Key Points
High protein or tryptophan in diet increases the risk of cancer-associated VTE in mice.
Colon cancer reprograms tryptophan metabolism to augment VTE risk in mice, which is mitigated by an IDO1 inhibitor.
Visual Abstract
Studies related to cardio-oncology remain a high priority, considering that venous thromboembolism (VTE) in cancer survivors is the second most common cause of death. Although diet-derived metabolites are emerging as contributors to VTE, the influence of specific dietary components, their underlying mechanisms, and means to mitigate cancer-associated VTE remain poorly investigated. This point is important because population studies point to a protein-rich diet associated with VTE. Leveraging a new colon cancer–VTE mouse model, we show that an imbalanced protein-rich diet augments venous thrombogenicity in tumor-bearing mice. Further probing showed that dietary tryptophan induces a procoagulant venous wall, characterized by upregulation of tissue factor, plasminogen activator inhibitor-1, and von Willebrand factor and downregulation of thrombomodulin. Targeted metabolomics of sera from tumor-bearing mice revealed a pattern consistent with increased biogenesis of kynurenine (Kyn) and its suppressed catabolism, despite equal diet consumption in all groups. Kyn levels positively correlated with venous clots. Indoleamine 2,3-dioxygenase 1 (IDO1) is a key rate-limiting enzyme converting tryptophan to Kyn. Sera and the inferior vena cava of tumor-bearing mice showed greater IDO1 activity and protein level, respectively. A specific IDO1 inhibitor reduced serum levels of Kyn, restored the balance of procoagulant and anticoagulant factors in the venous endothelium, and significantly suppressed venous thrombogenicity in tumor-bearing mice. Taken together, our results uncovered a prothrombotic effect of a protein- or tryptophan-rich diet in a syngeneic colon cancer model, which is significantly attenuated by an IDO1 inhibitor.
Introduction
Next to cancer recurrence or progression, cardiovascular diseases (CVDs) are the leading causes of death in cancer survivors.1-3 The Surveillance, Epidemiology, and End Results database revealed that among 3 234 256 US cancer survivors between 1973 and 2012, 38% died from cancer, and 11.3% died from CVDs.1,3 The cardiovascular complications in patients with cancer are grouped as cardio-oncology. Of various CVDs, such as cardiomyopathy and acute coronary events, cancer survivors are at a fourfold to sevenfold higher risk of venous thromboembolism (VTE), which proves fatal in 1 of 7 patients with cancer.1,4,5 The risk of cancer-associated VTE varies with cancer type and tumor burden and is the highest among patients with gastrointestinal cancers. Even after adjusting for confounders, VTE is associated with reduced overall survival during the first year of cancer diagnosis.6 Furthermore, patients with active cancer have a 52% cumulative incidence of VTE recurrence over 10 years.7 All these studies highlight cancer-associated VTE as an important cardio-oncology problem worthy of investigation.
Our previous work showed that mice with colon adenocarcinoma exhibit significantly higher inferior vena cava (IVC) clot weights, associated with increased blood levels of kynurenine (Kyn), a tryptophan (Trp) metabolite.8 The study was, to our knowledge, the first to imply that specific dietary components could influence cancer-associated thrombosis, although the mechanism remains unclear. There is a dearth of studies examining the effect of dietary proteins on CVD at the intersection of cancer.
Trp is an essential amino acid derived from dietary protein. The reported average dietary Trp intake by US adults is 826 mg/d, which is substantially higher than the average requirement of ∼280 mg/d for a 70-kg adult.9,10 Furthermore, a significant association between VTE and a protein-rich diet was found in 129 430 participants in the Nurses’ Health study and Health Professionals Follow-up study.11 Patients with cancer are often advised to increase their dietary protein intake to compensate for cancer cachexia and chemotherapy side effects, as well as during the periprocedural and postprocedural periods after esophagectomy, colectomy, or ileostomy.12-15 Approximately 3% to 8% of these patients receive parenteral nutrition at some point during their treatment for malnutrition and gastrointestinal dysfunction. Trp is an essential amino acid, constituting 0.5 to 1.0 g per 100 g of amino acids in infusion for patients on parenteral nutrition.16,17 A patient receiving 1 liter of parenteral nutrition with a 10% amino acid solution receives 1000 mg of Trp per 1-L solution. Such patients are exposed to ∼5 to 8 times more Trp than dietary recommendation.
In this study, we set out to understand the effect of imbalanced protein-rich, Trp-rich diets on cancer-associated VTE. Leveraging syngeneic and allogeneic tumor models, we examined the impact of such diets on venous thrombogenicity. Importantly, while examining systemic-level alterations in Trp metabolism in the presence of a tumor, we identified a key enzyme as a regulator of diet-induced venous thrombosis, which was further validated through pharmacological manipulation.
Methods
MC38 syngeneic and HT-29 allogeneic colon cancer models
The Institutional Animal Care and Use Committee at Boston University (430092001) approved all animal experiments. Mice from The Jackson Laboratory (Bar Harbor, ME; C57BL/6J [catalog no. 000664] and Nu/J [catalog no. 002019]) were used. In this study, tumor size was measured weekly for 4 weeks using a caliper (VWR International, Radnor, PA), and tumor volume was calculated as follows: V = L × (W2).
IVC stenosis model
An IVC ligation stenosis model was applied as previously described.18 Thrombi formed in the IVC distal to the ligation point were harvested for analysis. Clot weight and body weight of each mouse were recorded. Under our experimental conditions (a short 3- to 5-day diet period), there were no statistically significant differences in body weight among the 3 different diets administered to age- and sex-matched 8- to 12-week-old C57BL/6 mice. Body weights ranged from 19 to 22 g, including in both tumor-bearing and non–tumor-bearing mice. Thus, clot weight was normalized to body weight for each mouse as a readout of thrombogenicity.
Other methods
Diet composition, cells, reagents, antibodies, tissue factor (TF) activity assay, immunohistochemistry, targeted metabolomics, assay of coagulation factors, Kyn injection experiment, Trp tracing experiment, and statistical analysis are all fully described in supplemental Methods.
Results
A syngeneic mouse model of colon cancer–associated venous thrombosis
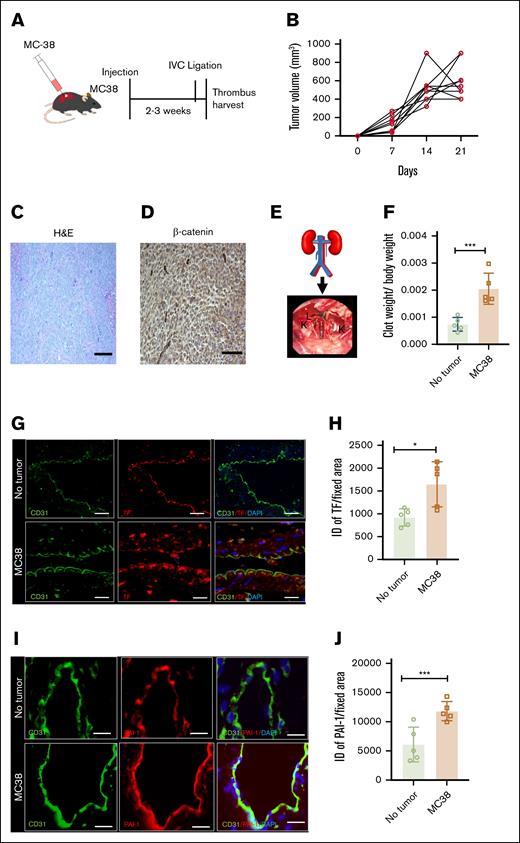
A syngeneic tumor mouse model using MC38 cells was generated in immunocompetent C57BL/6J female mice. Mice received subcutaneous injections of 1 million MC38 cells in the right flank (Figure 1A). Once the mice developed a tumor of size ∼500 mm3 without significant necrosis or hemorrhage and with tumor β-catenin expression (a mediator of oncogenesis; Figure 1B-D), they were subjected to partial IVC ligation, an established model of acute deep vein thrombosis (Figure 1E). The model is based on a partial stasis-induced flow reduction in the infrarenal IVC.18 Forty-eight hours after IVC ligation, clots were harvested, and clot weights normalized to body weight were considered biological readouts of thrombogenicity. Mice without tumors served as controls. With tumor, normalized clot weights increased by ∼70% compared to corresponding controls (∗∗∗P = .0016; Figure 1F).
Development of a syngeneic colon cancer tumor model with deep vein thrombosis. (A) A group of 8- to 12-week-old C57BL/6 female mice were injected at the flank with 1 million colon cancer MC38 cells or vehicle. Age-matched mice were compared between the tumor-bearing and control mice. Upon tumors reaching a certain size (typically within 2-3 weeks; see “Methods”), IVC ligation was performed, and clots were harvested 48 hours thereafter. (B) Averages of tumor growth in individual mice injected with MC38 over 3 weeks. (C) Histological analysis of MC38 cells; H&E stain was used. (D) Immunohistochemistry (IHC) of tumors using β-catenin antibody are shown at 100× original magnification (scale bar, 100 μm). (E) A schematic figure of the partial IVC ligation (see “Methods”) and an intraoperative feature of the IVC ligation model. (F) Averages of clot weights normalized to body weight from both groups are shown. Error bars represent the standard error of the mean (SEM; n = 5 mice per group; P = .0016). (G) Paraffin-embedded sections of IVC were stained using anti-TF and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Images are representative of IVCs from 5 mice in each group. Arrowheads point to endothelial cells. White arrowheads point to the TF expression on endothelial cells, and asterisk points to the subendothelial cells (scale bar, 100 μm). (H) Three images per mouse were analyzed, and a region of interest was marked corresponding to the endothelial cells. ID was normalized to the surface area of the region of interest measured in microns by using ImageJ. Averages of ID normalized to surface area are shown. Error bars represent SEM (P = .0154). (I) IVCs were stained by using anti–PAI-1 and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Representative images from 5 mice in each group (scale bar, 100 μm). (J) PAI-1 expression was quantified as previously described. Averages of ID normalized to the surface area are shown. Error bars represent SEM (P = .0056). DAPI, 4′,6-diamidino-2-phenylindole; H&E, hematoxylin and eosin; ID, integrated density; K, kidney; L, ligated IVC. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.0001, ∗∗∗∗P < 0.0001.
Development of a syngeneic colon cancer tumor model with deep vein thrombosis. (A) A group of 8- to 12-week-old C57BL/6 female mice were injected at the flank with 1 million colon cancer MC38 cells or vehicle. Age-matched mice were compared between the tumor-bearing and control mice. Upon tumors reaching a certain size (typically within 2-3 weeks; see “Methods”), IVC ligation was performed, and clots were harvested 48 hours thereafter. (B) Averages of tumor growth in individual mice injected with MC38 over 3 weeks. (C) Histological analysis of MC38 cells; H&E stain was used. (D) Immunohistochemistry (IHC) of tumors using β-catenin antibody are shown at 100× original magnification (scale bar, 100 μm). (E) A schematic figure of the partial IVC ligation (see “Methods”) and an intraoperative feature of the IVC ligation model. (F) Averages of clot weights normalized to body weight from both groups are shown. Error bars represent the standard error of the mean (SEM; n = 5 mice per group; P = .0016). (G) Paraffin-embedded sections of IVC were stained using anti-TF and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Images are representative of IVCs from 5 mice in each group. Arrowheads point to endothelial cells. White arrowheads point to the TF expression on endothelial cells, and asterisk points to the subendothelial cells (scale bar, 100 μm). (H) Three images per mouse were analyzed, and a region of interest was marked corresponding to the endothelial cells. ID was normalized to the surface area of the region of interest measured in microns by using ImageJ. Averages of ID normalized to surface area are shown. Error bars represent SEM (P = .0154). (I) IVCs were stained by using anti–PAI-1 and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Representative images from 5 mice in each group (scale bar, 100 μm). (J) PAI-1 expression was quantified as previously described. Averages of ID normalized to the surface area are shown. Error bars represent SEM (P = .0056). DAPI, 4′,6-diamidino-2-phenylindole; H&E, hematoxylin and eosin; ID, integrated density; K, kidney; L, ligated IVC. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.0001, ∗∗∗∗P < 0.0001.
TF is known to augment thrombogenesis, and plasminogen activator inhibitor-1 (PAI-1) is a thrombolysis inhibitor. Realizing that thrombotic factors will be dysregulated by surgical trauma induced by IVC ligation and wanting to probe the effect of cancer before IVC, the following measurements were performed on mice with or without tumors but with sham surgery. The vasculature was subjected to immunostaining using antibodies to TF, PAI-I, and to CD31, an endothelial cell marker (Figure 1G,I). Expression was quantitated and compared among samples using integrated density, which integrates the intensity of antibody staining and the number of pixels per surface area, using ImageJ, as previously described.8,19,20 In all cases, a corresponding immunoglobulin G (IgG) served as negative control (no staining observed). TF and PAI-1 expression in endothelial cells was upregulated in MC38 tumor–bearing mice compared to mice without tumors (∗P = .0154; ∗∗∗P = .0056, respectively; Figure 1H,J).
To probe a possible effect of secreted factors on a thrombotic phenotype, primary human umbilical vein endothelial cells exposed to 1% of individual mouse sera from both groups (tumors and nontumors) underwent a surface procoagulant TF assay. Endothelial cells grown in calf serum and sera from non–tumor-bearing mice served as controls. Sera from tumor-bearing mice increased TF activity by nearly 1.6-fold (supplemental Figure 1), pointing to possible mechanisms of effect associated with plasma factor(s).
Increased dietary protein increases cancer-associated venous thrombogenicity in mice
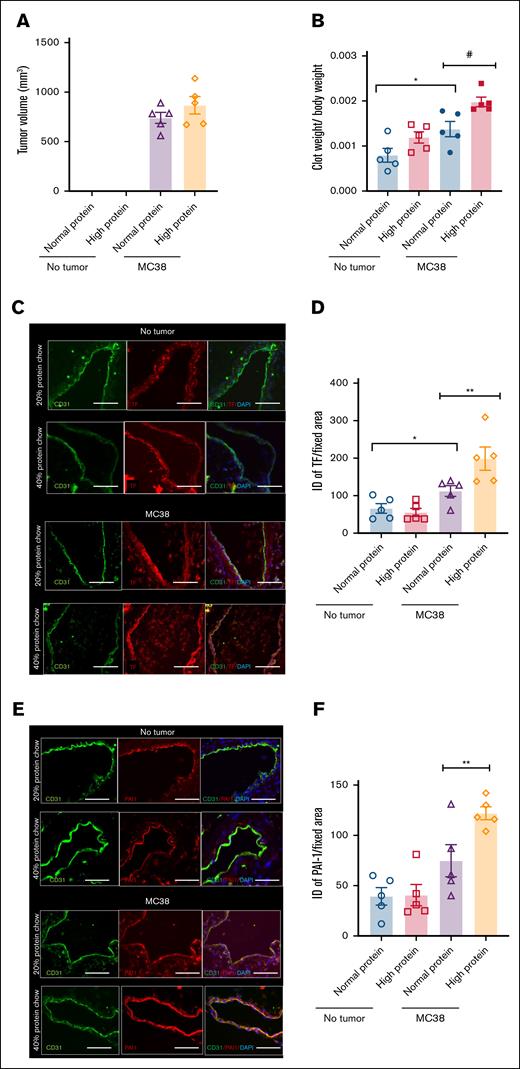
To examine the effects of protein diet modification, we first generated a set of purified diets. A standard mouse chow diet consists of 17 to 20 g of protein per 100 g of chow diet, with the primary source of protein being casein. It is difficult to alter individual dietary content in a normal mouse chow diet without disturbing other components. Hence, we resorted to a purified diet in which casein was the only source of protein. Mice were exposed to a purified diet containing either 20% (normal protein) or 40% protein (high protein; supplemental Table 1). Once tumor volume reached 500 mm3, the chow diet was shifted to high or low protein for 3 days, followed by an IVC ligation model. Clots were harvested 2 days after IVC ligation. As mentioned above, and for this and subsequent experiments, parallel groups of mice underwent sham surgery, and their IVCs were used for immunofluoresence (IF) staining of prothrombotic factors (as illustrated in supplemental Figure 2). We refrained from using longer periods of diet to avoid a confounding effect of diet on tumor size or body weight. There was no difference in the tumor volume among groups (Figure 2A). In the tumor group, the normalized clot weights increased by ∼40% in mice on high-protein diet (#P = .0164) compared to those on a normal protein diet (Figure 2B). In the nontumor group, a high-protein diet showed a trend toward higher normalized clot weights without tumors (P = .0807).
Influence of high-protein diet in a syngeneic colon cancer tumor model. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Averages of tumor volumes per group are shown. Error bars represent SEM. (B) Averages of normalized clot weights are shown. Two-factor analysis of variance (ANOVA; P = .0002). (C) Paraffin-embedded sections of IVC were stained using anti-TF and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Images are representative of IVCs from 5 mice in each group (scale bar, 100 μm). (D) ID was normalized to the surface area of the region of interest measured in microns by using ImageJ. Averages of ID normalized to surface area are shown. Error bars represent SEM. Two-factor ANOVA test (P = .0003). (E) IVCs were stained by using anti–PAI-1 and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Representative images from 5 mice in each group (scale bar, 100 μm). (F) PAI-1 expression was quantified as previously described. Averages of ID normalized to the surface area are shown. Error bars represent SEM. Two-factor ANOVA test (P = .0002; ∗∗P = .0255). #P < 0.05, ∗P < 0.05.
Influence of high-protein diet in a syngeneic colon cancer tumor model. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Averages of tumor volumes per group are shown. Error bars represent SEM. (B) Averages of normalized clot weights are shown. Two-factor analysis of variance (ANOVA; P = .0002). (C) Paraffin-embedded sections of IVC were stained using anti-TF and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Images are representative of IVCs from 5 mice in each group (scale bar, 100 μm). (D) ID was normalized to the surface area of the region of interest measured in microns by using ImageJ. Averages of ID normalized to surface area are shown. Error bars represent SEM. Two-factor ANOVA test (P = .0003). (E) IVCs were stained by using anti–PAI-1 and anti-CD31 antibodies. Alexa Fluor secondary antibodies and DAPI were used. Representative images from 5 mice in each group (scale bar, 100 μm). (F) PAI-1 expression was quantified as previously described. Averages of ID normalized to the surface area are shown. Error bars represent SEM. Two-factor ANOVA test (P = .0002; ∗∗P = .0255). #P < 0.05, ∗P < 0.05.
The presence of MC38 tumors led to an increase in TF expression with a normal protein diet (∗P = .0422). In the tumor group, TF expression (Figure 2C-D) and PAI-1 expression (Figure 2E-F) were about twofold (∗∗P = .0361) and 1.5-fold (∗∗P = .0255), respectively, higher in mice exposed to a high-protein diet than those on a normal diet. Our data suggest that only in the presence of tumor, a high-protein diet increases venous thrombogenicity.
Alteration of dietary Trp influences venous thrombogenicity in a syngeneic tumor model
A high protein diet will likely increase blood levels of several amino acids, of which Trp is particularly relevant considering its prothrombotic propensities.19 We examined the role of Trp on venous thrombogenicity using purified diets consisting of 3 different Trp concentrations: 0% (zero Trp), 0.2% (normal Trp; as in chow diet), and 1.2% (high Trp); other components were kept equal in all 3 diets (supplemental Table 2), as done in previous studies.21,22 No statistically significant changes were noted among the 3 diet groups in MC38 tumor size, weight, or histological characteristics (Figure 3A-D). In female mice without tumors, a significant difference in normalized clot weights was noted between normal and high Trp diets (####P < .0001). In the tumor-bearing group, compared to mice exposed to a normal Trp diet, those on high Trp diet showed a ∼30% increase in clot weights (∗P = .001). Mice on high Trp diet had greater clot weight (∼50%) than those on zero Trp diet (∗∗∗P = .006). Mice on normal Trp showed increased clots in tumor-bearing mice compared to nontumor controls (####P < .0001; Figure 3E). Similar to female mice, male mice on a normal diet with tumors showed a significantly increased normalized clot weight compared to mice on a normal diet without tumors (###P = .002). In the group without tumor, a significant difference in normalized clot weights was noted between normal and high Trp diets (∗∗∗P = .0001). However, no difference in clot weights was noted in tumor-bearing mice on a normal Trp compared to a high Trp diet (Figure 3F).
Dietary Trp alters the risk of venous thrombogenicity in a syngeneic colon cancer model. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Averages of tumor growth in all 3 dietary groups are shown. Error bars represent standard deviation. (B) Representative harvested tumors from the 3 groups are shown. (C) Averages of tumor weights in the 3 experimental groups are shown (zero Trp vs 0.2% Trp [normal] vs 1.2% Trp [high]). Error bars represent SEM. (D) Representative images of H&E-stained tumor tissue sections are shown at 40× and 100× original magnifications (scale bars, 25 μm [40×] and 50 μm [100×]). (E) Averages of normalized clot weights in female mice under 6 experimental conditions. Two-factor ANOVA showed P value <.001. Error bars represent SEM. (F) Averages of normalized clot weights in male mice under 6 experimental conditions. The number of mice analyzed under each condition is denoted by dots in each bar graph. Error bars represent SEM. Two-factor ANOVA test showed P value <.001. ns, nonsignificant. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.0001, ###P < 0.0001, ####P < 0.0001.
Dietary Trp alters the risk of venous thrombogenicity in a syngeneic colon cancer model. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Averages of tumor growth in all 3 dietary groups are shown. Error bars represent standard deviation. (B) Representative harvested tumors from the 3 groups are shown. (C) Averages of tumor weights in the 3 experimental groups are shown (zero Trp vs 0.2% Trp [normal] vs 1.2% Trp [high]). Error bars represent SEM. (D) Representative images of H&E-stained tumor tissue sections are shown at 40× and 100× original magnifications (scale bars, 25 μm [40×] and 50 μm [100×]). (E) Averages of normalized clot weights in female mice under 6 experimental conditions. Two-factor ANOVA showed P value <.001. Error bars represent SEM. (F) Averages of normalized clot weights in male mice under 6 experimental conditions. The number of mice analyzed under each condition is denoted by dots in each bar graph. Error bars represent SEM. Two-factor ANOVA test showed P value <.001. ns, nonsignificant. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.0001, ###P < 0.0001, ####P < 0.0001.
As orthogonal measures of thrombogenicity, we examined the vasculature for abundance of fibrin and the leukocyte common antigen CD45. In tumor-free mice, fibrin content increased by ∼80% to 90% on a high Trp diet, compared to the normal Trp diet (P < .0001). In tumor-bearing mice, fibrin content increased by 1.3- to 2.1-fold on high Trp diet compared to normal (P < .0001) or zero Trp diet (P = .00378; supplemental Figure 3A-C). There were no significant differences in CD45 appearance in the IVC of tumor-bearing mice under different diets. We also examined soluble and cellular factors in blood. Prothrombin, partial thromboplastin, and fibrinogen levels showed no significant differences across tumor and nontumor groups on respective diets (supplemental Table 3). Red blood cells and platelet counts were also similar across all groups. Neutrophil levels were higher in tumor-bearing mice than in non–tumor-bearing mice on all diets (P = .03 for normal Trp; P = .01 for zero Trp; P = .016 for high Trp), consistent with tumor-induced leukocytosis (supplemental Table 4).
A high Trp diet increases prothrombotic mediators in tumor-bearing mice
TF levels were upregulated in endothelial cells of IVC of mice with tumors and on high Trp diet compared to zero Trp diet (###P = .0002; Figure 4A-B). Mice with tumors on zero Trp diet showed lower TF and PAI-1 expression in the IVC compared to those on a normal Trp diet (∗∗∗P = .0002). Similarly, PAI-1 expression was higher in the endothelial cells (marked with CD31) of IVC of mice exposed to a high Trp diet than the normal and zero Trp groups (∗∗∗P = .0001; #####P < .0001, respectively; Figure 4C-D). Compared to mice on a normal diet, mice on a zero Trp diet had lower TF expression (∗∗∗∗P < .0001). We also examined other prothrombotic mediators, thrombomodulin (TM), an endothelial cell-surface receptor for thrombin, and von Willebrand Factor (VWF).23-26 Mice on a normal Trp diet showed approximately threefold (###P = .001) higher TM expression than those on a zero Trp diet, and those on a high Trp diet exhibited ∼3.8-fold (∗∗∗∗P < .001) lower TM expression than those on a zero Trp diet (Figure 4E-F). Mice on normal and low Trp diets showed significantly lower VWF expression than mice exposed to high Trp (P < .001 for both comparisons; Figure 4G-H).
The expressions of procoagulant and anticoagulant proteins are altered in the IVC of mice with colon cancer tumors and on a high Trp diet. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Representative images of paraffin-embedded sections of IVC from mice with MC38 tumor exposed to a normal, zero, or high Trp diets (n = 10 female mice per group). IVC tissues were stained with indicated antibodies. DAPI was used for nuclear stain (scale bar, 100 μm). (B) Averages of normalized ID of TF normalized to surface area in each of the examined sections. Three images per IVC were analyzed in each mouse. Two-factor ANOVA test (P < .0001). (C) Representative images of tissue section of IVC stained with anti–PAI-1 and anti-CD31 antibodies (scale bar, 100 μm). (D) Averages of normalized ID of PAI-1 normalized to surface area in each of the examined section. ANOVA (P < .0001). (E) Representative images of paraffin-embedded sections of IVC from female mice with MC38 tumor exposed to a normal, zero, or high Trp diets, stained with TM and CD31 (n = 10 mice per group). DAPI was used for nuclear stain (scale bar, 100 μm). (F) Averages of normalized ID of TM expression in endothelial cells normalized to surface area in each of the examined sections. ANOVA (P < .0001). (G) Representative images of paraffin-embedded sections of IVC from mice with MC38 tumor exposed to a normal, zero, or high Trp diets, stained with VWF and CD31 (scale bar, 100 μm). (H) Average normalized ID VWF expression in IVC was measured using a region of interest marked corresponding to the endothelial cells. ANOVA (P < .0001; ∗∗∗P = .0002 [zero vs normal Trp]; P = .0585 [not significant; high vs normal Trp]; ###P = .002 [high vs zero Trp]).
The expressions of procoagulant and anticoagulant proteins are altered in the IVC of mice with colon cancer tumors and on a high Trp diet. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Representative images of paraffin-embedded sections of IVC from mice with MC38 tumor exposed to a normal, zero, or high Trp diets (n = 10 female mice per group). IVC tissues were stained with indicated antibodies. DAPI was used for nuclear stain (scale bar, 100 μm). (B) Averages of normalized ID of TF normalized to surface area in each of the examined sections. Three images per IVC were analyzed in each mouse. Two-factor ANOVA test (P < .0001). (C) Representative images of tissue section of IVC stained with anti–PAI-1 and anti-CD31 antibodies (scale bar, 100 μm). (D) Averages of normalized ID of PAI-1 normalized to surface area in each of the examined section. ANOVA (P < .0001). (E) Representative images of paraffin-embedded sections of IVC from female mice with MC38 tumor exposed to a normal, zero, or high Trp diets, stained with TM and CD31 (n = 10 mice per group). DAPI was used for nuclear stain (scale bar, 100 μm). (F) Averages of normalized ID of TM expression in endothelial cells normalized to surface area in each of the examined sections. ANOVA (P < .0001). (G) Representative images of paraffin-embedded sections of IVC from mice with MC38 tumor exposed to a normal, zero, or high Trp diets, stained with VWF and CD31 (scale bar, 100 μm). (H) Average normalized ID VWF expression in IVC was measured using a region of interest marked corresponding to the endothelial cells. ANOVA (P < .0001; ∗∗∗P = .0002 [zero vs normal Trp]; P = .0585 [not significant; high vs normal Trp]; ###P = .002 [high vs zero Trp]).
We next validated the above findings in an allogeneic colon cancer model with HT-29 adenocarcinoma cells grown on an immunodeficient background. Tumor growth, final volume, histology, and β-catenin expression were similar across experimental groups (supplemental Figures 4A-D). An example of IVC ligation causing complete vessel occlusion is shown in supplemental Figure 4F. The highest clot weights were observed in HT-29 tumor–bearing mice on a high Trp diet (supplemental Figure 4G). In non–tumor-bearing mice, no clot size differences were noted in the different groups. On a normal Trp diet, tumor-bearing mice showed a higher normalized clot weight than non–tumor-bearing mice (∗P = .0130). In the tumor-bearing group, a high Trp diet led to significantly higher clot weights than normal and zero Trp diets (∗P = .0133 and ∗∗∗P = .0037, respectively). IVCs of sham-operated mice showed higher TF, PAI-1, and VWF expression and lower TM expression in HT-29–bearing mice on a high Trp diet than those on a normal diet (supplemental Figures 5 and 6).
Alterations in Trp metabolome in tumor-bearing mice on different diets
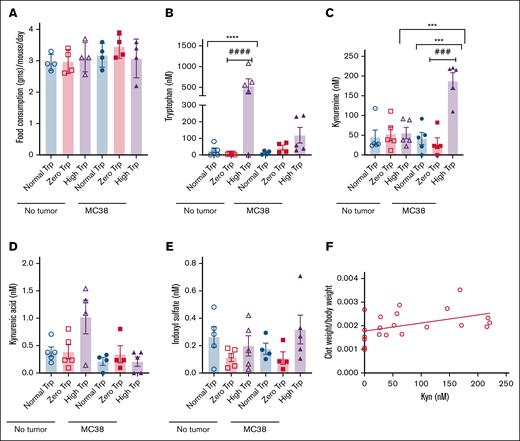
Approximately 80% to 90% of dietary Trp is metabolized by indoleamine 2,3-dioxygenase (IDO) and Trp 2,3-dioxygenase. IDO1 and IDO2 convert Trp to N-formyl Kyn, which is converted to Kyn by formamidase. Kyn is subsequently catabolized as illustrated in supplemental Figure 7. Once we noted no significant difference in diet consumption among different groups of mice (Figure 5A), we used a prevalidated liquid chromatography/mass spectrometry method27,28 to measure Trp metabolites in sera after IVC ligation. In the nontumor group, serum Trp levels were several-fold higher in mice exposed to a high Trp (mean ± standard error of the mean, 531.7 ± 174.9 nM) diet than normal Trp (27.64 ± 14.79 nM; ∗∗∗∗P = .0208) and zero Trp diets (6.610 ± 1.846 nM; ####P = .0170; Figure 5B). Intriguingly, mice with tumors under a high Trp diet had lower serum Trp levels (118.9 ± 47.44 nM; P = .0508) than those without tumors. Tumor-bearing group on a high Trp diet had approximately threefold to fourfold higher Kyn levels (2.081 ± 0.5455 nM) than those on normal (0.5188 ± 0.1467 nM; ∗∗∗P = .0306) or low Trp diets (0.3723 ± 0.150 nM; ###P = .0269). Moreover, in the high Trp diet group, Kyn levels were approximately fivefold higher in tumor-bearing mice than non–tumor-bearing mice on a high Trp diet (∗∗∗P = .0009; Figure 5C). No significant differences were noted in the kynurenic acid and indoxyl sulfate levels among the 3 diet groups in non–tumor-bearing or tumor-bearing mice (Figure 5D-E). Intriguingly, normalized clot weights positively correlated with blood Kyn levels (R2 = 0.2; P = .04; Figure 5F).
Alterations in Trp metabolome in mice with syngeneic colon cancer tumors. (A) Average diet intake in grams was measured in 4 age-matched female mice subjected to different Trp diets over 4 days. The y-axis denotes the diet intake per day per mouse. Error bars represent SEM. (B-E) Average levels of Trp metabolites in 4 to 5 mice per group are shown. Error bars represent SEM. ANOVA was performed to compare all the groups. P = .0006 (Trp levels ANOVA); P = .0017 (Kyn ANOVA); P = .0147 (kynurenic acid ANOVA); indoxyl sulfate ANOVA was not significant. Student t test was performed to compare individual groups. For kynurenic acid, in the nontumor group, P = .0631 (between normal and high Trp groups); P = .0778 (between zero and high Trp groups). No significant differences were noted between different diets in the tumor groups. (F) A linear correlation of normalized clot weights with Kyn levels.
Alterations in Trp metabolome in mice with syngeneic colon cancer tumors. (A) Average diet intake in grams was measured in 4 age-matched female mice subjected to different Trp diets over 4 days. The y-axis denotes the diet intake per day per mouse. Error bars represent SEM. (B-E) Average levels of Trp metabolites in 4 to 5 mice per group are shown. Error bars represent SEM. ANOVA was performed to compare all the groups. P = .0006 (Trp levels ANOVA); P = .0017 (Kyn ANOVA); P = .0147 (kynurenic acid ANOVA); indoxyl sulfate ANOVA was not significant. Student t test was performed to compare individual groups. For kynurenic acid, in the nontumor group, P = .0631 (between normal and high Trp groups); P = .0778 (between zero and high Trp groups). No significant differences were noted between different diets in the tumor groups. (F) A linear correlation of normalized clot weights with Kyn levels.
Kyn can originate from various sources, including tumors. Metabolomics analysis of lysates extracted from tumors showed high levels of Kyn in MC38 tumors of mice on a high Trp diet (supplemental Table 5; P = .0242 [high Trp vs zero Trp]; P = .0259 [high Kyn vs zero Trp]; P = .0618 [high kynurenic acid vs zero Trp]). Tumors stained with TF also showed the highest TF expression in mice on a high Trp diet (∗∗∗P = .0011 [high vs zero Trp]; ∗∗∗P = .0067 [normal vs zero Trp]; supplemental Figure 8A-B). These results suggest that tumors are an additional source of Kyn, possibly contributing to TF levels in tumor cells.
Finally, to establish the causality of Kyn as a promoter of venous thrombogenesis, mice were injected intraperitoneally with Kyn, and its elevated plasma concentration was confirmed by a metabolomic assay (supplemental Table 6). IVC ligation was performed on day 3 of Kyn injection, and tissues were harvested on day 5. In nonligated mice, IVC tissues were analyzed for TF, PAI-1, TM, and VWF (supplemental Figure 9). Our data showed that the normalized clot weights were 80% higher in mice exposed to Kyn than vehicle-injected mice (∗P = .0267; supplemental Figure 9A). TF expression increased by ∼70% (∗P = .016), PAI-1 by ∼60% (∗∗P = .0031), and VWF by threefold (∗∗P = .0005), whereas TM decreased by >60% (∗∗∗P = .0008; supplemental Figure 9B-I). These findings causally link Kyn to increased venous thrombogenicity.
The causality found between Kyn levels in circulation and thrombosis prompted a comprehensive tracing of Trp metabolism. To this end, mice with and without MC38 tumors were starved and then injected with deuterium-labeled Trp (L-Trp-[D8]); sera was harvested after 30 minutes, and organs were collected after 3 hours for liquid chromatography/mass spectrometry analysis, as previously described29-31 (supplemental Tables 7-11). Mice without tumors had higher Trp and Kyn levels in the brain (P = .007 [Trp]; P = .0518 [Kyn]) than mice with tumors. Tumor-bearing mice also showed a 10-fold higher Kyn in vessels (P = .0064 [aorta + IVC]), higher Trp and Kyn in the spleen (P = .024 [Trp]; P = .0030 [Kyn]), and lower Kyn in the lungs (4.2-fold; P = .0030) than non–tumor-bearing mice. Kidneys of tumor-bearing mice had higher quinolinic acid and picolinic acid and lower Trp (P = .0172 [quinolinic acid]; P = .004 [picolinic acid]; P = .014 [Trp]; supplemental Tables 7 and 8). Sera from tumor-bearing mice showed a decrease in Trp (from 87.18 to 25.87 nM) and an increase in Kyn levels (from 103.80 to 591.89 nM; supplemental Tables 9 and 10) within 30 minutes of L-Trp-[D8] injection, compared to non–tumor-bearing mice. The presence of tumors suppressed downstream Kyn catabolites, as manifested by anthranilic acid sera levels. Tumor lysates also showed high Kyn levels as traced by L-Trp-[D8] injection (supplemental Table 11). These data suggest a rapid Trp clearance from blood, its conversion to Kyn, and altered metabolism of Trp in different organs in the presence of tumors, all of which have potential to affect thrombosis in different organs.
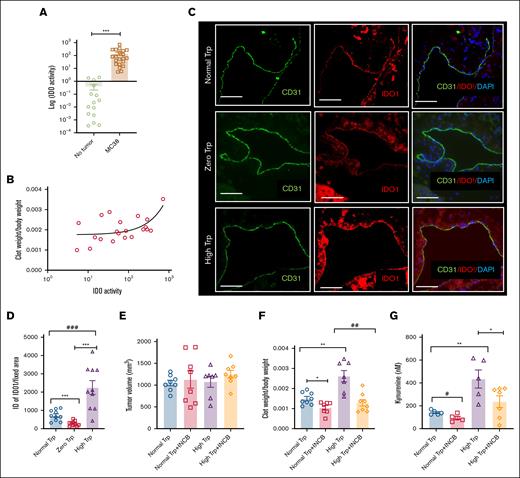
IDO1 activity is elevated in mouse cancer models under Trp-rich diets
Because IDO1 is the rate-limiting enzyme in the conversion of Trp to Kyn,32 IDO activity was measured in blood as previously described.32-34 IDO activity was significantly higher in tumor-bearing mice than controls (P = .0037; Figure 6A), and it positively correlated with normalized clot weights (R2 = 0.324; P = .007; Figure 6B). IDO1 is expressed in endothelial cells and vascular smooth muscle cells.32 The group of mice with tumor and on a high Trp diet had a higher IDO expression in endothelial cells and subendothelial region mice exposed to a low (∗∗∗P < .001) or normal Trp diet (###P < .0001; Figure 6C-D). IDO vascular expression in mice exposed to a normal Trp diet was significantly higher than those on a zero Trp diet (∗∗∗P < .0021). Similarly, MC38 or HT-29 tumors in mice on a high Trp diet showed significantly higher IDO1 expression than those on a normal diet (∗∗P = .0082 for MC38; P = .005 for HT-29; supplemental Figure 10A-B).
IDO1 inhibition attenuates thrombosis in a mouse cancer model under different Trp diets. (A) IDO activity measured in the sera of mice with and without MC38 tumor. A group of age-matched 8- to 12-week-old C57BL/6 female mice were used for this experiment. The y-axis is depicted in a log scale. Error bars represent SEM. Student t test was used to compare the groups (P = .0037). (B) Clot weights measured under different diets, as described in Figure 3E, were plotted against values of IDO1 activity measured in the sera of mice described in panel A. A linear correlation between normalized clot weights and IDO activity levels. (C) Representative IF images of IVC from IVC of mice on different Trp diets, stained with antibodies to IDO1 and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). (D) ID of IDO1 labeling normalized to a uniform surface area in all samples. ANOVA (P < .001). Student t test was used to compare groups. (E) Averages of tumor volume in all groups are shown. Error bars represent SEM. (F) Averages of clot weights normalized to the body weight across different groups of mice are shown. Error bars represent SEM. ANOVA was done to compare all groups; P < .0001. Student t test was performed to compare groups. ∗P = .0131; ∗∗P = .0020; ##P = .0011. (G) Average levels of Kyn are shown. Error bars represent SEM. Kyn level measured here under a normal or high Trp diet is somewhat higher than the one recorded in Figure 5C, likely owing to a 3-day longer duration of diet under this experimental condition (see “Results”). The number of mice analyzed under each condition is denoted by dots in each bar graph. Age-matched C57BL/6 female mice were used in all experiments, similar to Figure 1. ANOVA was performed to compare all groups. P = .0012. Student t test was performed to compare groups. #P = .0199; ∗P = .0501; ∗∗P = .0079.
IDO1 inhibition attenuates thrombosis in a mouse cancer model under different Trp diets. (A) IDO activity measured in the sera of mice with and without MC38 tumor. A group of age-matched 8- to 12-week-old C57BL/6 female mice were used for this experiment. The y-axis is depicted in a log scale. Error bars represent SEM. Student t test was used to compare the groups (P = .0037). (B) Clot weights measured under different diets, as described in Figure 3E, were plotted against values of IDO1 activity measured in the sera of mice described in panel A. A linear correlation between normalized clot weights and IDO activity levels. (C) Representative IF images of IVC from IVC of mice on different Trp diets, stained with antibodies to IDO1 and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). (D) ID of IDO1 labeling normalized to a uniform surface area in all samples. ANOVA (P < .001). Student t test was used to compare groups. (E) Averages of tumor volume in all groups are shown. Error bars represent SEM. (F) Averages of clot weights normalized to the body weight across different groups of mice are shown. Error bars represent SEM. ANOVA was done to compare all groups; P < .0001. Student t test was performed to compare groups. ∗P = .0131; ∗∗P = .0020; ##P = .0011. (G) Average levels of Kyn are shown. Error bars represent SEM. Kyn level measured here under a normal or high Trp diet is somewhat higher than the one recorded in Figure 5C, likely owing to a 3-day longer duration of diet under this experimental condition (see “Results”). The number of mice analyzed under each condition is denoted by dots in each bar graph. Age-matched C57BL/6 female mice were used in all experiments, similar to Figure 1. ANOVA was performed to compare all groups. P = .0012. Student t test was performed to compare groups. #P = .0199; ∗P = .0501; ∗∗P = .0079.
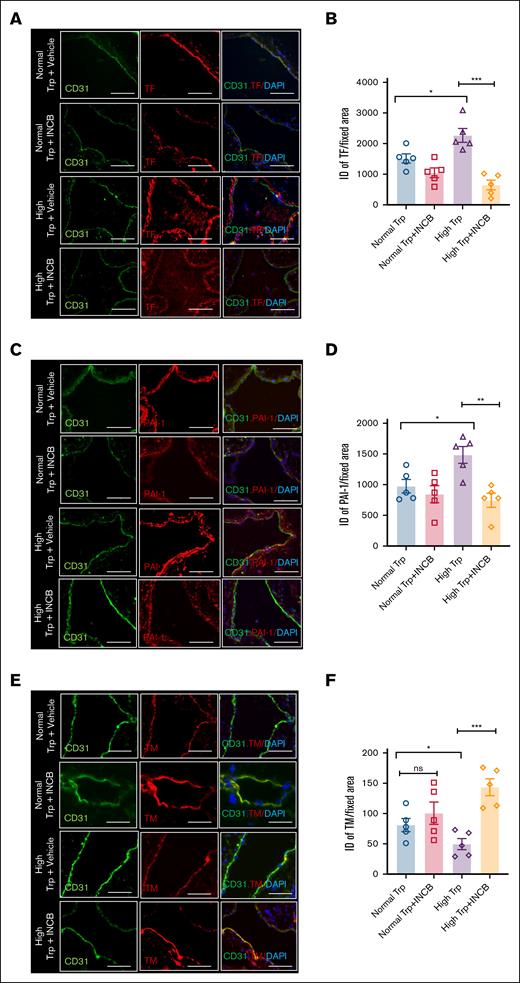
To test the functional significance of elevated IDO1, mice were subjected to a 5-day normal or high Trp diet after tumor volume reached 400 to 500 mm3 (Figure 6E). The mice were then randomized to receive either the highly potent and specific IDO1 inhibitor INCB2346032,35 or a vehicle for 3 days before IVC ligation and were continued till IVC harvest (total duration, 5 days). In the normal Trp diet group, compared to vehicle treatment, INCB23460 suppressed normalized clot weights by ∼25% to 32% (∗P = .0133). A similar comparison in the high Trp diet group showed ∼70% reduction in normalized clot weights upon INCB23460 administration, compared to vehicle-treated mice (##P = .0011; Figure 6F). Sera samples of mice on a normal or high Trp diet treated with INCB24360 showed a significant reduction in Kyn levels (#P = .0199; ∗P = .0501, respectively), supporting the reduced conversion of Trp to Kyn (Figure 6G). Compared to vehicle-treated mice, INCB23460-treated mice on a high Trp diet showed reduced TF (∗∗∗P = .004) and PAI-1 expression (∗∗P = .03) and higher TM levels in the IVC (∗∗∗ P = .005; Figures 7). However, no change in VWF was noted with INCB23460 (supplemental Figure 10C) in the high Trp group. Finally, INCB024360 treatment also reduced TF expression in the tumors of mice on a high Trp diet by 80% (P = .005) compared to the vehicle-treated group (supplemental Figure 10D).
IDO1 inhibition alters TF, PAI-1, and TM expression in IVC of mice with tumors exposed to a high Trp diet. (A) Representative images of tissue sections of IVC from MC38 tumor–bearing mice stained with TF and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). White arrowheads point to the TF expression on endothelial cells, and asterisk points to the subendothelial cells (B) Averages of normalized ID are shown. ANOVA (P < .0001). Student t test was used to compare groups. (C) Representative images of tissue sections of IVC from mice in different groups stained with PAI-1 and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). White arrowheads point to the PAI-1 expression on endothelial cells, and asterisk points to the subendothelial cells. (D) Averages of normalized ID are shown. ANOVA (P = .0036). Student t test was used to compare groups. (E) Representative images of tissue sections of IVC from MC38 tumor–bearing mice stained with TM and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). (F) Averages of normalized ID are shown. Data are representative of 5 age-matched female mice per group. ANOVA (P = .0015). Student t test was used to compare groups. ns, nonsignificant.
IDO1 inhibition alters TF, PAI-1, and TM expression in IVC of mice with tumors exposed to a high Trp diet. (A) Representative images of tissue sections of IVC from MC38 tumor–bearing mice stained with TF and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). White arrowheads point to the TF expression on endothelial cells, and asterisk points to the subendothelial cells (B) Averages of normalized ID are shown. ANOVA (P < .0001). Student t test was used to compare groups. (C) Representative images of tissue sections of IVC from mice in different groups stained with PAI-1 and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). White arrowheads point to the PAI-1 expression on endothelial cells, and asterisk points to the subendothelial cells. (D) Averages of normalized ID are shown. ANOVA (P = .0036). Student t test was used to compare groups. (E) Representative images of tissue sections of IVC from MC38 tumor–bearing mice stained with TM and CD31. DAPI was used for nuclear stain (scale bar, 100 μm). (F) Averages of normalized ID are shown. Data are representative of 5 age-matched female mice per group. ANOVA (P = .0015). Student t test was used to compare groups. ns, nonsignificant.
Discussion
Cardio-oncology is an emerging field of research focused on the intertwined relationship between CVDs and cancer. In this realm, this study probes the effect of small metabolites and the contribution of their regulating enzyme in cancer-associated VTE. Although the effects of high-fat diets have been studied in the context of cancer and CVD, sparse information is available on the impact of high-protein diets and cancer-associated thrombosis. Our work shows that an imbalanced diet characterized by high-protein content augments venous thrombogenicity in 2 discrete colon cancer mouse models. In this context, Trp-derived metabolites contribute to converting the endothelial surface from an anticoagulant to a more procoagulant state. Increased serum levels of Kyn induced by a high Trp diet is associated with upregulation of IDO1 expression in the venous walls of tumor-bearing mice as well as in tumors. As illustrated in the graphic abstract, this work demonstrates an antithrombotic property of IDO1 inhibitor used in tumor-bearing mice, which is a desirable effect considering the cancer-associated risk of VTE. Overall, our study highlights a tripartite interaction between an imbalanced protein diet, cancer, and cardiovascular complications.
In our study, we observed a significant effect of a high Trp diet, compared to a normal Trp diet, on clot formation in both male and female non–tumor-bearing mice. However, in contrast to female mice, male mice bearing tumors had higher clots under a high Trp diet only when compared to the extreme condition of a zero Trp diet. Future studies focused on potential differences in Trp metabolism in male vs female mice could explain this observation. Cancer is known to alter fat, carbohydrate, and protein metabolism and reprogram several metabolic pathways to its advantage.36-38 Combining observations from targeted metabolomics analysis and tracing L-Trp-[D8] metabolism suggest a rapid conversion of Trp to Kyn in different organs of tumor-bearing mice, perhaps increasing the likelihood of thrombosis in multiple sites. One would have also expected an increase in Kyn downstream catabolites.39 However, sera of tumor-bearing mice did not show a consistent increase in downstream Kyn catabolites (xanthurenic acid, anthranilic acid, and kynurenic acid). This pattern of Trp metabolome in tumor-bearing mice points to a possible dysregulation of enzymes in the catabolic pathway.
As we reported earlier, Kyn upregulates TF level through the aryl hydrocarbon receptor–TF axis.40 However, this work did not identify the source of dysregulated Kyn in cancer or the effect of diet on Kyn levels. Although this work addresses this knowledge gap, it raises the question regarding factor(s) driving elevated IDO1 expression in the vessel wall and tumors. Considering reports of IDO1 upregulation by inflammatory factors,41,42 it is quite possible that a tumor-induced inflammatory milieu contributes to its upregulation.
This study has potential implications at individual patient and population levels. Nutrition management is an integral component of care of patients with cancer to counteract cancer-induced catabolism and cachexia, especially during chemotherapy.43 In head-neck and gastrointestinal cancers, up to 1.5 g/kg per day of protein is recommended to support protein balance (normal recommended protein intake, 0.8 g/kg per day).14,44 Furthermore, interventions with amino acids were also tested in cancer to improve the nutritional status.45 The results of our findings using mice raise a point of caution for higher protein administration in cancer cases with risk of CVD and motivate future studies in human cohorts with a variety of cancers.
Chemotherapeutic agents, such as cisplatin, hormonal therapy, and, to a lesser extent, fluorouracil, are associated with thrombosis.46,47 Thromboembolic complications can be a major concern for US Food and Drug Association rejection of some anticancer agents such as bevacizumab.48 The antithrombotic property of an IDO1 inhibitor, as uncovered in our study, offers an advantage because its combination with other chemotherapeutic agents with prothrombotic propensities may reduce the overall VTE risk. Taken together, this work further encourages comprehensive investigations of different dietary components in the context of different cancers for their contribution to cardio-oncology.
Acknowledgments
The authors thank Michael Kirber at the Boston University Medical Center (BUMC) Imaging Core facility for his assistance in Nikon Deconvolutional microscopy and Howard Cabral for statistical input.
This project was funded by the AAmerican Heart Association (AHA) Cardio-Oncology Strategically Focused Research Network (SFRN) Cancer-Associated Thromboembolism as Affected by Health Disparities (CAT-HD) Center grant 857078 (S.L., A.J., X.Y., K.R., and V.C.C.); National Institute of Health (NIH) grants R01HL166608 (V.C.C. and K.R.) and T32HL125232 (S.L.); Center of Cross Organ Vascular Pathology; and by the Thrombosis and Hemostasis Affinity Research Collaborative at Boston University Chobanian & Avedesian School of Medicine.
Authorship
Contribution: V.C.C. and K.R. developed the hypothesis and designed the research; S.L. performed all animal experiments; X.Y., A.J., T.B., A.L., and I.H. assisted in animal studies; T.B. and A.J. performed immunofluorescence studies; S.L., A.J., V.C.C., and K.R. reviewed and analyzed different parts of data and wrote the manuscript; S.L., A.J., and V.C.C. prepared the figures; M.D.C.P. and N.L. performed metabolomics studies; H.C. assisted with statistical considerations; and all authors contributed conceptually to different degrees and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vipul C. Chitalia, Center of Cross-Organ Vascular Pathology, Department of Medicine, Boston University Medical Center Boston, 650 Albany St, EBRC X-540, Boston, MA 02118; email: vichital@bu.edu; and Katya Ravid, Evans Center for Interdisciplinary Biomedical Research, Department of Medicine, Boston University Medical Center, 650 Albany St, EBRC X-540, Boston, MA 02118; email: kravid@bu.edu.
References
Author notes
S.L. and A.J. contributed equally to this study.
All data are available for sharing, including supplemental Methods available with the online version of this article. All other requests can be directed to the corresponding authors, Vipul C. Chitalia (vichital@bu.edu) and Katya Ravid (kravid@bu.edu).
The full-text version of this article contains a data supplement.

![Dietary Trp alters the risk of venous thrombogenicity in a syngeneic colon cancer model. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Averages of tumor growth in all 3 dietary groups are shown. Error bars represent standard deviation. (B) Representative harvested tumors from the 3 groups are shown. (C) Averages of tumor weights in the 3 experimental groups are shown (zero Trp vs 0.2% Trp [normal] vs 1.2% Trp [high]). Error bars represent SEM. (D) Representative images of H&E-stained tumor tissue sections are shown at 40× and 100× original magnifications (scale bars, 25 μm [40×] and 50 μm [100×]). (E) Averages of normalized clot weights in female mice under 6 experimental conditions. Two-factor ANOVA showed P value <.001. Error bars represent SEM. (F) Averages of normalized clot weights in male mice under 6 experimental conditions. The number of mice analyzed under each condition is denoted by dots in each bar graph. Error bars represent SEM. Two-factor ANOVA test showed P value <.001. ns, nonsignificant. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.0001, ###P < 0.0001, ####P < 0.0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/19/10.1182_bloodadvances.2025017079/2/m_blooda_adv-2025-017079-gr3.jpeg?Expires=1769841357&Signature=OYfoSwGaSa~lsx-WSENdBJCHaX0pYG3ShbJeD0up7kSdxbbsOlufiMc1g-jpr7-yIAJVm-cLXLCAeMqwGnCUDHFS4BXxS7JrCclpN8LSR0F02X-I~bjmcHTqTtndkl8T2AcI7h7oX42eWxoD982XNr2vraaaiXuEKJqMcUiLdrCEFNbrHb8aZqemiG1TKJFh2hsUyhK--NItBEWZNu6W~ZuyMMDwHKtxrspa8H8vvTTCIlyxTjFx7c749zbZj-5~4xPti-fe5ATpTLlStVya~935BJgxgAIXFAie-my~vCkpJrxX3E1F04LCm5t1tVsqPc9L93ou1paIyn~G4n07Cg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![The expressions of procoagulant and anticoagulant proteins are altered in the IVC of mice with colon cancer tumors and on a high Trp diet. Age-matched C57BL/6 female mice were used as in Figure 1. (A) Representative images of paraffin-embedded sections of IVC from mice with MC38 tumor exposed to a normal, zero, or high Trp diets (n = 10 female mice per group). IVC tissues were stained with indicated antibodies. DAPI was used for nuclear stain (scale bar, 100 μm). (B) Averages of normalized ID of TF normalized to surface area in each of the examined sections. Three images per IVC were analyzed in each mouse. Two-factor ANOVA test (P < .0001). (C) Representative images of tissue section of IVC stained with anti–PAI-1 and anti-CD31 antibodies (scale bar, 100 μm). (D) Averages of normalized ID of PAI-1 normalized to surface area in each of the examined section. ANOVA (P < .0001). (E) Representative images of paraffin-embedded sections of IVC from female mice with MC38 tumor exposed to a normal, zero, or high Trp diets, stained with TM and CD31 (n = 10 mice per group). DAPI was used for nuclear stain (scale bar, 100 μm). (F) Averages of normalized ID of TM expression in endothelial cells normalized to surface area in each of the examined sections. ANOVA (P < .0001). (G) Representative images of paraffin-embedded sections of IVC from mice with MC38 tumor exposed to a normal, zero, or high Trp diets, stained with VWF and CD31 (scale bar, 100 μm). (H) Average normalized ID VWF expression in IVC was measured using a region of interest marked corresponding to the endothelial cells. ANOVA (P < .0001; ∗∗∗P = .0002 [zero vs normal Trp]; P = .0585 [not significant; high vs normal Trp]; ###P = .002 [high vs zero Trp]).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/19/10.1182_bloodadvances.2025017079/2/m_blooda_adv-2025-017079-gr4.jpeg?Expires=1769841357&Signature=aLTHmO4CjOCfQ15eMGoWdMx0~Hy87EfpBzHgx8umjMTa6I6nF0-JdrXLDUPp7dKzNbW1CWQDXHcFvQI-lc9cHhlCARtntZ4OLC-L~11FETqzyArZ~AlbvIFbDwHzZvhnkqJ7lrjvCWOmG00Iql56UtIgFwL0nfEq6kUbPSRKVmgXoiaO61FzwHihFwg6M0ZF9P9qt1LyR1yTbOlEHgdA~V-RYLKrvnd37Bz~DT9N8z57auxZRFhHz~1p-tyuT0IvWfYx3R7zHfC6MbkNd2qeJA9v9IrvVMApD8qZqe0KfuFLPur2CSSMl-1kZxIffQpjsmJVv6bSpfMqWozl1NrnWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)