TO THE EDITOR:
We read with interest the correspondence by Gutmair et al,1 who did not find major differences between R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone) and R-bendamustine in frontline therapy of transplant-ineligible mantle cell lymphoma (MCL). We agree that patients with early progression of disease who were treated with R-CHOP might have an advantage if indicated for T-cell engaging therapies.
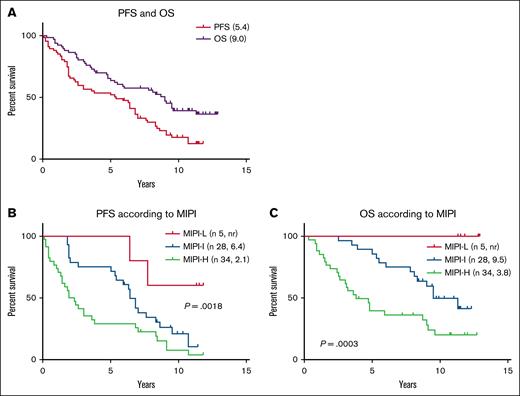
In younger patients with MCL, the study by Hermine et al2 demonstrated prolongation of survival with the alternation of R-CHOP and R-DHAP (high-dose cytarabine and cisplatin) compared to R-CHOP. The prospective observational study by the Czech Lymphoma Study Group (CLSG-MCL-01; ClinicalTrials.gov identifier: NCT03054883) in transplant-ineligible MCL modified this protocol for older/comorbid patients who were treated with the alternation of R-CHOP and R-cytarabine.3,4 In the subcohort of patients treated at the centers implementing maintenance rituximab (n = 67), median progression-free survival (mPFS) and overall survival reached 5.4 and 9 years (see figure), which is comparable to both the R-CHOP (mPFS, 4.1 years) and R-bendamustine group (mPFS, 2.9 years).1 Baseline characteristics of the patients enrolled in the CLSG-MCL-01 study were like those reported for the R-CHOP and R-bendamustine cohorts (median age, 70 years; using MCL International Prognostic Index categorized into high/intermediate/low risk in 51%, 42% and 7% patients, respectively; and Ki-67 proliferation index ≥30% in 54% patients). The numbers of patients who achieved minimal residual disease (MRD) negativity in peripheral blood and bone marrow at end of induction in the CLSG-MCL-01 trial were also comparable to the results from the R-CHOP cohort of the European MCL Elderly Trial (peripheral blood: 78% compared to 64%; bone marrow: 55% compared to 52%).5 In the European MCL R2 Elderly trial (CinicalTrials.gov identifier: NCT01865110), progression-free survival and overall survival were also not different between the R-CHOP-only and R-CHOP/RHA + dexamethasone induction.6 No unexpected safety signals were reported in either study.
Survival parameters of transplant-ineligible patients treated with the alternation of R-CHOP and R-cytarabine. (A) PFS and overall survival (OS) of 67 patients treated with the alternation of R-CHOP and R-cytarabine (2 g/m2, 2 doses at 24 hours), 3+3 cycles, and maintenance rituximab (every 3 months for 2 years, for a total of 8 doses). (B) PFS according to MIPI. (C) OS according to MIPI. MIPI, Mantle Cell Lymphoma International Prognostic Index; MIPI-L, MIPI-low risk; MIPI-I, MIPI-intermediate risk; MIPI-H, MIPI-high risk; nr, not reached; PFS, progression-free survival.
Survival parameters of transplant-ineligible patients treated with the alternation of R-CHOP and R-cytarabine. (A) PFS and overall survival (OS) of 67 patients treated with the alternation of R-CHOP and R-cytarabine (2 g/m2, 2 doses at 24 hours), 3+3 cycles, and maintenance rituximab (every 3 months for 2 years, for a total of 8 doses). (B) PFS according to MIPI. (C) OS according to MIPI. MIPI, Mantle Cell Lymphoma International Prognostic Index; MIPI-L, MIPI-low risk; MIPI-I, MIPI-intermediate risk; MIPI-H, MIPI-high risk; nr, not reached; PFS, progression-free survival.
The data from the CLSG-MCL-01 and MCL R2 Elderly trials suggest that R-CHOP/R-cytarabine (±dexamethasone) represents a relevant alternative to R-CHOP or R-bendamustine.
Acknowledgments: Funding for this study was provided by the Ministry of Health of the Czech Republic grant AZV NU23-03-00172 (P.K., M.T.), National Institute for Cancer Research, Programme EXCELES, ID Project No. LX22NPO5102 (P.K.), and the Leukemia & Lymphoma Society (LLS), grant MCL 7005-24 (P.K.).
Contribution: P.K. and M.T. wrote the manuscript; and L.T. and P.B. prepared the figure.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavel Klener, First Department of Internal Medicine-Hematology, University General Hospital in Prague and First Faculty of Medicine, Charles University, U Nemocnice 2, 12808 Prague 2, Czech Republic; email: pavel.klener2@vfn.cz.