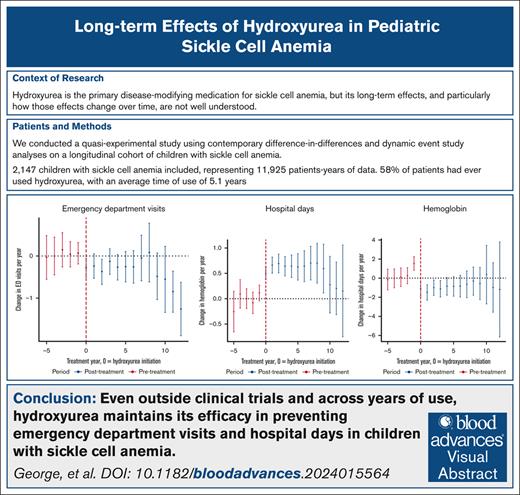
Key Points
Long-term effects of medications like hydroxyurea are hard to capture with traditional clinical trial data.
Using contemporary quasi-experimental methods with real-world data, we showed hydroxyurea’s sustained benefits over years of use.
Visual Abstract
Hydroxyurea is the primary disease-modifying medication for sickle cell anemia (SCA), but its long-term effects, particularly how these effects change over time, are not well understood. This study aimed to quantify the effects of hydroxyurea on clinical and laboratory outcomes in children with SCA over a prolonged period of use. We conducted a quasi-experimental study using contemporary difference-in-differences and dynamic event study analyses on a longitudinal cohort of 2147 children with SCA (hemoglobin SS or hemoglobin SSβ0, HbSS/HbSβ0) from 2010 to 2021. The primary outcomes included emergency department (ED) visits per year, hospital days per year, and annual average hemoglobin concentration. Hydroxyurea use was associated with fewer ED visits per year (average treatment effect on the treated [ATT], −0.36 visit per year; 95% confidence interval [CI], −0.57 to −0.16) and fewer hospital days per year (ATT, −0.84 d/y; 95% CI, −1.51 to −0.17) with sustained effects over time. On average, the hemoglobin concentration increased with hydroxyurea use (ATT, 0.56 g/dL; 95% CI, 0.39-0.73), but the sustained effect was observed only among the subgroup with laboratory markers of good adherence. This study demonstrates that hydroxyurea has sustained clinical benefits in reducing ED visits and hospital days across years of use in children with SCA. These findings provide perspective for clinicians and families regarding the long-term efficacy of hydroxyurea in pediatric SCA management and underscore the importance of ongoing adherence counseling to optimize clinical benefit. Furthermore, this study design provides a methodological framework for rigorously and causally evaluating other SCA-specific treatments, such as stem cell transplant and gene therapy, in real-world settings.
Introduction
Hydroxyurea is the first-line disease-modifying medication in sickle cell anemia (SCA).1 Hydroxyurea therapy confers significant clinical benefits when compared with no hydroxyurea therapy and has become the primary disease-modifying treatment modality in SCA.2-5 The National Heart, Lung, and Blood Institute expert panel report for the management of sickle cell disease recommends that hydroxyurea should be offered for all children aged 9 to 12 months and older with SCA (hemoglobin SS or hemoglobin SSβ0, HbSS/HbSβ0), regardless of clinical severity.6 Currently, hydroxyurea is recommended as a lifelong medication for patients who meet the treatment criteria, and there is no similarly efficacious second-line medication.2
Observational studies document the long-term efficacy of hydroxyurea relative to never using hydroxyurea. Among a cohort of patients followed for 17.5 years, long-term use of hydroxyurea was associated with reductions in pulmonary complications and overall mortality.7 A separate study documented that, when compared with historic controls who never took hydroxyurea, infants and young children with SCA had fewer episodes of acute chest syndrome and better growth after 4 years on hydroxyurea,8 whereas another documented that hydroxyurea is a viable alternative to chronic transfusion therapy in primary (although not secondary) stroke prevention.9,10 Other observational studies have confirmed long-term safety in varied settings.8,11-15
Of note, there are no published data on the time-varying effect of hydroxyurea. Although it has been shown that long-term use of hydroxyurea is beneficial when compared with no hydroxyurea use, the available studies did not examine whether the relative effect of hydroxyurea changes over time. It is possible that decreasing efficacy over time may be observed, as described for long-term use of other anti-inflammatory medications and opioids.11 Conversely, it is possible that the benefits of long-term hydroxyurea use would compound, leading to increasing effect over time. Furthermore, adherence to daily hydroxyurea is a known barrier to optimal response, and poor clinical outcomes in the setting of difficulty with adherence are reasons to consider curative therapy.16,17
Thus, the primary objective of this study was to quantify the time-varying effects of hydroxyurea on outcomes in children with SCA. Specifically, using real-world (ie, outside of a clinical trial) data on a longitudinal cohort of children with SCA, we implemented a quasi-experimental observational study design (difference-in-differences [DiD] and dynamic event study) that analyzed how the effects of hydroxyurea change over time.
Methods
Our study included data from a longitudinal cohort of children with SCA who were followed at Children’s Healthcare in Atlanta (CHOA), which has been described in detail elsewhere.18,19 Briefly, all children seen at CHOA with a laboratory-confirmed diagnosis of SCD were added to the CHOA SCD registry, and those who received care from 2010 to 2021 were included in this study. Clinical, laboratory, and sociodemographic details were abstracted from the CHOA electronic records with manual chart review by research epidemiologists for confirmation of the hospitalizations and hydroxyurea use. CHOA has the only comprehensive pediatric SCA program in the Atlanta metropolitan area; therefore, ∼95% of all hospitalizations and all pediatric SCA specialty outpatient appointments were captured in the database, thereby representing a nearly complete population of children with SCA in Atlanta.4 The inclusion criteria for the study were children with SCA (HbSS/HbSβ0, because this is the only group of patients with sickle cell disease in whom hydroxyurea is regularly used) younger than 18.0 years of age (to focus on a population exclusively treated within the pediatric care system and to minimize variability introduced by transitions to adult care). Children were excluded from the analysis if they had <3 total clinical visits, and they were censored at their last clinical visit if they went >2 years without a clinical visit; if they underwent a bone marrow transplant or gene therapy; or if they started chronic transfusion therapy or other disease modifying medications (specifically voxelotor or crizanlizumab).
The unit of observation is the patient-year with years corresponding to the patient age (eg, from 0 to 1 year of age, 1-2 years, etc). The primary outcomes of interest were clinical (health care utilization, including emergency department [ED] visits per year and hospital days per year) and laboratory findings (average annual hemoglobin value, mean corpuscular volume [MCV], and absolute neutrophil count). These outcomes were chosen a priori with consideration for the clinical relevance and data availability. Although hemoglobin F is an important treatment effect of hydroxyurea, it was not chosen a priori as an outcome, because data on it were not consistently or uniformly collected during the study period. ED visits and hospital days were summed over a given age-year. Laboratory variables were averaged within each patient age-year, but the laboratory results from ED visits and hospitalizations were excluded to provide nonsick annual averages. Baseline (ie, before hydroxyurea) values were established by retrieving data from the initiation date of hydroxyurea treatment. If laboratory results from this date were not available, the most recent laboratory values from a nonsick clinic visit within 1 year before starting hydroxyurea were used.
The exposure of interest was hydroxyurea use during a specific age-year. Research epidemiologists reviewed the clinical notes and prescription details from each office visit and marked a patient as taking or not taking hydroxyurea accordingly. Specifically, patients were classified as taking hydroxyurea if they had an active prescription at the visit, including newly issued or sufficient refill prescriptions to cover the visit timeframe, and there was no indication in the clinical note that hydroxyurea had been discontinued. Notably, however, we did not have data on whether patients filled these hydroxyurea prescriptions. Patients were classified as hydroxyurea users for a specific age-year if their electronic medical record indicated that they were prescribed hydroxyurea at >50% of their clinical visits during that year. At each visit, the use of hydroxyurea was recorded as a binary variable, namely as “yes” if hydroxyurea was prescribed and “no” otherwise. To assess the robustness of this classification, we conducted sensitivity analyses by increasing the threshold from 50% to 80%. In other words, patients were considered hydroxyurea users for a given age-year only if they were prescribed hydroxyurea at 80% or more of their clinical visits within that year. This higher threshold provides a stricter definition of hydroxyurea use, thereby enabling us to examine whether our findings remain consistent under more conservative assumptions. For all analyses within a specific age-year, observations with missing values for variables relevant to that analysis were excluded.
To evaluate the impact of hydroxyurea on clinical and laboratory outcomes in children with SCA, we used both traditional and dynamic DiD methods. The traditional DiD analysis provided an overall average effect of hydroxyurea by comparing changes in outcomes over time between children treated with hydroxyurea and those who were untreated. The dynamic DiD approach, also known as an event study, examined outcomes on a year-by-year basis, thereby allowing us to assess how hydroxyurea’s effects varied over time, both before and after treatment initiation. This dynamic approach provided a detailed understanding of hydroxyurea’s time-varying impact.20
To address potential biases associated with staggered treatment initiation and time-varying responses, we employed a framework that used children who never started hydroxyurea and those who had not yet started hydroxyurea as control groups.20 This approach helped to account for confounders that could influence both treated and untreated groups, thereby ensuring more accurate estimates of hydroxyurea’s effects. To validate the parallel trends assumption, a key requirement for DiD analyses, we visually assessed the pretreatment trends in the outcomes of interest and quantified these trends statistically by comparing pretreatment trajectories between groups.21,22 The supplemental Methods contain more detailed information.
Several sensitivity analyses were conducted to address specific biases. First, to minimize confounding from hydroxyurea initiation prompted by severe SCA complications, we restricted analyses to children who began hydroxyurea at 1 year of age when significant disease manifestations are uncommon because of the protective effects of fetal hemoglobin. Second, to account for the role of adherence in hydroxyurea’s effectiveness, we used MCV as a proxy for adherence. Good adherence was defined as an MCV of at least 110% from baseline after adjusting for age-related increases.23 These methods enabled us to capture both the overall and time-specific effects of hydroxyurea on clinical outcomes, while ensuring robustness of our findings.21,24,25
All analyses were performed in R, v4.2. This study was approved by the Children's Healthcare of Atlanta Institutional Review Board. No identifying information was published, and all results are presented in aggregate. Because of the observational nature of this study, individual patient consent was not required.
Results
Of the 2444 patients with SCA (HbSS/HbSβ0) seen for a clinical encounter at CHOA from 2010 to 2021, 2147 (88%) met the inclusion criteria (supplemental Figure 1). The average follow-up time was 5.5 years, providing a total of 11 925 patient-years of data. In total, 1240 patients (58%) had ever used hydroxyurea; of those, the average time on hydroxyurea was 5.1 years with 304 children with ≥8 years of continuous hydroxyurea therapy. Hydroxyurea use increased steadily over time, from 27% use in 2010 to 65% use in 2021. On average, there were 1.1 ED visits per patient-year, 0.9 hospitalization per year, and 3.5 hospital days per patient-year for the cohort. See Tables 1 and 2 for more details.
Summary statistics by hydroxyurea use (ever)
| Variable . | n . | Never used hydroxyurea (n = 907) . | Used hydroxyurea (n = 1240) . | P value∗ . |
|---|---|---|---|---|
| Sex | 2146 | 0.4 | ||
| Female | 469 (52%) | 618 (50%) | ||
| Male | 437 (48%) | 622 (50%) | ||
| Years in dataframe (sd) | 2147 | 4.2 (3.8) | 7.1 (3.8) | <0.001 |
| Years on hydroxyurea (sd) | 2147 | 0.0 (0.0) | 5.1 (3.2) | <0.001 |
| Insurance | 2147 | 0.002 | ||
| Commercial | 209 (23%) | 330 (27%) | ||
| Medicaid | 501 (55%) | 711 (57%) | ||
| Uninsured/none listed | 197 (22%) | 199 (16%) |
| Variable . | n . | Never used hydroxyurea (n = 907) . | Used hydroxyurea (n = 1240) . | P value∗ . |
|---|---|---|---|---|
| Sex | 2146 | 0.4 | ||
| Female | 469 (52%) | 618 (50%) | ||
| Male | 437 (48%) | 622 (50%) | ||
| Years in dataframe (sd) | 2147 | 4.2 (3.8) | 7.1 (3.8) | <0.001 |
| Years on hydroxyurea (sd) | 2147 | 0.0 (0.0) | 5.1 (3.2) | <0.001 |
| Insurance | 2147 | 0.002 | ||
| Commercial | 209 (23%) | 330 (27%) | ||
| Medicaid | 501 (55%) | 711 (57%) | ||
| Uninsured/none listed | 197 (22%) | 199 (16%) |
Unit of analysis for this table is individual person.
Mean (standard deviation [sd]) for continuous variables; n (%) for categorical variables.
1945 children were classified as HbSS, 71 as HbSβ0, and 131 as SCA unspecified (HbSS or HbSβ0).
Pearson's Chi-squared test; Wilcoxon rank sum test.
Summary statistics per patient-year by hydroxyurea use (current)
| Variable . | n . | Hydroxyurea use this year (n = 6308) . | No hydroxyurea use this year (n = 5554) . | P value∗ . |
|---|---|---|---|---|
| Age (years) | 11 925 | 9.9 (4.5) | 6.5 (5.2) | <0.001 |
| Sex | 11 924 | <0.001 | ||
| Female | 3089 (49%) | 3013 (54%) | ||
| Male | 3219 (51%) | 2540 (46%) | ||
| MCV (fL) | 9744 | 95.1 (12.2) | 83.2 (8.9) | <0.001 |
| Hgb (g/dL) | 9738 | 9.1 (1.2) | 9.0 (1.3) | <0.001 |
| ANC (103/μL) | 9655 | 4.5 (1.9) | 4.8 (2.4) | <0.001 |
| ED visits per year | 11 925 | 1.1 (1.9) | 1.1 (1.7) | 0.2 |
| Hospital days per year | 11 925 | 3.8 (5.7) | 2.8 (4.4) | <0.001 |
| Adherence hydroxyurea | 4790 | 2516 (53%) | NA | NA |
| Variable . | n . | Hydroxyurea use this year (n = 6308) . | No hydroxyurea use this year (n = 5554) . | P value∗ . |
|---|---|---|---|---|
| Age (years) | 11 925 | 9.9 (4.5) | 6.5 (5.2) | <0.001 |
| Sex | 11 924 | <0.001 | ||
| Female | 3089 (49%) | 3013 (54%) | ||
| Male | 3219 (51%) | 2540 (46%) | ||
| MCV (fL) | 9744 | 95.1 (12.2) | 83.2 (8.9) | <0.001 |
| Hgb (g/dL) | 9738 | 9.1 (1.2) | 9.0 (1.3) | <0.001 |
| ANC (103/μL) | 9655 | 4.5 (1.9) | 4.8 (2.4) | <0.001 |
| ED visits per year | 11 925 | 1.1 (1.9) | 1.1 (1.7) | 0.2 |
| Hospital days per year | 11 925 | 3.8 (5.7) | 2.8 (4.4) | <0.001 |
| Adherence hydroxyurea | 4790 | 2516 (53%) | NA | NA |
Unit of analysis for this table is individual person-year, where year is by age.
Mean (standard deviation [sd]) for continuous variables; N (%) for categorical variables.
ANC, absolute neutrophil count; ED, emergency department; Hgb, hemoglobin; MCV, mean corpuscular volume.
Wilcoxon rank sum test; Pearson's Chi-squared test; Fisher's exact test.
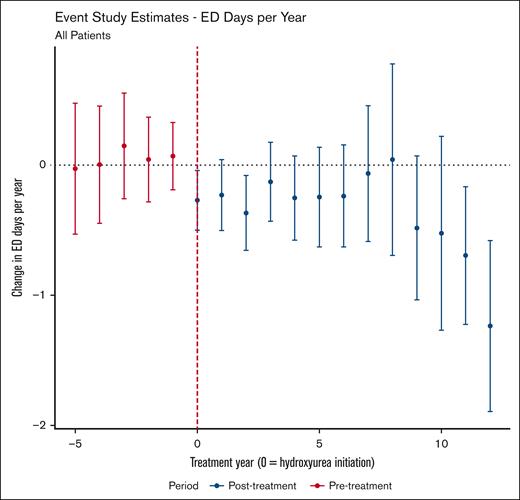
In our primary models, hydroxyurea use was significantly associated with fewer ED visits per patient-year (average treatment effect on the treated [ATT], −0.36 visit per year; 95% confidence interval [CI], −0.57 to −0.16). The results of the event study analysis can be found in Figure 1. Similar findings were observed for the ATT and the time-varying effect of hydroxyurea in our sensitivity analyses (supplemental Figures 3 and 4), reinforcing the robustness of our findings.
Event study estimates of the effect of hydroxyurea use on annual ED visits. Points with 95% CIs (y-axis) show the estimated average treatment effect on those treated with hydroxyurea for the given year of therapy (x-axis) relative to not using hydroxyurea. Year 0 is the year of hydroxyurea initiation. Of note, the DiD estimate for this analysis was −0.36 (95% CI, −0.57 to −0.16), meaning, on average (across treatment year and age group), that hydroxyurea use was associated with 0.36 fewer ED visit per year.
Event study estimates of the effect of hydroxyurea use on annual ED visits. Points with 95% CIs (y-axis) show the estimated average treatment effect on those treated with hydroxyurea for the given year of therapy (x-axis) relative to not using hydroxyurea. Year 0 is the year of hydroxyurea initiation. Of note, the DiD estimate for this analysis was −0.36 (95% CI, −0.57 to −0.16), meaning, on average (across treatment year and age group), that hydroxyurea use was associated with 0.36 fewer ED visit per year.
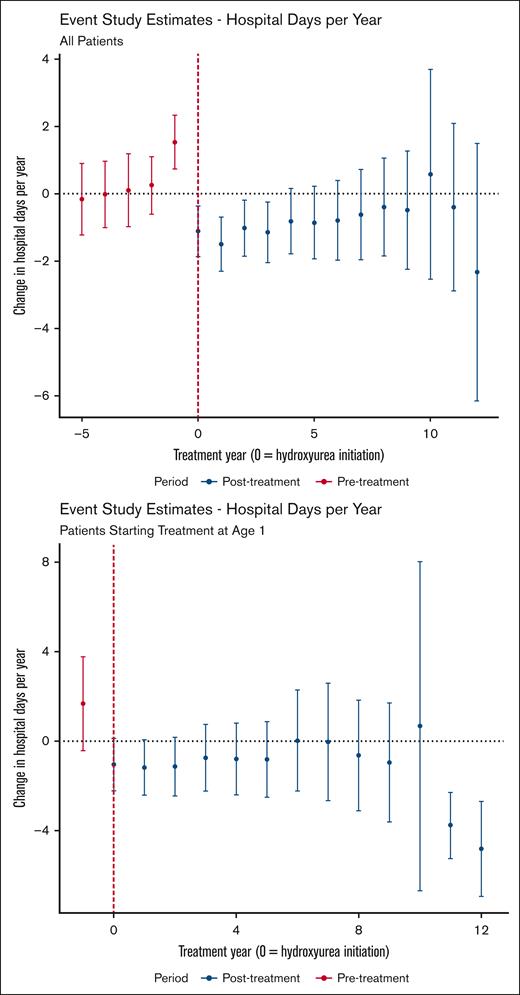
Hydroxyurea use was also associated with a reduction in hospital days per patient-year with an ATT of −0.84 visit per year (95% CI, −1.51 to −0.17). The event study analysis, depicted in Figure 2 (top panel), indicates a steady effect over time. Notably, the effect at year −1 (ie, the year before treatment initiation) is positive. This statistically significant finding suggests that a higher number of hospital days is associated with subsequent initiation of hydroxyurea, which violates the parallel trends assumption. To mitigate this potential bias, we performed an additional analysis that limited the treatment group to those who began hydroxyurea treatment at 1 year of age. With this limitation in place, we did not find a statistically significant difference in the outcomes before hydroxyurea initiation (Figure 2, bottom panel).
Event study estimates of the effect of hydroxyurea use on annual hospital days. Data for the entire sample (top panel) and for the treatment group limited to those who began treatment at 1 year of age (bottom panel) are shown. Points with 95% CIs (y-axis) show the estimated average treatment effect on those treated with hydroxyurea for the given year of therapy (x-axis) relative to never using hydroxyurea. Year 0 is the year of hydroxyurea initiation.
Event study estimates of the effect of hydroxyurea use on annual hospital days. Data for the entire sample (top panel) and for the treatment group limited to those who began treatment at 1 year of age (bottom panel) are shown. Points with 95% CIs (y-axis) show the estimated average treatment effect on those treated with hydroxyurea for the given year of therapy (x-axis) relative to never using hydroxyurea. Year 0 is the year of hydroxyurea initiation.
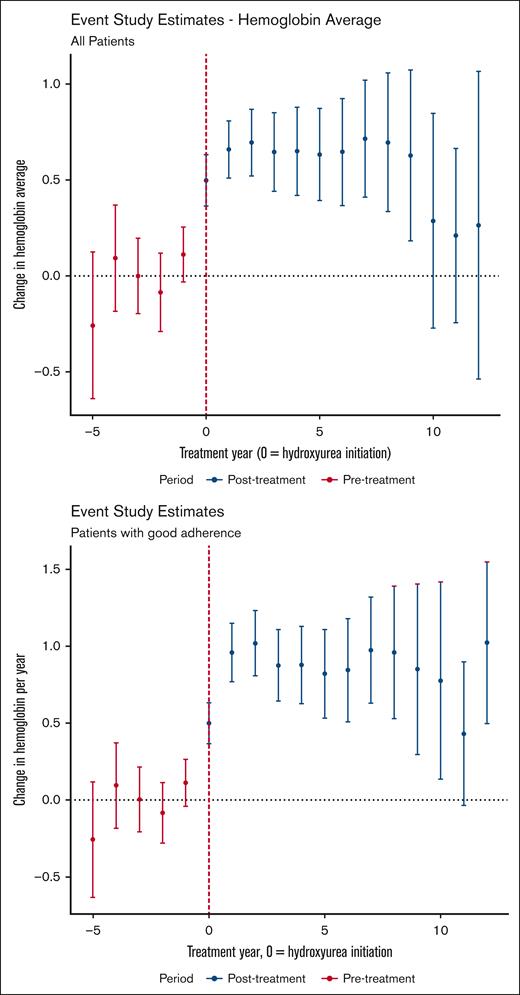
Our laboratory outcome of interest was annual hemoglobin concentration. The DiD estimator led to an ATT of 0.56 g/dL (95% CI, 0.39-0.73). The event study analysis is depicted in Figure 3. In contrast with the clinical outcomes, we found that the effect of hydroxyurea use on hemoglobin concentration decreases with time. This trend in decreasing effect on hemoglobin concentration is not observed among the subgroup of treated patients with laboratory markers of good adherence. Supplemental Figure 5 shows the unadjusted data on the average hemoglobin values by hydroxyurea across time. Hemoglobin values are higher on average for males than females, and show an overall slight increase that begins in adolescence for males.
Event study estimates of the effect of hydroxyurea use on annual hemoglobin values. Data for the entire sample (top panel) and for the treatment group limited to those with laboratory evidence (ie, increase in MCV) of hydroxyurea adherence (bottom panel) are shown. Points with 95% CIs (y-axis) show the estimated average treatment effect for those treated with hydroxyurea for the given year of therapy (x-axis) relative to those who never used hydroxyurea. Year 0 is the year of hydroxyurea initiation.
Event study estimates of the effect of hydroxyurea use on annual hemoglobin values. Data for the entire sample (top panel) and for the treatment group limited to those with laboratory evidence (ie, increase in MCV) of hydroxyurea adherence (bottom panel) are shown. Points with 95% CIs (y-axis) show the estimated average treatment effect for those treated with hydroxyurea for the given year of therapy (x-axis) relative to those who never used hydroxyurea. Year 0 is the year of hydroxyurea initiation.
Discussion
In this quasi-experimental analysis of hydroxyurea in 2147 children and adolescents with SCA, we found that hydroxyurea use had sustained impact on ED visits and hospital days across prolonged use. These results remained consistent even after addressing potential confounding from disease severity (by including only those who began hydroxyurea at age 1) and from suboptimal medication intake (by limiting the sample to patients with laboratory markers of good adherence). Interestingly, the initial improvements seen in hemoglobin concentration with hydroxyurea use were not sustained over time in this cohort; however, when limiting the sample to patients with markers of good adherence, the improvements in hemoglobin concentration are more sustained. These results reflect real-world use of hydroxyurea.
Our study addresses a significant gap in the existing literature on the long-term, time-varying effects of hydroxyurea in children with SCA. Randomized controlled trials provide strong and consistent evidence of the short-term benefits of hydroxyurea.5,26,27 Previous studies provide strong evidence on the short-term impact of hydroxyurea, but because of limited follow-up, they do not estimate the time-varying effect over more extended periods.
Observational studies have documented the prolonged impact of hydroxyurea over longer periods. When comparing those on hydroxyurea with those not taking hydroxyurea, reductions in mortality are consistently documented, even over follow-up periods of 5 to 20 years.7,12,28 Similarly, studies have reported sustained hematologic efficacy for years following hydroxyurea initiation.8,29,30 However, observational studies are susceptible to potential biases that can obscure the true effect of hydroxyurea. For example, children prescribed hydroxyurea may be those with more severe disease at baseline or come from families with more proactive healthcare management, leading to different outcomes regardless of hydroxyurea use. In addition, long-term follow-up must account for evolving SCA management practices that can influence outcomes; traditional observational studies often do not control for such changes.
To address these challenges, DiD analysis compares changes in outcomes over time between treatment and control groups, accounting for time-invariant and time-varying unobserved confounders. This approach enabled a more robust evaluation of hydroxyurea’s long-term effects in real-world settings, providing insight into its sustained impact beyond what is typically observed in traditional observational studies. Furthermore, previous studies have reported average effects of hydroxyurea without examining how its impact evolved over time. Understanding these temporal trends is crucial for guiding treatment plans, managing expectations, and addressing concerns about long-term efficacy and safety. Families may worry whether the initial benefits of hydroxyurea will persist or diminish with extended use, and commonly reported barriers to hydroxyurea use are concerns about long-term efficacy and safety.2,31,32 By analyzing hydroxyurea’s clinical and laboratory effects over time, this study provides evidence that its benefits are sustained, thereby helping to reassure families and providers, alleviate concerns, and encourage adherence.
We defined hemoglobin concentrations using stable-state values only, excluding those obtained during ED visits or hospitalizations to avoid inflating differences caused by fewer acute events among hydroxyurea users. Despite this approach, and the continued reduction in acute healthcare utilization, our findings show that hydroxyurea’s impact on steady-state hemoglobin concentration waned over time. However, when limiting the sample to those with laboratory markers of good adherence, we observed more sustained hemoglobin concentration improvements, even at 9 to 12 years of use. These findings suggest that adherence counseling among patients should remain ongoing, even years into taking the medication. The discrepancy between waning hemoglobin effects and continued improvement in ED visits and hospital days may reflect the fact that acute events are influenced by a combination of protective effects of hydroxyurea, including increases in fetal hemoglobin and decreases in inflammatory markers, such as absolute neutrophil count, that persist over time. Several additional factors might underlie the attenuation observed, including age-related changes in baseline hemoglobin, partial tolerance to hydroxyurea, or differences in the rates of transfusions between hydroxyurea users and non–hydroxyurea users. Although our study was not designed to pinpoint these mechanisms, future work with more precise adherence measures and transfusion data could help to clarify hydroxyurea’s effect on hemoglobin concentrations.
Limitations
A key limitation of this study, as with other DiD and event studies, was the inability to directly verify the parallel trends assumption. In this context, it is plausible that hydroxyurea users and non–hydroxyurea users could exhibit differing trajectories in outcomes in the absence of treatment. In addition, if patients initiated hydroxyurea use as a response to adverse outcomes in a previous year, this would violate the parallel trends assumption and introduce bias into the results.33 However, our pretreatment trajectory analysis offers some reassurance; before hydroxyurea initiation, outcomes seem similar between groups. Furthermore, the observed postinitiation changes in outcomes, most notably the clear improvements in ED visits and hemoglobin levels, strongly indicate that hydroxyurea drove these changes rather than an unobserved confounder. That said, a notable exception arose in the analysis of hospital days in that we observed a statistically significant increase in hospital days 1 year before hydroxyurea initiation (Figure 2). This finding suggests potential bias, because it indicates that patients may begin hydroxyurea in response to worsening clinical trajectories, specifically increased hospital utilization.
Our data were collected from a single health care center, CHOA, which although providing a nearly complete population-wide picture of pediatric SCA in metropolitan Atlanta, may limit the generalizability of our findings to other settings or populations. However, because CHOA is the only institution that provides multidisciplinary specialty care for SCA in Atlanta, it minimizes selection bias, because patients of all severities are included and follow-up is comprehensive and not restricted to only the most severe cases. Another limitation is relying on ED visits and hospital days as outcome measures, which do not fully capture the complexity of SCA severity. Moreover, because these analyses did not include data that occurred outside of CHOA, and we could have underestimated the true severity of some patients’ condition by not capturing such data (such as ED visits at adult hospitals), which could bias the results if these values differed systematically based on hydroxyurea use. Similarly, censoring children who went >2 years without a clinical visit may introduce bias, because this approach could exclude individuals who were not on hydroxyurea treatment and doing well enough to avoid seeking acute or routine care, potentially underestimating favorable outcomes in this group. In addition, the limited number of patients with ≥8 years of hydroxyurea use is a notable limitation, as shown in supplemental Table 3 and reflected in the widening confidence intervals for later treatment years.
Another significant limitation was that the analysis did not account for the potential impact of (1) intermittent transfusions used to treat acute SCD complications and (2) acute events causing ongoing anemia (eg, vaso-occlusive event) on the annual average hemoglobin. Data on intermittent transfusions were unavailable. This unaccounted factor may bias the results, because hydroxyurea users may have had less frequent acute events that required intermittent transfusions, which may also vary with time on hydroxyurea. In addition, as the duration of hydroxyurea use increased, the number of observations decreased, leading to wider confidence intervals. This limitation hinders our ability to definitively determine whether the effect of hydroxyurea on hemoglobin diminishes over time. Further studies with longer follow-up and more robust data on transfusion practices are needed to address these gaps. Our adherence measure, based on MCV increases as a proxy, is not the gold standard and may conflate poor adherence with a limited biologic response to hydroxyurea.34 Without direct measures of adherence, our results could be biased, because patients with suboptimal adherence might show diminished hydroxyurea effects, leading to an underestimation of its true efficacy. Incorporating more robust adherence data in future studies would help to clarify the relationship between hydroxyurea use and long-term outcomes.
Conclusions
This quasi-experimental study demonstrates that hydroxyurea has a sustained clinical impact over years of use in children and adolescents with SCA and provides significant long-term benefits in reducing hospitalizations and ED visits. Interestingly, we observed an initial but not sustained improvement in hemoglobin. However, among the subset of patients with laboratory markers of good adherence, the hemoglobin response remained sustained. These results underscore the potential for even greater clinical benefits through programmatic efforts to improve hydroxyurea adherence and to optimize dosing strategies. These findings reinforce the importance of hydroxyurea as a key treatment modality in SCA and provide insights for clinicians and families regarding its long-term efficacy. Given the low mortality rate in the pediatric population, our study was not powered to determine an impact on mortality; future studies should examine the impact of hydroxyurea on this and other key health-related quality of life measures. In addition, future research should continue to explore these effects in diverse settings and populations, notably in adults with SCA, to further validate and expand our results.
Despite the inherent challenges and imperfect data capture typical of observational studies, our methodological framework successfully identified clinically and statistically significant effects of hydroxyurea over time by using use and observational measures. This demonstrates the robustness of our approach in handling real-world, messy data. By effectively measuring treatment effects longitudinally, our study provides a valuable framework that can be employed in other health care settings to identify causal effects for which randomized controlled trials are not feasible, practical, or ethical. Examples include evaluating gene therapy, hydroxyurea use in adults, or treatment effects in patients with hemoglobin SC. Moreover, our current study would be further enhanced with more precise transfusion data, adherence measures, and patient-reported outcomes, such as self-reported pain scores and other quality of life measures. Routinely incorporating these data into electronic medical records would make this framework even more robust, thereby enabling a more comprehensive evaluation of treatment effects in future research.
Acknowledgments
The authors thank the dedicated staff of the Sickle Cell Disease program at Children’s Healthcare of Atlanta for their assistance in data collection and management.
This work was supported, in part, by a grant from the Abraham J. & Phyllis Katz Foundation.
Authorship
Contribution: P.G. contributed to conceptualization, statistical analyses, and manuscript writing; G.K. was responsible for data review, data abstraction, and manuscript editing; P.A.L. edited the manuscript and provided oversight; W.L. conceptualized the study and provided oversight; J.L. conceptualized the study and edited the manuscript; and D.H. was responsible for conceptualization, manuscript editing, and statistical analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul George, Pediatrics, Emory University School of Medicine, 1518 Clifton Rd NE, Atlanta, GA 30322; email: pegeorg@emory.edu.
References
Author notes
Because of the inclusion of identifying patient information, the data from this study cannot be shared. All code used for the analysis will be publicly available in a GitHub repository maintained by the corresponding author, Paul George (pegeorg@emory.edu).
The full-text version of this article contains a data supplement.