Patients with hemophilia A can develop antifactor antibodies to factor VIII. The incidence is ∼30%, and such patients suffer worse morbidity and mortality. The only proven method to eradicate these inhibitors is via immune tolerance induction therapy, which consists of infusing factor VIII concentrates at regular intervals. This approach is effective ∼65% of the time, leaving at least a third of patients who develop inhibitors with this lifelong problem. Although emicizumab has greatly improved the quality of life of inhibitor patients, eradicating the inhibitor remains an important treatment goal. Animal models have shown the potential for gene therapy to induce tolerance. A recent abstract describing a study in humans demonstrated the potential for successful tolerance induction. This article will describe the rationale for using gene therapy to induce tolerance and provide this author's viewpoint on the importance and possible historic significance of attempting to eradicate inhibitors with this approach.
Introduction
Hemophilia A is an inherited, lifelong bleeding disorder characterized by the deficiency of factor VIII (FVIII) and caused by mutations in the F8 gene. Untreated, patients experience recurrent spontaneous and traumatic bleeding, primarily in joints.1 Treatment with clotting factor concentrate to replace the missing or defective FVIII, although effective in preventing and treating bleeds, is invasive and costly, and up to 30% may develop inhibitor antibodies. Anti-FVIII antibodies disrupt clot formation and result in poorly controlled bleeding and significant morbidity. Thus, a major goal of hemophilia therapy has been to develop effective and safer therapies for hemophilia with and without inhibitors.
The inhibitor antibody response directed against exogenous FVIII is a T-cell–dependent, B-cell–immune response that requires T-cell help2-5 and is regulated by FoxP3+ T regulatory (T reg) cells.4,6-8 In early studies of hemophilia animal models, it was recognized that experimental disruption of B- and T-cell pathways could reduce or prevent inhibitors,9 leading to interest in eradicating inhibitors by nonfactor and gene transfer approaches.
Among the novel nonfactor therapies developed in the past decade, one, emicizumab, a bispecific monoclonal antibody that mimics FVIII, has been licensed for bleed prevention in patients with and without inhibitors.10-12 Although effective and safe in preventing bleeds, ultimately all these nonfactor therapies require perpetual administration, and none eradicate inhibitors. Although the ultimate “cure” would be the permanent correction of a factor deficiency to normal levels from a young an age, it is recognized this would require correction of the defective gene, for example, by gene editing, which is some years away.
Significant progress has recently been made with FVIII gene transfer. In a phase 3 clinical trial,13 valoctocogene roxaparovovec (Roctavian; BioMarin, Novato, CA), an adeno-associated virus (AAV) vector–based FVIII gene transfer, has been shown to be safe and effective in preventing bleeds in adults with hemophilia A aged ≥18 years without inhibitors, similar to findings in hemophilia B. Individuals with hemophilia and a history of inhibitors have been excluded from gene trial participation. This is because they may not have a therapeutic response due to the inhibitor or due to the potential that circulating FVIII produced by the FVIII transgene might lead either to recurrence of inhibitors in those previously tolerized or to an anamnestic response increasing inhibitor titer, either of which could increase bleeding risk and morbidity. However, based on the continuous, uninterrupted FVIII expression possible in recipients of gene transfer and the mounting evidence in preclinical studies of gene transfer in hemophilia animal models, the potential for gene transfer as a means of tolerance induction to the transgene was recognized.4,14-16
Inhibitor eradication in animal models of gene transfer
Although the exact mechanism of clinical immune tolerance induction (ITI) is not fully elucidated, it was recognized that regular administration of an antigen (ie, FVIII) exerts a tolerogenic effect on the immune system. So, if continual exposure to that antigen could be accomplished over time, it was reasoned that tolerance to a foreign protein, for example, FVIII, could be achieved.4,5 Thus, the concept arose that continuous endogenous factor production by gene transfer would be more effective in achieving clinical tolerance than intermittent exposure to exogenous FVIII concentrate with clinical ITI.
The initial demonstration that tolerance induction by gene transfer could reduce inhibitor formation was performed in a mouse model of hemophilia B (FIX deficiency).14 Because of the smaller size of FIX, early studies were performed in the hemophilia B (FIX deficient) mouse model. Mingozzi et al demonstrated that hepatic FIX gene transfer eradicated the anti-IX antibody response in this mouse. They further showed that the induction of CD4+ T reg cells were responsible for inhibitor eradication in this model.17 It was postulated that the immunological milieu of the liver was an important component of this tolerizing response, and they showed that the reduced anti-IX formation after hepatic gene transfer was due to FIX-specific immune tolerance.
Subsequent studies in hemophilia A dogs with preexisting inhibitors receiving AAV-canine FVIII transgene experienced a reduction of anti-FVIII formation and successfully induced tolerance to FVIII.18 The time to inhibitor eradication was shorter, 4 to 5 weeks, in younger (<2-year-old) dogs and longer, 100 weeks, in an older (5-year-old) dog. Although FVIII levels ultimately were modest, bleeding events became less frequent after inhibitor titers were no longer detected. The tolerization was again accompanied by an increase in T regs, CD4+CD25+FoxP3+ T reg cells, which suppress antibody formation in these models.19
Inhibitor eradication after gene transfer in hemophilia B dogs and mice was also associated with concurrent loss of anaphylaxis,19,20 and furthermore, gene transfer was also successful in tolerizing inhibitor-prone dogs, even before inhibitor formation.21 Although limited by small numbers, lower titers, and other differences between canine and human ITI data,6,22,23 these models provided preliminary data for the design of human studies.
Inhibitor eradication in humans with gene transfer
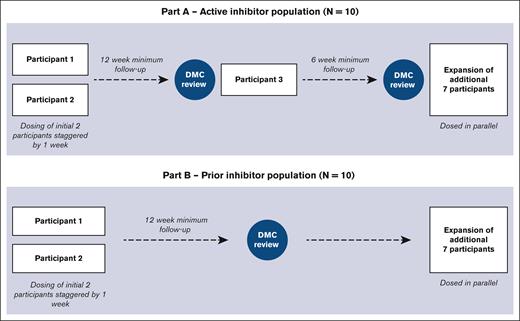
Whether AAV FVIII gene transfer can eradicate inhibitors in individuals with hemophilia A and inhibitors is the goal of the GENEr8-INH study (ClinicalTrials.gov identifier: NCT04684940). Valoctocogene roxaparvovec will be evaluated in individuals with hemophilia A and inhibitors, previously excluded from gene transfer trials. This trial will use the same product, dose, and outcome measures as the phase 3 GENEr8-3 study.13 Although prophylactic corticosteroids were originally used to prevent immune responses to the vector, it has been discontinued because new evidence indicates prophylactic steroids are not an effective strategy.24 Ten patients each will be enrolled on this 2-part study, including 10 with severe hemophilia A with active FVIII inhibitors currently on emicizumab for prophylaxis (part A) and 10 with a history of FVIII inhibitors previously successfully tolerized (part B).25 The trial design is shown in Figure 1. A chromogenic substrate assay will be used to measure FVIII levels, and a chromogenic Bethesda assay will be performed to measure FVIII inhibitor titers.
The design of GENEr8-INH study. Part A includes patients with active inhibitors, and part B includes patients with prior inhibitors that have been tolerized. The first 4 patients received prophylactic steroids as indicated.
The design of GENEr8-INH study. Part A includes patients with active inhibitors, and part B includes patients with prior inhibitors that have been tolerized. The first 4 patients received prophylactic steroids as indicated.
It is hypothesized that outcomes in previously tolerized patients (part B) will be similar to patients without a history of inhibitors, and importantly, their inhibitors will not recur after gene transfer. In addition, it is hypothesized that gene transfer will result in immune tolerance. If successful in inhibitor patients, this approach would allow for such individuals to resume FVIII concentrate for managing bleeds and surgery, rather than by less reliable bypass agents, and potentially experience factor utilization and other outcomes similar to those without an inhibitor.
Data on 2 patients enrolled thus far in part B are consistent with previous trial data in patients without inhibitors: at 36 weeks after gene transfer, 1 patient reached a FVIII peak of 7.8 IU/dL and the other 67.2 IU/dL. Alanine aminotransferase (ALT) elevations occurred in both patients and required immunosuppressive therapy similar to those without inhibitors. The outcomes have been both expected and satisfactory. An additional 8 patients are planned to complete a cohort of 10 patients for part B.
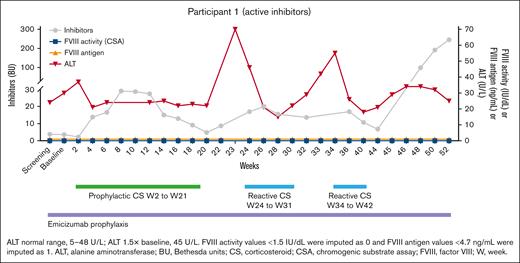
The first 2 patients enrolled in part A included a 30-year-old patient with hemophilia A and a lifelong history of inhibitors (Figure 2) with no past ITI. As a child, he lived in a country where ITI was not possible, and as an adult, his hemophilia center health care team decided not to pursue ITI. His peak inhibitor titer is 200 Bethesda unit (BU), and his titer at screening was 3.8 BU. He has had numerous bleeds and chronic arthropathy, most notably in one knee. Once emicizumab became available, he began it to prevent bleeds. After gene transfer, his FVIII inhibitor titer rose as anticipated (similar to the rise in inhibitor titer typical of traditional ITI) and then fell to <5 BU, at which time there was a rise in ALT. This was managed with protocol-driven corticosteroid treatment and was followed by a rise in inhibitor titer. The steroids were tapered off, and there was 1 more rise in his inhibitor titer, followed by normalization of ALT. Then, his inhibitor peaked at 52 weeks and persists. Although the cause of this response is not known, it is assumed the patient began to produce endogenous FVIII, which stimulated a rise in inhibitor titer, as if he were again in the early stages of tolerization. The corticosteroids given for the rise in ALT may have blunted a T reg cell response, but it is hoped in his second year after infusion after stopping corticosteroids the assumed endogenous FVIII production will lead to tolerance. His immunologic assessments will hopefully provide mechanistic evidence of tolerance.
The time course for participant 1. At the interim analysis, he had an increase in FVIII inhibitor, as seen in early stages of immune tolerance and suggesting it arose from endogenous FVIII production. The patient had 2 courses of reactive steroids, which may have impeded tolerization due to their negative impact on T reg cells.
The time course for participant 1. At the interim analysis, he had an increase in FVIII inhibitor, as seen in early stages of immune tolerance and suggesting it arose from endogenous FVIII production. The patient had 2 courses of reactive steroids, which may have impeded tolerization due to their negative impact on T reg cells.
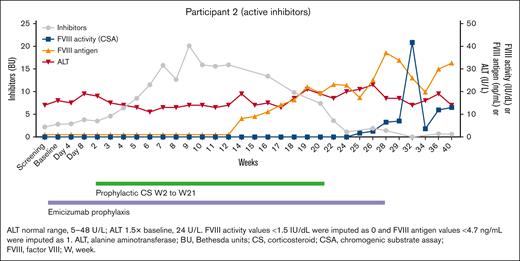
The second patient (Figure 3), 27 years old, had never had ITI treatment. His peak inhibitor titer is 72 BU, and his titer at screening was 2.2 BU. He had a significant rise in his inhibitor to 20 BU at week 9 after the infusion, followed by a steady decline to an unmeasurable level at week 32. Commensurate with this, he had a rise first in FVIII antigen at week 12, followed by a rise in FVIII activity at week 25. At his final follow-up of the interim analysis, his FVIII inhibitor is unmeasurable, and his FVIII activity is at 13 IU/dL by chromogenic substrate assay. It is noted that he had a spike in his FVIII level at week 32, and although it is clear from the data that this is not due to exogenous FVIII, we do not know for certain the reason for this; and for now, we assume there was an assay issue with that 1 sample. An additional 8 patients are planned to complete this active inhibitor cohort of 10 patients. Similar to the first patient, immunological laboratory testing will be performed in the future.
The time course for participant 2. This patient achieved tolerance as noted by a negative inhibitor titer, presence of FVIII antigen, and an FVIII activity of 15%.
The time course for participant 2. This patient achieved tolerance as noted by a negative inhibitor titer, presence of FVIII antigen, and an FVIII activity of 15%.
One important point regarding the first 2 patients is that neither had been treated with ITI due to their specific situations; however, in well-resourced countries, the majority of adult active inhibitor patients likely had at least attempted ITI and failed. Are those patients immunologically different than the 2 presented above? Could gene transfer to induce tolerance in this situation result in different outcomes? Currently, there is no answer to this question; however, additional enrolled patients, some of whom undoubtedly will have had ITI, could shed light on these important questions.
Viewpoint
These findings are among the most important and compelling implications of gene transfer, given the high morbidity and difficulty of clinical management of hemophilia inhibitor patients. Although nonfactor therapies such as emicizumab provide an effective therapeutic alternative to bypass therapy, bleeding events and their associated complications still occur. With the development and recent licensure of gene transfer, by contrast, not only is there cost-effective bleed reduction and improvement in quality of life in patients with hemophilia without inhibitors, but now there is the potential for inhibitor eradication in patients with hemophilia with inhibitors. The anticipated immunologic correlates of these findings will be of great interest.
Authorship
Contribution: G.Y. conceived and wrote the manuscript.
Conflict-of-interest disclosure: G.Y. has received consulting fees from BioMarin, Centessa, CSL Behring, Genentech/Roche, Hema Biologics/LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi Genzyme, Spark, and Takeda, and funds for research support from Genentech/Roche, Sanofi, and Takeda.
Correspondence: Guy Young, Children’s Hospital Los Angeles, 4650 Sunset Blvd, Mail Stop 54, Los Angeles, CA 90027; email: gyoung@chla.usc.edu.