 105/μL) purified from the fresh blood of healthy controls (n = 20), patients who are aPL+ (n = 38), and patients with aPL-negative VTE [VTE (aPL−), n = 8]. (B) Flow cytometric evaluation of platelet surface P selectin (CD62P+ events within the CD41+ population) and (C) activated αIIbβ3 receptor (PAC-1+ events within the CD41+ population) in fresh blood. (D) ELISA-based estimation of plasma PF4. Spearman correlation for platelet cAMP of aPL+ patients with surface CD62P (E), activated αIIbβ3 receptor (F), plasma PF4 (G), and anti-PS/PT IgG (H). Data represent mean ± standard deviation (SD). ∗P < .05, ∗∗P < .01, ∗∗∗∗P < .0001, and nonsignificant (ns) by 1-way analysis of variance (ANOVA) with the Tukey multiple comparisons correction. MFI, Mean Fluorescence Intensity.
We hypothesized that the lower platelet cAMP content of aPL+ patients might be associated with a tendency toward activation. Indeed, we found that platelet cAMP was negatively correlated with the surface expression of CD62P (r = −0.619, P = .0001) (Figure 1E) and active integrin αIIbβ3 (r = −0.4, P = .012) (Figure 1F). A similar relationship was observed for plasma PF4 (r = −0.38, P = .018) (Figure 1G). We observed a nonsignificant trend toward lower platelet cAMP (P = .204) in aPL+ patients with a history of thrombosis compared with those without such a history (supplemental Figure 1). Platelets from VTE (aPL–) patients displayed increased surface CD62P without a corresponding decrease in cAMP levels. Nonetheless, a moderate association between the 2 variables was still apparent (r = −0.595) (supplemental Figure 2). We also compared platelet cAMP to autoantibodies potentially associated with platelet activation in APS, specifically the IgG and IgM isotypes of aβ2GPI and anti-PS/PT antibodies. Though no associations were significant in this relatively small cohort, there was a trend toward an inverse association between platelet cAMP levels and anti-PS/PT IgG (r = −0.31, P = .056) (Figure 1H). A significant positive correlation was found between plasma PF4 and anti-PS/PT IgG (r = 0.38, P = .016) (supplemental Figure 3). Taken together, these data confirm a tendency toward increased platelet activation in aPL+ patients, which is associated with lower platelet cAMP content.
APS IgG induces activation of platelets via FcγRIIa
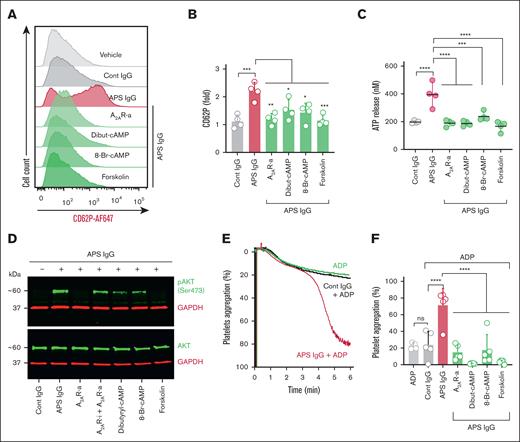
Next, we determined whether total IgG fractions isolated from healthy controls or triple-positive APS patients could induce platelet activation and decrease cAMP content. We observed a dose-dependent decrease in platelet cAMP upon treatment with APS IgG, whereas control IgG had no effect (Figure 2A). Furthermore, APS IgG significantly increased platelet activation markers, such as surface CD62P (indicating alpha granule release), extracellular ATP (indicating dense granule release), and activated integrin αIIbβ3 (indicating inside-out signaling), compared with control IgG (supplemental Figure 4A-C). We also found more tyrosine-phosphorylated proteins, indicative of activation-associated signaling, in APS IgG–treated platelet lysates (supplemental Figure 4D).
FcγRIIa-mediated platelet activation and lowering of cAMP by IgG purified from APS plasma. (A) Purified platelets obtained from healthy volunteers (n = 4) were treated with various concentrations of control IgG (cont IgG) or APS IgG for 1 hour, and cAMP was measured by assay kit. Platelets pretreated with either a monoclonal anti-FcγRIIa antibody (aFcγRIIa, clone IV.3, 1μg) or U73122 (PLC-i, 1μM), followed by stimulation with 100 μg/mL APS IgG for 1 hour. Platelets were evaluated for surface CD62P (α-granules) using a flow cytometer (B), released ATP (dense granule) using luminescent-based assay (C), and cellular cAMP using an assay kit (D). (E-H) CD62P and cAMP were measured in platelets pretreated with aFcγRIIa (1 μg) or PLC-i (1 μM). After incubation, platelets were stimulated with affinity-purified aβ2GPI IgG (20 μg/mL) or affinity-purified aPT IgG (10 μg/mL) for 1 hour. Data represent mean ± SD. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons correction (n = 4). PLC-I, phospholipase C-inhibitor.
FcγRIIa-mediated platelet activation and lowering of cAMP by IgG purified from APS plasma. (A) Purified platelets obtained from healthy volunteers (n = 4) were treated with various concentrations of control IgG (cont IgG) or APS IgG for 1 hour, and cAMP was measured by assay kit. Platelets pretreated with either a monoclonal anti-FcγRIIa antibody (aFcγRIIa, clone IV.3, 1μg) or U73122 (PLC-i, 1μM), followed by stimulation with 100 μg/mL APS IgG for 1 hour. Platelets were evaluated for surface CD62P (α-granules) using a flow cytometer (B), released ATP (dense granule) using luminescent-based assay (C), and cellular cAMP using an assay kit (D). (E-H) CD62P and cAMP were measured in platelets pretreated with aFcγRIIa (1 μg) or PLC-i (1 μM). After incubation, platelets were stimulated with affinity-purified aβ2GPI IgG (20 μg/mL) or affinity-purified aPT IgG (10 μg/mL) for 1 hour. Data represent mean ± SD. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons correction (n = 4). PLC-I, phospholipase C-inhibitor.
To determine whether APS IgG–induced platelet activation is mediated through FcγRIIa, we blocked FcγRIIa using a monoclonal antibody (IV.3). We also inhibited PLC, a downstream mediator of FcγRIIa-dependent signaling, using U73122. The FcγRIIa blocker and the PLC inhibitor significantly reduced APS IgG–induced platelet activation, as evidenced by measurements of surface CD62P, released ATP, and cellular cAMP (Figure 2B-D). Conversely, blocking FcγRIIa did not affect thrombin-induced CD62P expression or ATP release (supplemental Figure 5). We also tested affinity-purified aβ2GPI IgG in the same context. aβ2GPI IgG significantly increased CD62P expression (Figure 2E) and ATP release (supplemental Figure 6) while simultaneously decreasing cAMP (Figure 2F), all dependent on FcγRIIa. Similar results were observed when platelets were treated with affinity-purified aPT IgG from patients who were anti-PS/PT IgG-positive (Figure 2G-H).
A2AR agonism limits platelet activation induced by APS IgG
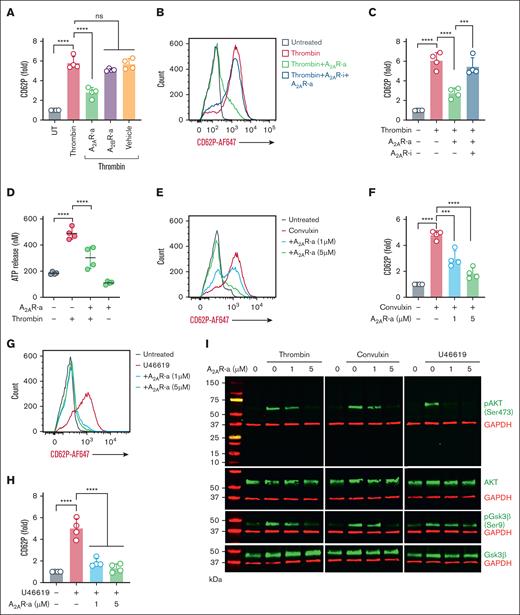
Given that adenosine receptor agonism tempers platelet activation by increasing intracellular cAMP, we aimed to determine the extent to which the specific A2AR agonist CGS21680 and the specific A2BR agonist BAY 60-6583 might prevent platelet activation triggered by various platelet activators that signal through protease-activated receptors (thrombin), glycoprotein VI (convulxin), and the thromboxane A2 receptor (U46619). Platelets pretreated with an A2AR agonist, but not an A2BR agonist, significantly reduced CD62P expression when platelets were stimulated with thrombin (Figure 3A). As expected, there was no change in platelet CD62P expression when the A2AR agonist was combined with a specific A2AR inhibitor (Figure 3B-C). The A2AR agonist blunted thrombin-induced ATP release (Figure 3D) and significantly reduced platelet CD62P expression induced by convulxin (Figure 3E-F) and U46619 (Figure 3G-H). Because the Akt pathway is known to be involved in both outside-in and inside-out signaling during platelet activation,25 we performed immunoblots for Akt signaling proteins to determine the impact of A2AR activation on this pathway. Preincubation of platelets with the A2AR agonist downregulated thrombin-, convulxin-, and U46619-mediated phosphorylation of Akt and GSK3β (Figure 3I; supplemental Figure 7).
Induction of A2AR reduces platelet activation by enhancing cAMP. (A) Healthy platelets were pretreated with either vehicle or CGS21680 (A2AR-a, 5μM) or BAY 60-6583 (A2BR-a, 5μM), followed by stimulation with thrombin (0.05 U/mL) for 15 minutes. CD62P was assessed using flow cytometry. (B-C) Flow cytometric analysis of surface CD62P in platelets preincubated with vehicle or A2AR inhibitor (SCH-442416, 5μM) for 20 minutes, followed by A2AR-a and thrombin (0.05 U/mL) stimulation. (D) Quantification of released ATP in A2AR-a and thrombin-treated platelets. (E-F) A2AR-a (1μM and 5μM) pretreated platelets were stimulated with either convulxin (20 ng/mL) or (G-H) U46619 (5μM) and assessed for surface CD62P using a flow cytometer. (I) Purified platelets were pretreated with vehicle or A2AR-a for 15 minutes before stimulation with thrombin (0.05 U/ml), or convulxin (20 ng/mL), or U46619 (5μM) for 15 minutes. The representative immunoblots show the phosphorylated-Akt (pAkt, Ser 473), total Akt, (pGSK3β, Ser9), and total GSK3β. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also used as an internal loading control for all the samples. Data represent mean ± SD. ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons correction (n = 4).
Induction of A2AR reduces platelet activation by enhancing cAMP. (A) Healthy platelets were pretreated with either vehicle or CGS21680 (A2AR-a, 5μM) or BAY 60-6583 (A2BR-a, 5μM), followed by stimulation with thrombin (0.05 U/mL) for 15 minutes. CD62P was assessed using flow cytometry. (B-C) Flow cytometric analysis of surface CD62P in platelets preincubated with vehicle or A2AR inhibitor (SCH-442416, 5μM) for 20 minutes, followed by A2AR-a and thrombin (0.05 U/mL) stimulation. (D) Quantification of released ATP in A2AR-a and thrombin-treated platelets. (E-F) A2AR-a (1μM and 5μM) pretreated platelets were stimulated with either convulxin (20 ng/mL) or (G-H) U46619 (5μM) and assessed for surface CD62P using a flow cytometer. (I) Purified platelets were pretreated with vehicle or A2AR-a for 15 minutes before stimulation with thrombin (0.05 U/ml), or convulxin (20 ng/mL), or U46619 (5μM) for 15 minutes. The representative immunoblots show the phosphorylated-Akt (pAkt, Ser 473), total Akt, (pGSK3β, Ser9), and total GSK3β. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also used as an internal loading control for all the samples. Data represent mean ± SD. ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons correction (n = 4).
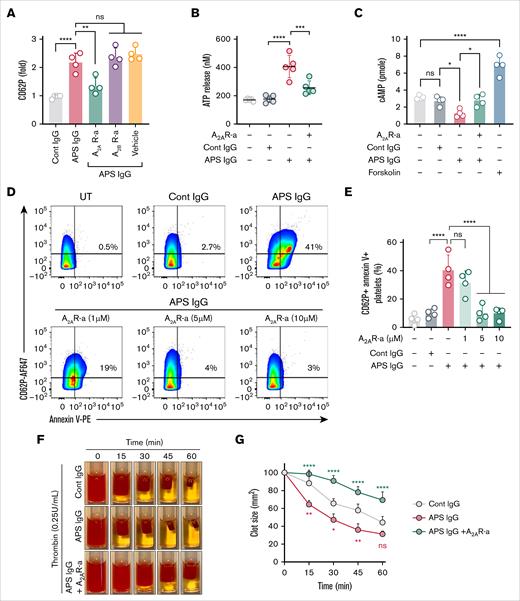
We next investigated whether adenosine receptor agonism could protect platelets from activation induced by APS IgG. When A2AR and A2BR agonist-pretreated platelets were challenged with APS IgG for 1 hour, only the A2AR agonist significantly reduced APS IgG–induced surface CD62P expression (Figure 4A). Furthermore, the A2AR agonist blunted ATP release and boosted cAMP content (Figure 4B-C). Specific subsets of activated platelets become “procoagulant” by exposing negatively charged PS on the outer surface of the plasma membrane. We assessed whether APS IgG induced the formation of procoagulant platelets. Flow cytometry revealed that 25% to 50% of platelets exposed PS upon APS IgG treatment, which was dose-dependently reduced by the A2AR agonist (Figure 4D-E). We also measured APS IgG–dependent fibrin clot retraction, which is dependent on integrin αIIbβ3 signaling, in healthy platelet-rich plasma. As expected, clot retraction was significantly faster in the presence of APS IgG (Figure 4F-G; supplemental Figure 8), an effect that the A2AR agonist abolished.
Inhibition of APS IgG–accelerated platelet activation, clot retraction, and procoagulant platelet formation by an A2AR agonist. (A) Purified platelets were pretreated with A2AR-a (5 μM) and A2BR-a (5 μM), followed by stimulation with 100 μg/mL cont IgG or APS IgG for 1 hour, and surface CD62P was assessed using flow cytometry. (B-C) Using assay kits, released ATP in supernatant and cellular cAMP were measured in platelets pretreated with A2AR-a (5 μM) followed by stimulation with 100 μg/mL of cont IgG or APS IgG for 1 hour. Forskolin (1 μM) was used as a positive cont for cAMP quantification. (D-E) Flow cytometric quantification of PS exposure along with CD62P in platelets treated with either vehicle or various concentrations of A2AR-a, followed by stimulation with 100 μg/mL cont IgG or APS IgG. (F) The representative images show the clot retraction was measured for 1 hour in platelet-rich plasma, supplemented with red blood cells, incubated with 50 μg/mL of cont IgG or APS IgG in the presence or absence of A2AR-a (5 μM) before adding thrombin (0.25 U/mL). (G) Quantified clot size over time and the values are presented as mean ± standard error of the mean (SEM). Statistical significance was determined by 2-way ANOVA with Tukey multiple comparisons tests, n = 3 individual donors per group. Data represent mean ± SD. ∗P <.05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4).
Inhibition of APS IgG–accelerated platelet activation, clot retraction, and procoagulant platelet formation by an A2AR agonist. (A) Purified platelets were pretreated with A2AR-a (5 μM) and A2BR-a (5 μM), followed by stimulation with 100 μg/mL cont IgG or APS IgG for 1 hour, and surface CD62P was assessed using flow cytometry. (B-C) Using assay kits, released ATP in supernatant and cellular cAMP were measured in platelets pretreated with A2AR-a (5 μM) followed by stimulation with 100 μg/mL of cont IgG or APS IgG for 1 hour. Forskolin (1 μM) was used as a positive cont for cAMP quantification. (D-E) Flow cytometric quantification of PS exposure along with CD62P in platelets treated with either vehicle or various concentrations of A2AR-a, followed by stimulation with 100 μg/mL cont IgG or APS IgG. (F) The representative images show the clot retraction was measured for 1 hour in platelet-rich plasma, supplemented with red blood cells, incubated with 50 μg/mL of cont IgG or APS IgG in the presence or absence of A2AR-a (5 μM) before adding thrombin (0.25 U/mL). (G) Quantified clot size over time and the values are presented as mean ± standard error of the mean (SEM). Statistical significance was determined by 2-way ANOVA with Tukey multiple comparisons tests, n = 3 individual donors per group. Data represent mean ± SD. ∗P <.05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4).
Boosting intracellular cAMP inhibits APS IgG–induced Akt pathway–mediated platelet activation and aggregation
To investigate whether cAMP boosters beyond A2AR agonists could also limit APS IgG–mediated platelet activation, we pretreated healthy platelets with cell-permeable dibutyryl-cAMP and 8-Br-cAMP, as well as forskolin, a direct activator of adenylate cyclase. Thrombin-induced expression of CD62P was significantly reduced by all 3 of dibutyryl-cAMP, 8-Br-cAMP, and forskolin (supplemental Figure 9). APS IgG–induced surface CD62P expression was also reduced by all cAMP boosters (Figure 5A-B), with similar results observed for ATP release (Figure 5C), Akt phosphorylation (Figure 5D; supplemental Figure 10), and platelet aggregation (Figure 5E-F).
cAMP inducers decrease APS IgG–induced platelet activation and aggregation. Purified platelets were preincubated with A2AR-i (5μM), A2AR-a (5 μM), dibutyryl-cAMP (Dibut-cAMP, 0.5 mM), 8-Br-cAMP (1 mM), or forskolin (1 μM) for 20 minutes, followed by stimulation with 100 μg/mL cont IgG or APS IgG for 1 hour. (A-B) Platelets were then assessed for surface CD62P using flow cytometry, and (C) released ATP in the supernatant from the treated platelets using the luminescence-based assay. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001, by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4). (D) Representative immunoblots for (pAkt, Ser 473), total Akt, and GAPDH in the treated platelets (n = 3). (E and F) Representative curves and quantification of washed platelet aggregation in response to 100 μg/mL cont IgG or APS IgG treatment in the presence or absence of cAMP inducers, followed by stimulation with ADP (Effective concentration of 5-10 μM). Data represent mean ± SD. ∗∗∗∗P < .0001 and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 5).
cAMP inducers decrease APS IgG–induced platelet activation and aggregation. Purified platelets were preincubated with A2AR-i (5μM), A2AR-a (5 μM), dibutyryl-cAMP (Dibut-cAMP, 0.5 mM), 8-Br-cAMP (1 mM), or forskolin (1 μM) for 20 minutes, followed by stimulation with 100 μg/mL cont IgG or APS IgG for 1 hour. (A-B) Platelets were then assessed for surface CD62P using flow cytometry, and (C) released ATP in the supernatant from the treated platelets using the luminescence-based assay. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001, by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4). (D) Representative immunoblots for (pAkt, Ser 473), total Akt, and GAPDH in the treated platelets (n = 3). (E and F) Representative curves and quantification of washed platelet aggregation in response to 100 μg/mL cont IgG or APS IgG treatment in the presence or absence of cAMP inducers, followed by stimulation with ADP (Effective concentration of 5-10 μM). Data represent mean ± SD. ∗∗∗∗P < .0001 and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 5).
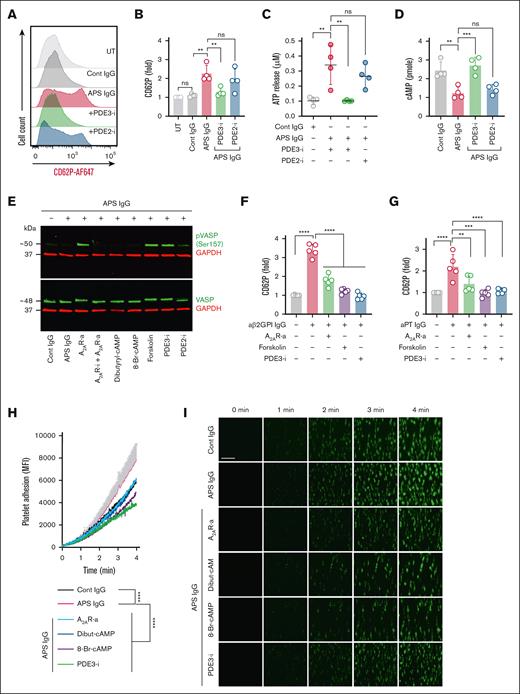
We next asked whether stabilizing cAMP levels through phosphodiesterase inhibition might limit platelet activation. We used BAY 60-7550 and cilostazol to inhibit PDE2 and PDE3, respectively. PDE3, but not PDE2, inhibition blocked APS IgG–induced CD62P expression (Figure 6A-B), with similar results seen for ATP release (Figure 6C). Furthermore, PDE3 inhibition boosted cAMP in the presence of APS IgG (Figure 6D) and decreased total tyrosine-phosphorylated proteins triggered by thrombin, convulxin, and U46619 (supplemental Figure 11). We further evaluated the downstream effects of cAMP, particularly protein kinase A activation, by assessing the phosphorylation of the critical protein kinase A substrate vasodilator-stimulated phosphoprotein (VASP) at serine 157. Phosphorylated VASP constitutes an essential brake on platelet activation. Treatment with an A2AR agonist, forskolin, or a PDE3 inhibitor promoted VASP phosphorylation (Figure 6E; supplemental Figure 12). These agents also effectively negated aβ2GPI IgG–induced CD62P expression (Figure 6F) and total-tyrosine phosphorylated proteins (supplemental Figure 13), as well as aPT IgG–induced CD62P expression (Figure 6G). To assess the functional consequences of APS IgG on platelet adhesion under arterial shear, we performed a collagen-coated microfluidic flow chamber assay with shear set to 1800 s–1. APS IgG–treated platelets exhibited significantly increased surface accumulation compared to control IgG–treated platelets (Figure 6H-I). cAMP-boosting agents, including an A2AR agonist, dibutyryl-cAMP, 8-Br-cAMP, and a PDE3 inhibitor attenuated this enhanced adhesion (Figure 6H-I).
Inhibition of PDE3 ameliorates APS IgG–induced platelet activation by increasing cAMP. (A-B) Surface CD62P, (C) released ATP, and (D) cellular cAMP were measured in platelets treated with cilostazol (PDE3-i, 10 μM) and BAY 60-7550 (PDE2-i, 10 μM) before stimulation with 100 μg/mL cont IgG or APS IgG. ∗∗P < .01, ∗∗∗P < .001, and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4). (E) Representative immunoblots for (pVASP, Ser 157), total VASP, and GAPDH in platelets treated with A2AR-i (5μM), A2AR-a (5 μM), dibutyryl-cAMP (0.5 mM), 8-Br-cAMP (1 mM), forskolin (1 μM), PDE3-i (10 μM), or PDE2-i (10 μM) for 20 minutes, followed by stimulation with 100 μg/mL of either cont IgG or APS IgG for 1 hour (n = 3). (F-G) Flow cytometric evaluation of surface CD62P in platelets pretreated with A2AR-a (5 μM), forskolin (1 μM), or PDE3-i (10 μM), followed by stimulation with affinity-purified (F) aβ2GPI IgG (20 μg/mL) or (G) aPT IgG (10 μg/mL) for 1 hour. (H-I) Quantification and representative images of platelet adhesion and accumulation in human whole blood stained with DiOC6 and treated with 100 μg/mL cont IgG or APS IgG along with A2AR-a (5 μM), dibutyryl-cAMP (0.5 mM), 8-Br-cAMP (1 mM), PDE3-i (10 μM) perfused through a collagen-coated chamber at arterial shear (n = 5). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. Data represent mean ± SD for panels A-G. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001, by a 1-way ANOVA with the Tukey multiple comparisons test (n = 5). MFI, Mean fluorescence intensity; UT, Untreated.
Inhibition of PDE3 ameliorates APS IgG–induced platelet activation by increasing cAMP. (A-B) Surface CD62P, (C) released ATP, and (D) cellular cAMP were measured in platelets treated with cilostazol (PDE3-i, 10 μM) and BAY 60-7550 (PDE2-i, 10 μM) before stimulation with 100 μg/mL cont IgG or APS IgG. ∗∗P < .01, ∗∗∗P < .001, and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4). (E) Representative immunoblots for (pVASP, Ser 157), total VASP, and GAPDH in platelets treated with A2AR-i (5μM), A2AR-a (5 μM), dibutyryl-cAMP (0.5 mM), 8-Br-cAMP (1 mM), forskolin (1 μM), PDE3-i (10 μM), or PDE2-i (10 μM) for 20 minutes, followed by stimulation with 100 μg/mL of either cont IgG or APS IgG for 1 hour (n = 3). (F-G) Flow cytometric evaluation of surface CD62P in platelets pretreated with A2AR-a (5 μM), forskolin (1 μM), or PDE3-i (10 μM), followed by stimulation with affinity-purified (F) aβ2GPI IgG (20 μg/mL) or (G) aPT IgG (10 μg/mL) for 1 hour. (H-I) Quantification and representative images of platelet adhesion and accumulation in human whole blood stained with DiOC6 and treated with 100 μg/mL cont IgG or APS IgG along with A2AR-a (5 μM), dibutyryl-cAMP (0.5 mM), 8-Br-cAMP (1 mM), PDE3-i (10 μM) perfused through a collagen-coated chamber at arterial shear (n = 5). Data represent mean ± SEM. Two-way ANOVA. Scale bars represent 100 μm. Data represent mean ± SD for panels A-G. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001, by a 1-way ANOVA with the Tukey multiple comparisons test (n = 5). MFI, Mean fluorescence intensity; UT, Untreated.
Ecto-5’-nucleotidase (CD73) activity is inversely associated with platelet activation
CD73 is a surface ectonucleotidase that hydrolyzes AMP to release homeostatic adenosine. To quantify platelet CD73 activity, we measured released inorganic phosphate when washed platelets from aPL+ patients, VTE (aPL–) patients, and healthy controls were exposed to 100-μM AMP. Platelets from aPL+ patients demonstrated significantly lower AMP hydrolysis than healthy controls (Figure 7A), and a positive correlation was observed between platelet CD73 activity and cAMP (r = 0.495, P = .0016) (Figure 7B). There was a nonsignificant trend toward an inverse relationship between platelet CD73 activity and CD62P (r = −0.309, P = .058) (Figure 7C). Platelet CD73 activity correlated inversely with anti-PS/PT antibodies (r = −0.327, P = .045).
Association between platelet hyperactivity and low CD73 activity in APS. (A) Estimation of ectonucleotidase activity by measuring free inorganic phosphate (μM) using the Malachite Green assay kit, after the addition of AMP (100 μM) to purified platelets from the fresh blood of healthy controls (n = 20), patients who are aPL+ (n = 38), or patients with aPL-negative VTE disease [VTE (aPL−), n = 8]. (B) Spearman correlation of CD73 activity with cAMP level and (C) surface CD62P for aPL+ patients as indicated. ∗∗∗∗P < .0001 and ns by a 1-way ANOVA with the Tukey multiple comparisons test. (D) Flow cytometric evaluation of surface expression of CD73 on platelets treated with 100 μg/mL cont IgG or APS IgG. ∗P < .05 by unpaired t test (n = 4). (E) Evaluation of CD73 expression using flow cytometer in platelets treated with aFcγRIIa (1 μg) or PLC-i (1 μM) before inducing 100 μg/mL of APS IgG for 1 hour (n = 4). (F) Schematic representation of healthy platelets treated with affinity-purified antibodies followed by AMP to measure surface CD73 activity. (G-H) Platelets were pretreated with aFcγRIIa (1 μg) or PLC-i (1 μM). Platelets were then stimulated with affinity-purified aβ2GPI IgG (20 μg/mL) (G) or affinity-purified aPT IgG (10 μg/mL) (H) for 1 hour. Platelet surface CD73 activity was measured using the Malachite Green assay kit after adding AMP (100 μM). (I) Schematic representation of the degradation of AMP to adenosine by CD73, including inhibition of CD73 by the selective inhibitor PSB 12379. (J) Platelet CD62P measured on PSB 12379 (20 μM) treated platelets in the presence or absence of APS IgG (100 μg/mL) (n = 5). Data represent mean ± SD. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4).
Association between platelet hyperactivity and low CD73 activity in APS. (A) Estimation of ectonucleotidase activity by measuring free inorganic phosphate (μM) using the Malachite Green assay kit, after the addition of AMP (100 μM) to purified platelets from the fresh blood of healthy controls (n = 20), patients who are aPL+ (n = 38), or patients with aPL-negative VTE disease [VTE (aPL−), n = 8]. (B) Spearman correlation of CD73 activity with cAMP level and (C) surface CD62P for aPL+ patients as indicated. ∗∗∗∗P < .0001 and ns by a 1-way ANOVA with the Tukey multiple comparisons test. (D) Flow cytometric evaluation of surface expression of CD73 on platelets treated with 100 μg/mL cont IgG or APS IgG. ∗P < .05 by unpaired t test (n = 4). (E) Evaluation of CD73 expression using flow cytometer in platelets treated with aFcγRIIa (1 μg) or PLC-i (1 μM) before inducing 100 μg/mL of APS IgG for 1 hour (n = 4). (F) Schematic representation of healthy platelets treated with affinity-purified antibodies followed by AMP to measure surface CD73 activity. (G-H) Platelets were pretreated with aFcγRIIa (1 μg) or PLC-i (1 μM). Platelets were then stimulated with affinity-purified aβ2GPI IgG (20 μg/mL) (G) or affinity-purified aPT IgG (10 μg/mL) (H) for 1 hour. Platelet surface CD73 activity was measured using the Malachite Green assay kit after adding AMP (100 μM). (I) Schematic representation of the degradation of AMP to adenosine by CD73, including inhibition of CD73 by the selective inhibitor PSB 12379. (J) Platelet CD62P measured on PSB 12379 (20 μM) treated platelets in the presence or absence of APS IgG (100 μg/mL) (n = 5). Data represent mean ± SD. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4).
Next, we aimed to determine whether IgG purified from patients with APS could reduce CD73 expression by healthy platelets. Although platelets express relatively low levels of CD73 (supplemental Figure 14), flow cytometry still demonstrated a significant decrease in CD73 expression in platelets treated with APS IgG compared with control IgG (Figure 7D). Blocking FcγRIIa or inhibiting PLC significantly reversed the CD73 changes caused by APS IgG (Figure 7E; supplemental Figure 14). Additionally, treating platelets with affinity-purified aβ2GPI IgG or aPT IgG reduced platelet CD73 activity (Figure 7F-H), which could be restored by the FcγRIIa blocker or PLC inhibitor. To investigate the mechanism behind reduced platelet CD73 expression, we measured soluble CD73 in the supernatants of control platelets stimulated with APS IgG. Stimulation caused a modest but consistent increase in soluble CD73 levels. Pretreatment with an FcγRIIa antagonist significantly decreased CD73 release (supplemental Figure 15), indicating that CD73 cleavage is at least partially dependent on FcγRIIa signaling.
We next evaluated adenosine generation in platelets treated with APS IgG. To enhance the stability of extracellular adenosine, we pharmacologically inhibited equilibrative nucleoside transport (ENT) with the selective ENT1 inhibitor nitrobenzylthioinosine (NBMPR). Platelets were incubated with AMP to facilitate CD73-mediated adenosine production, and extracellular adenosine levels were quantified. NBMPR treatment significantly increased basal adenosine accumulation following AMP supplementation (supplemental Figure 16A). APS IgG and thrombin-stimulated platelets exhibited a marked reduction in adenosine production compared to control IgG and untreated platelets (supplemental Figure 16B). Notably, ENT1 expression remained unchanged in platelets treated with either APS IgG or thrombin (supplemental Figure 17), suggesting that the observed decreases in extracellular adenosine were not due to increased uptake via ENT1, but rather impaired CD73-dependent production.
To further investigate the role of CD73 in restraining platelet signaling induced by APS IgG, we treated control platelets with a specific inhibitor of CD73 and assessed platelet activation by measuring the surface expression of CD62P. Although the CD73 inhibitor, N-(phenylmethyl)-adenosine, 5′-[hydrogen P-(phosphonomethyl)phosphonate] (PSB) 12379, did not significantly activate platelets on its own, it resulted in a significant decrease in cAMP levels (supplemental Figure 18). After pretreating the platelets with the CD73 inhibitor for 20 minutes and then stimulating them with APS IgG for 1 hour, we observed significantly higher levels of platelet activation compared to platelets treated with APS IgG alone (Figure 7I-J). Additionally, control platelets were stimulated with submaximal concentrations (effective concentration ∼25%) of thrombin, convulxin, and U46619, and, in this context, we also found more platelet activation in the presence of the CD73 inhibitor (supplemental Figure 19).
Finally, based on prior reports of increased P2Y12 receptor expression in APS patients,20 we assessed the role of P2Y12 signaling in platelet activation and cAMP regulation. P2Y12 blockade with AR-C 69931 (cangrelor) significantly reduced APS IgG–induced platelet activation (supplemental Figure 20A), confirming its involvement in platelet hyperactivation. However, inhibition of P2Y12 did not significantly modulate cAMP levels (supplemental Figure 20B), indicating that the cAMP reduction observed in APS is more likely to be attributable to CD73 dysfunction than to P2Y12 overexpression.
Discussion
Here, we identified a mechanism by which IgG aPL prime platelets for activation by reducing cAMP. Furthermore, we demonstrated that restoring cAMP through various mechanisms can help platelets resist APS-relevant stimuli and that blocking FcγRIIa significantly inhibits platelet activation induced by affinity-purified aβ2GPI and aPT IgG.
The binding of IgG aPL to the platelet FcγRIIa receptor leads to the phosphorylation of the immunoreceptor tyrosine–based activation motif.26 This phosphorylation event creates a docking site for the signaling protein Syk, which contains an SH2 domain. Once bound, the tyrosine kinase activity of Syk promotes the activation of various isoforms of PLC. PLC then produces second messengers like inositol 1,4,5-trisphosphate and 1,2-diacylglycerol, increasing intracellular calcium and activating protein kinase C.27 Here, we found that APS IgG–treated platelets have significantly higher levels of total tyrosine phosphorylated proteins compared with resting platelets or platelets exposed to control IgG. Additionally, inhibiting PLC reduced platelet activation and reversed the decreased cAMP triggered by aPL.
A recent study focused on aPL-induced signaling pathways in platelets and found that both the (Phosphoinositide 3-kinase) PI3K/Akt and mechanistic target of rapamycin complex 2 (mTORC2)/Akt signaling pathways were upregulated in the platelet transcriptome of APS patients.13 Their findings underscore the potential role of the Akt pathway in activating platelets by aPL,13 which would not be surprising given that the Akt pathway plays a significant role in both inside-out and outside-in signaling during agonist-induced platelet activation.28-30 Here, we found that APS IgG–treated platelets showed higher levels of Akt phosphorylation at S473. Beyond signaling pathways as potential therapeutic targets, recent work has emphasized that procoagulant platelets are a subpopulation of highly activated platelets that express PS and P-selectin on their surface in many thromboinflammatory disease states.31 This expression allows coagulation factors to bind and thrombin to be generated. Indeed, we found that aPL-treated platelets demonstrated more procoagulant activity.
Prior work has demonstrated that platelets express functional CD73, and inhibition of its activity enhances intracellular calcium and total tyrosine phosphorylation.32 Our study found that the CD73 activity of platelets from APS patients is significantly lower than that of healthy control platelets and is inversely associated with platelet activation. Additionally, when purified platelets from healthy controls were treated with a CD73 inhibitor, there was a notable potentiation of platelet activation in response to not only APS IgG but also submaximal doses of other platelet agonists, such as thrombin and convulxin. As CD73 is a GPI-anchored protein, activated PLC may cleave CD73, releasing it from the membrane,33 which aligns with our data demonstrating that we can prevent the APS IgG–mediated loss of CD73 activity by either blocking FcγRIIa or inhibiting PLC. Our findings suggest that the loss of membrane-bound CD73 on platelets in APS disrupts local adenosine production and thereby impairs antithrombotic signaling. Although soluble CD73 retains its enzymatic activity,34 it may lack the spatial proximity to platelet A2AR to enable efficient receptor engagement. Furthermore, we observed a significant reduction in extracellular adenosine levels in APS IgG–treated platelets, without a corresponding change in the expression of ENTs,35 indicating that the defect is more likely to lie in adenosine production rather than uptake. These results underscore the unique importance of surface-tethered CD73 in sustaining adenosine signaling and restraining platelet reactivity in the thromboinflammatory setting of APS. Taken together, it appears that the deficiency of CD73 activity in APS platelets contributes to their heightened susceptibility to activation.
The CD73-adenosinergic axis is a biological pathway in which the ectoenzyme CD73 (ecto-5′-nucleotidase) plays a vital role in producing vascular adenosine.36,37 This locally available adenosine can inhibit platelet activation by stimulating A2 receptor subtypes (A2AR or A2BR), increasing intracellular cAMP levels.38 Here, we found that specific A2AR agonism significantly abolished aPL-induced platelet activation, platelet aggregation, procoagulant platelet formation, and clot retraction. A2BR agonism was not similarly effective, possibly due to lower platelet expression compared with A2AR.39 Platelet cAMP regulates various aspects of platelet function, including protein kinase A (PKA) phosphorylation, degranulation, calcium release, and integrin activation. Accordingly, we found an inverse association between cAMP level and platelet activation markers in freshly isolated platelets from APS patients. Although our study primarily focused on platelet CD73, it is important to consider that multiple cell types, including leukocytes and endothelial cells produce adenosine.40,41 Given the complex regulation of adenosinergic pathways, it is plausible that broader dysregulation may contribute to the prothrombotic state observed in APS. Future studies examining CD73 expression and adenosine production in these other cellular compartments will be crucial in determining whether a systemic adenosinergic defect underlies the thromboinflammatory phenotype of APS.
Platelet cAMP levels are also regulated by PDEs, which mediate cAMP breakdown, thereby serving as a counterpoint to cAMP-mediated signaling. Platelets express 3 types of PDEs, PDE2, PDE3, and PDE5.42 PDE2 and PDE3 primarily control cAMP levels in platelets. Past work has suggested that the PDE3 inhibitor cilostazol could be more effective than aspirin in preventing recurrent ischemic stroke while having a lower risk of bleeding.43 Furthermore, the combination of cilostazol and clopidogrel, a P2Y12 blocker, significantly reduced the recurrence of ischemic stroke without increasing the risk of bleeding beyond clopidogrel alone in high-risk, noncardioembolic patients.44 In the context of APS IgG–induced platelet activation, we found that specific PDE3 inhibition with cilostazol, but not PDE2 inhibition, significantly reduced activation by boosting cAMP. This could be due to the relative abundance of PDE3 in platelets.45 VASP is a vital signaling molecule regulated by intracellular cAMP. Increased cAMP leads to VASP phosphorylation, inhibiting platelet activation and aggregation by modulating the platelet cytoskeleton and integrin function. Here, we found that A2AR agonism, direct adenylate cyclase activation, and PDE3 inhibition were all highly effective strategies for blunting APS IgG–induced platelet activation by increasing cAMP and subsequent phosphorylation of VASP at serine-157.
In summary, we identified several mechanism-informed therapeutic targets, including FcγRIIa and PLC, the inhibition of which prevents CD73 activity from being lost from the platelet surface. Furthermore, A2AR agonists and PDE3 inhibitors emerge as promising strategies for restoring platelet homeostasis in APS, offering the potential to reduce thrombotic risk while minimizing bleeding compared to traditional antiplatelet therapies.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute, grant number R01HL134846 to J.S.K. S.K.N. was supported by a grant from the Arthritis National Research Foundation. B.M.F. was supported by the São Paulo Research Foundation (FAPESP), Brazil. Process number 2022/06868-1. A.T. was supported by a grant from the Rheumatology Research Foundation. Y.Z. was supported by NIH, National Institute of Arthritis & Musculoskeletal & Skin Diseases, grant number K08AR080205.
Authorship
Contribution: S.K.N., T.G.N., B.M.F., S.Y., M.C.F.N., K.S., K.K., E.C., K.S., C.H.R., and M.P.T. performed experiments and collected and analyzed data; C.S., J.A.M., A.T., J.K.S., and Y.Z. identified and recruited participants; S.K.N., M.H., Y.Z., and J.S.K. conceived the study; and all authors participated in drafting the manuscript and approved the final version for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason S. Knight, Division of Rheumatology Department of Internal Medicine, University of Michigan, 1150 W. Medical Center Dr, Ann Arbor, MI 48109-5680; email: jsknight@umich.edu.
References
Author notes
Data are available on request from the corresponding author, Jason S. knight (jsknight@umich.edu) or Somanthapura K. NaveenKumar (nsomanat@med.umich.edu).
The full-text version of this article contains a data supplement.

![Relationship between platelet activation and low cAMP levels in patients with aPL+. (A) Estimation of cAMP in platelets (2 × 105/μL) purified from the fresh blood of healthy controls (n = 20), patients who are aPL+ (n = 38), and patients with aPL-negative VTE [VTE (aPL−), n = 8]. (B) Flow cytometric evaluation of platelet surface P selectin (CD62P+ events within the CD41+ population) and (C) activated αIIbβ3 receptor (PAC-1+ events within the CD41+ population) in fresh blood. (D) ELISA-based estimation of plasma PF4. Spearman correlation for platelet cAMP of aPL+ patients with surface CD62P (E), activated αIIbβ3 receptor (F), plasma PF4 (G), and anti-PS/PT IgG (H). Data represent mean ± standard deviation (SD). ∗P < .05, ∗∗P < .01, ∗∗∗∗P < .0001, and nonsignificant (ns) by 1-way analysis of variance (ANOVA) with the Tukey multiple comparisons correction. MFI, Mean Fluorescence Intensity.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/21/10.1182_bloodadvances.2025016162/2/m_blooda_adv-2025-016162-gr1.jpeg?Expires=1767706661&Signature=YzRpD3rZ--wJa-7Y2ldeUyY14vym0GUQXPkJrk9ZqKWrsVkHzuCe9VL1FWuJ9jWFssggN6gdljrYCcMaLwzhFZv~mE2Kfzh-aDF-a1mxgW3nsUKZPFp0UitpywTUYwzn2fy8VVE2iUP9S4G6YFArqg5Y0~S~7aVFew3~TEEdLjXMeD27IRHoTjPy76Bb9WGNwMcmeoJqdUuRAetYASCi6fFyIBggbAVTLDKW~B08tqFdIYeJNn5Xfk3epazHDA9Pirz9wLYHmsw90-xFhCe5WtwfP7~4CLkN79Dc4abKuGE39j~V54TugxDQ52H5-JqgB61M2nwZx3sHXKwbma9H2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Association between platelet hyperactivity and low CD73 activity in APS. (A) Estimation of ectonucleotidase activity by measuring free inorganic phosphate (μM) using the Malachite Green assay kit, after the addition of AMP (100 μM) to purified platelets from the fresh blood of healthy controls (n = 20), patients who are aPL+ (n = 38), or patients with aPL-negative VTE disease [VTE (aPL−), n = 8]. (B) Spearman correlation of CD73 activity with cAMP level and (C) surface CD62P for aPL+ patients as indicated. ∗∗∗∗P < .0001 and ns by a 1-way ANOVA with the Tukey multiple comparisons test. (D) Flow cytometric evaluation of surface expression of CD73 on platelets treated with 100 μg/mL cont IgG or APS IgG. ∗P < .05 by unpaired t test (n = 4). (E) Evaluation of CD73 expression using flow cytometer in platelets treated with aFcγRIIa (1 μg) or PLC-i (1 μM) before inducing 100 μg/mL of APS IgG for 1 hour (n = 4). (F) Schematic representation of healthy platelets treated with affinity-purified antibodies followed by AMP to measure surface CD73 activity. (G-H) Platelets were pretreated with aFcγRIIa (1 μg) or PLC-i (1 μM). Platelets were then stimulated with affinity-purified aβ2GPI IgG (20 μg/mL) (G) or affinity-purified aPT IgG (10 μg/mL) (H) for 1 hour. Platelet surface CD73 activity was measured using the Malachite Green assay kit after adding AMP (100 μM). (I) Schematic representation of the degradation of AMP to adenosine by CD73, including inhibition of CD73 by the selective inhibitor PSB 12379. (J) Platelet CD62P measured on PSB 12379 (20 μM) treated platelets in the presence or absence of APS IgG (100 μg/mL) (n = 5). Data represent mean ± SD. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, and ns by a 1-way ANOVA with the Tukey multiple comparisons test (n = 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/21/10.1182_bloodadvances.2025016162/2/m_blooda_adv-2025-016162-gr7.jpeg?Expires=1767706661&Signature=3jpdUnndkUxhFoG44LMqyeaJXvr734a~JnNH8Cxg0n-EaIYNUl2NP~wBv1Rul-u38He5PA0ATe~fidfttZiiuvF-D9ME-EZw33702Jue7rk36F2NsQmpPVI7Zng2ho64MbGgfpXmDDGTbLFFHe6sk11fhB~tXH4vClyWUYrvVE0QtZ2dknYJ2UHmqc9BqbVNFHjG-F~Lshuuxpnrf7liHU38rcQW2yqFTa1J0Bevq2PViU4auiMDSKteXF6oq6ghXrLz9s9nqfwusqfSnLTVHpHFVXT9wXlR8sSFAhi0dyY~Jfyzac~9haQS7e44wD8Ks-86S-Hgn~4-sqb7bPwCPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)