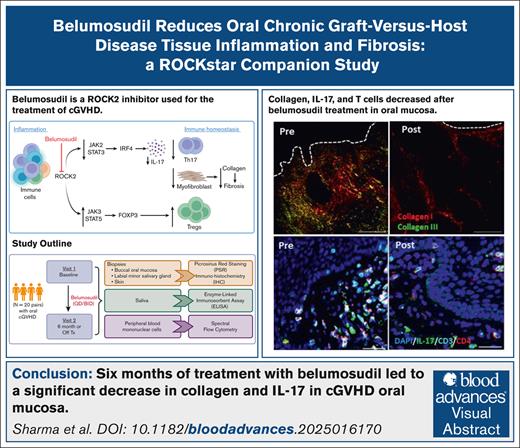
Key Points
Belumosudil treatment for cGVHD decreased collagen content in the OM.
Belumosudil reduced IL-17 production and inflammation in the OM and MSGs in oral cGVHD.
Visual Abstract
Belumosudil (KD025), an oral, selective, Rho-associated, coiled-coil–containing protein kinase 2 (ROCK2) inhibitor, is approved for third-line treatment of chronic graft-versus-host disease (cGVHD). Previous studies demonstrated that ROCK2 inhibition reduces blood interleukin-17 (IL-17) activity and promotes regulatory T-cell (Treg cell) recovery. However, these studies did not evaluate immune responses within cGVHD-affected tissues. This study assessed tissue-level immune dynamics in 20 patients with oral cGVHD from the phase 2 ROCKstar trial, before and after 6 months of belumosudil treatment, focusing on key effector sites (oral mucosa [OM], minor salivary glands [MSGs], and skin) and the peripheral blood. After belumosudil treatment, reduction in collagen was observed in OM in parallel with decreased IL-17+ cell frequency in both OM (n = 14 pairs) and MSG (n = 11 pairs). IL-17 was primarily produced by non–T cells in the oral tissues. Immune cell frequencies in the OM decreased after treatment, whereas CD4 Treg cells increased in both the MSG and blood. Per overall or mouth-specific clinical response criteria, responders to belumosudil exhibited a reduction in collagen type I and IL-17 in the OM. Additionally, salivary transforming growth factor β1 (TGF-β1), a critical driver of fibrosis, decreased significantly, with a strong correlation observed between TGF-β1 and IL-17 levels. These findings illustrate the tissue-level response to belumosudil therapy and suggest that there is a reduction in tissue fibrosis and inflammation, thereby highlighting the therapeutic impact of ROCK2 inhibition in mitigating cGVHD. The ROCKstar study was registered at www.ClinicalTrials.gov as #NCT03640481.
Introduction
Chronic graft-versus-host disease (cGVHD) is an immune-mediated condition characterized by heterogenous inflammation, tissue damage, and fibrosis that is a serious late complication in survivors of allogeneic hematopoietic cell transplant (allo-HCT) for treatment of hematologic malignancies, bone marrow failure syndromes, and genetic immunodeficiencies.1,2 High-dose steroids are used as first-line treatment for cGVHD; several novel agents have now been approved for use in subsequent lines of therapy based on data from trials focusing on clinical and blood response measures.3 However, despite knowledge of the target mechanisms of these novel agents, their impact at cGVHD effector sites such as oral mucosa (OM), minor salivary glands (MSGs), and skin have, to our knowledge, not been examined. Thus, the tissue-level biology driving the clinical changes observed in clinical trials remains to be described.
Interleukin-17 (IL-17) plays a key role in inflammation4 and contributes to cGVHD pathogenesis5 by increasing the frequency of proinflammatory T helper 17 (Th17) cells and reducing the frequency of regulatory T (Treg) cells,6 driving pathogenic differentiation, function, and tissue sequestration of macrophages.7 When Rho-associated, coiled-coil–containing protein kinase 2 (ROCK2) is physiologically activated in T cells, IL-17 and IL-21 secretion is increased.8-11 ROCK2 signaling is further linked to fibrosis, collagen type I production, and the development of cellular adhesion, through direct and indirect effects on macrophage function.12,13
Belumosudil (Rezurock, KD025) is an orally administered, selective inhibitor of ROCK2 approved by the US Food and Drug Administration for the treatment of refractory cGVHD.14-16 Inhibition of ROCK2 by belumosudil downregulates proinflammatory responses via suppression of signal transducer and activator of transcription 3 (STAT3) phosphorylation, reducing peripheral blood IL-17, Th17 cells, and T follicular helper cells, and upregulating STAT5 phosphorylation, thereby increasing the frequency of Treg cells9,14,17 and restoring the Th17/Treg cell balance.14,17,18 In addition, belumosudil is reported to reverse organ fibrosis via modulation of macrophages and tissue remodeling.12 Belumosudil also significantly reduced expression of collagen type I in the liver.12 The clinical safety, tolerability, and efficacy of belumosudil was evaluated in the phase 2 ROCKstar study (ClinicalTrials.gov identifier: NCT03640481).19 Patients with cGVHD after allo-HCT were randomized to treatment with belumosudil at doses of either 200 mg once daily or 200 mg twice daily. After a median follow-up of 14 months, overall response rates were 74% and 77%, respectively.19
This study was undertaken to evaluate the impact of ROCK2 inhibition at the tissue level in patients with oral cGVHD who were enrolled in a ROCKstar companion study before and after belumosudil treatment. Because the frequency of IL-17 producing cells in oral tissues of individuals with cGVHD has been associated with increased disease severity,20 and these cells are also present in the skin, assessment of matched skin samples was included as an exploratory end point. Here, we report these longitudinal findings from the ROCKstar study, highlighting the effects of belumosudil treatment on immune cell and tissue profiles at cGVHD effector sites and the peripheral blood from patients enrolled across 4 study centers.
Methods
Study design and patient population
Full details of the study design of the phase 2, randomized, multicenter ROCKstar study have been published previously.19 This companion study randomized 20 allo-HCT recipients aged ≥12 years with persistent oral cGVHD manifestations after receiving ≥2 to 5 previous systemic lines of treatment. Patients were enrolled from 4 centers in the United States (National Institutes of Health [NIH], Dana-Farber Cancer Institute, MD Anderson Cancer Center, and Washington University in St. Louis) after the data cutoff date (19 August 2020) for the main ROCKstar study publication.19 In addition to meeting the eligibility criteria for the primary study, patients for this companion study had oral cGVHD and no contraindication(s) for biopsy collection. Oral cGVHD was defined as lichenoid changes or distinctive features of oral cGVHD (including mucoceles, mucosal atrophy, ulcers, or pseudomembranes) sufficient for diagnosis, with or without clinical symptoms, provided there was no suspicion for alternative causes. Disease definitions were based on the 2014 NIH Consensus Development Project on Criteria for Clinical Trials in cGVHD (referred to as the 2014 NIH Consensus Criteria).21
Patients were randomized to receive belumosudil either 200 mg once daily or twice daily, administered orally. Belumosudil treatment ended early if there was unacceptable toxicity or clinically significant progression of cGVHD. Progression of cGVHD was based on an organ-specific cGVHD response assessment, using the 2014 NIH Consensus Criteria.22 Severity of GVHD in the oral cavity was assessed according to the NIH-modified OM rating scale (OMRS; range, 0-12).22 Clinical mouth response was defined by change from baseline to the primary end point (6 months) in the NIH-modified OMRS: a score of 0 indicated complete response (CR), a decrease of ≥2 points was considered a partial response (PR), and an increase of ≥2 points was defined as progressive disease (PD). The study protocol (supplemental Appendix23) was approved by the institutional review board at each center, and written informed consent was provided by all patients. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines.
This investigation was conducted with the primary objective of characterizing tissue-level changes in IL-17 signaling in the oral cavity and the local immune cell profile before and after 6 months of treatment with belumosudil. The secondary objective to assess the feasibility of including detailed biospecimen collection in a multicenter study was assessed by the proportion of usable specimens obtained and is reported with 95% confidence intervals (CIs). Exploratory objectives included analysis of changes in the following parameters before and after treatment with belumosudil: skin and peripheral blood immune cellular profile, with a focus on IL-17 production, and local saliva cytokine and chemokine profiles; followed by relevant comparisons among sites.
Biopsies from the buccal OM, labial MSGs, and cutaneous skin were obtained before initiation of belumosudil and after 6 months of belumosudil therapy or at the time of last treatment (whichever came earlier). At the same time points, unstimulated 5-minute whole saliva and the peripheral blood were sampled using previously described methodology.24 Details of sample processing, tissue stains, multiplex cytokine assays on saliva, and spectral flow cytometry on peripheral blood mononuclear cells (PBMCs) are detailed in the supplemental Materials and methods.
Statistical analysis
Paired (pretreatment and posttreatment) samples were assessed using a paired t test if the paired differences were normally distributed (P > .05 by a Shapiro-Wilks test); otherwise, a Wilcoxon signed-rank test was used.
Results
A total of 20 patients were enrolled in the ROCKstar study between December 2020 and May 2022, as detailed in the CONSORT diagram (supplemental Figure 1).
Baseline demographics and clinical characteristics
Demographic and clinical characteristics of patients were assessed according to clinical NIH mouth response (Table 1). At baseline, clinical mouth responders (CR/PR) had a higher mouth score, higher organ involvement and global severity ratings, and a lower Karnofsky performance score than nonresponders. At baseline, all enrolled patients (summarized individually in supplemental Table 1) had oral cGVHD, with an average score of 3.5 (range, 1-7) on the 12-point NIH-modified mouth OMRS. Skin involvement was not required for study participation, but, at baseline, 80% of patients had some skin involvement based on an NIH skin score of ≥1 (on a scale of 0-3). Using the 2014 NIH Consensus Criteria,21 85% of patients had severe cGVHD and 15% had moderate cGVHD. In total, 65% of patients had involvement of ≥4 organs at baseline.
Baseline patient demographics and clinical characteristics between clinical mouth responders and nonresponders
| Characteristic . | Mouth responders (n = 14) . | Mouth nonresponders (n = 6) . |
|---|---|---|
| Age, median (range), y | 49.5 (18-70) | 51.5 (27-71) |
| Sex, female, n (%) | 7 (50.0) | 2 (33.3) |
| Race/ethnicity, n (%) | ||
| White | 13 (92.9) | 5 (83.3) |
| Black | — | 1 (16.7) |
| Other | 1 (7.1) | — |
| HSCT indication, n (%) | ||
| AML | 6 (42.9) | 3 (50.0) |
| ALL | 1 (7.1) | 2 (33.3) |
| MDS | 2 (14.3) | — |
| CML | 1 (7.1) | — |
| Myelofibrosis | 1 (7.1) | — |
| CLL | 1 (7.1) | — |
| NHL | 1 (7.1) | — |
| Other | 1 (7.1) | 1 (16.7) |
| Conditioning, n (%) | ||
| Myeloablative | 11 (78.6) | 6 (100) |
| Nonmyeloablative | 2 (14.3) | — |
| TBI | — | — |
| Unknown | 1 (7.1) | — |
| HSCT graft source, peripheral blood, n (%) | 14 (100) | 6 (100) |
| HLA matching of donor/recipient, n (%) | ||
| Matched (related) | 6 (42.9) | 2 (33.3) |
| Matched (unrelated) | 7 (50.0) | 4 (66.7) |
| Partially matched (related) | 1 (7.1) | — |
| CMV serostatus (donor/recipient), n (%) | ||
| Positive/positive | — | 3 (50.0) |
| Positive/negative | 4 (28.6) | 1 (16.7) |
| Negative/positive | 3 (21.4) | 2 (33.3) |
| Negative/negative | 5 (35.7) | — |
| Unknown | 2 (14.3) | — |
| Previous aGVHD, n (%) | 10 (71.4) | 5 (83.3) |
| NIH cGVHD severity, n (%) | ||
| Moderate | 3 (21.4) | — |
| Severe | 11 (78.6) | 6 (100) |
| Mouth involvement per NIH-modified OMRS, mean (range) | ||
| Erythema score | 1.0 (0-2) | 1.0 (1-2) |
| Lichenoid score | 1.0 (0-3) | 0.5 (0-3) |
| Ulcer score | 0 (0-3) | 0 (0) |
| Total mouth score | 3.5 (1-7) | 1.5 (1-5) |
| Organ involvement | ||
| No. of organs involved, median (range) | 4.5 (3-6) | 3 (1-5) |
| ≥4 organs involved, n (%) | 11 (78.6) | 2 (33.3) |
| Skin, n (%) | 12 (85.7) | 4 (66.7) |
| Joints/fascia, n (%) | 12 (85.7) | 4 (66.7) |
| Eyes, n (%) | 9 (64.3) | 2 (33.3) |
| Lungs, n (%) | 6 (42.9) | 0 |
| Esophagus, n (%) | 4 (28.6) | 1 (16.7) |
| Upper GI tract, n (%) | 1 (7.1) | 0 |
| Lower GI tract, n (%) | 2 (14.3) | 0 |
| Liver, n (%) | 1 (7.1) | 1 (16.7) |
| Baseline global severity rating (0-10), n (%) | ||
| 3 | — | 1 (16.7) |
| 4 | — | — |
| 5 | 2 (14.3) | 1 (16.7) |
| 6 | 3 (21.4) | 1 (16.7) |
| 7 | 6 (42.9) | 3 (50.0) |
| 8 | 3 (21.4) | — |
| Median Karnofsky performance status, n (%) | ||
| 60-70 | 2 (14.3) | — |
| 80-90 | 12 (85.7) | 6 (100) |
| 100 | — | — |
| Time from HSCT to study enrollment, median (range), mo | 43.0 (13.6-89.7) | 33.2 (12.9-102.4) |
| Time from cGVHD diagnosis to study enrollment, median (range), mo | 13.9 (3.6-38.9) | 12.2 (5.1-38.7) |
| Previous therapies, median (range), n | 3.5 (2-7) | 3 (2-5) |
| Patients per treatment arm, n (%) | ||
| Once daily | 8 (57.1) | 3 (50.0) |
| Twice daily | 6 (42.9) | 3 (50.0) |
| Characteristic . | Mouth responders (n = 14) . | Mouth nonresponders (n = 6) . |
|---|---|---|
| Age, median (range), y | 49.5 (18-70) | 51.5 (27-71) |
| Sex, female, n (%) | 7 (50.0) | 2 (33.3) |
| Race/ethnicity, n (%) | ||
| White | 13 (92.9) | 5 (83.3) |
| Black | — | 1 (16.7) |
| Other | 1 (7.1) | — |
| HSCT indication, n (%) | ||
| AML | 6 (42.9) | 3 (50.0) |
| ALL | 1 (7.1) | 2 (33.3) |
| MDS | 2 (14.3) | — |
| CML | 1 (7.1) | — |
| Myelofibrosis | 1 (7.1) | — |
| CLL | 1 (7.1) | — |
| NHL | 1 (7.1) | — |
| Other | 1 (7.1) | 1 (16.7) |
| Conditioning, n (%) | ||
| Myeloablative | 11 (78.6) | 6 (100) |
| Nonmyeloablative | 2 (14.3) | — |
| TBI | — | — |
| Unknown | 1 (7.1) | — |
| HSCT graft source, peripheral blood, n (%) | 14 (100) | 6 (100) |
| HLA matching of donor/recipient, n (%) | ||
| Matched (related) | 6 (42.9) | 2 (33.3) |
| Matched (unrelated) | 7 (50.0) | 4 (66.7) |
| Partially matched (related) | 1 (7.1) | — |
| CMV serostatus (donor/recipient), n (%) | ||
| Positive/positive | — | 3 (50.0) |
| Positive/negative | 4 (28.6) | 1 (16.7) |
| Negative/positive | 3 (21.4) | 2 (33.3) |
| Negative/negative | 5 (35.7) | — |
| Unknown | 2 (14.3) | — |
| Previous aGVHD, n (%) | 10 (71.4) | 5 (83.3) |
| NIH cGVHD severity, n (%) | ||
| Moderate | 3 (21.4) | — |
| Severe | 11 (78.6) | 6 (100) |
| Mouth involvement per NIH-modified OMRS, mean (range) | ||
| Erythema score | 1.0 (0-2) | 1.0 (1-2) |
| Lichenoid score | 1.0 (0-3) | 0.5 (0-3) |
| Ulcer score | 0 (0-3) | 0 (0) |
| Total mouth score | 3.5 (1-7) | 1.5 (1-5) |
| Organ involvement | ||
| No. of organs involved, median (range) | 4.5 (3-6) | 3 (1-5) |
| ≥4 organs involved, n (%) | 11 (78.6) | 2 (33.3) |
| Skin, n (%) | 12 (85.7) | 4 (66.7) |
| Joints/fascia, n (%) | 12 (85.7) | 4 (66.7) |
| Eyes, n (%) | 9 (64.3) | 2 (33.3) |
| Lungs, n (%) | 6 (42.9) | 0 |
| Esophagus, n (%) | 4 (28.6) | 1 (16.7) |
| Upper GI tract, n (%) | 1 (7.1) | 0 |
| Lower GI tract, n (%) | 2 (14.3) | 0 |
| Liver, n (%) | 1 (7.1) | 1 (16.7) |
| Baseline global severity rating (0-10), n (%) | ||
| 3 | — | 1 (16.7) |
| 4 | — | — |
| 5 | 2 (14.3) | 1 (16.7) |
| 6 | 3 (21.4) | 1 (16.7) |
| 7 | 6 (42.9) | 3 (50.0) |
| 8 | 3 (21.4) | — |
| Median Karnofsky performance status, n (%) | ||
| 60-70 | 2 (14.3) | — |
| 80-90 | 12 (85.7) | 6 (100) |
| 100 | — | — |
| Time from HSCT to study enrollment, median (range), mo | 43.0 (13.6-89.7) | 33.2 (12.9-102.4) |
| Time from cGVHD diagnosis to study enrollment, median (range), mo | 13.9 (3.6-38.9) | 12.2 (5.1-38.7) |
| Previous therapies, median (range), n | 3.5 (2-7) | 3 (2-5) |
| Patients per treatment arm, n (%) | ||
| Once daily | 8 (57.1) | 3 (50.0) |
| Twice daily | 6 (42.9) | 3 (50.0) |
aGVHD, acute GVHD; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; GI, gastrointestinal; HSCT, hematopoietic stem cell transplant; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; TBI, total body irradiation.
Among experienced centers, it was feasible to conduct a multicenter cGVHD study with detailed biospecimen collection
The study plan was to obtain 20 pairs of biospecimens before starting belumosudil and after 6 months of therapy for each type of specimen. Because of the COVID-19 pandemic and resultant logistical complications, a total of 16 paired OM biopsies (80%; 95% CI, 62-98), 15 paired labial MSG biopsies (75%; 95% CI, 56-94), 14 paired skin biopsies (70%; 95% CI, 50-90), 12 saliva pairs (60%; 95% CI, 36-81), and 10 blood pairs (50%; 95% CI, 28-72), were usable. Shipment of samples stable at ambient temperatures, local processing of specimens, and shipment of samples in small batches rather than in bulk at the end of study to protect against lost specimens due to shipping errors are recommended for future studies.
Belumosudil elicited tissue-specific effects on IL-17 and collagen types I and III
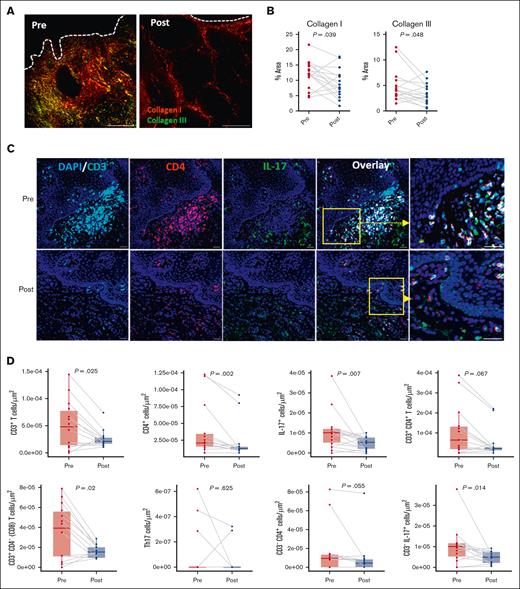
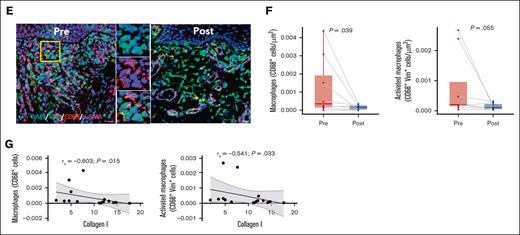
Significant reductions in type I (P = .039) and type III (P = .048) collagen in the oral buccal mucosa were observed, suggesting regression of fibrosis (Figure 1A-B). Immune cell number changes were assessed by immunofluorescence staining (Figure 1C), which revealed significant reductions in CD3+ T cells (P = .025), CD4+ cells (P = .002), and CD3+CD4− (CD8) T cells (P = .02), and a nonsignificant trend for decreases in CD3+CD4+ T cells (P = .067) and CD3−CD4+ cells (P = .055; Figure 1D). At baseline, immune cells were predominantly located near the basement membrane. Consistent with previous reports that ROCK2 inhibition downregulates IL-17 expression,17 overall IL-17 protein levels were significantly decreased after treatment (P = .007), although Th17 cell frequency was unchanged (UN). Non–T cells (CD3–IL-17+), which were identified as the primary IL-17 producers, showed a significant decline after treatment (P = .014; Figure 1D). These cells evaded comprehensive phenotyping. However, other specific immune cell types known to be potent IL-17 producers, including natural killer (NK) cells, myeloid cells, and NK T (NKT) cells remained UN in the OM; there was also no change in interferon gamma (IFN-γ) production (supplemental Figure 2; supplemental Table 2). Macrophages are known contributors to the inflammatory microenvironment in fibrotic cGVHD.25 In the OM, tissue macrophages (CD68+) were decreased (P = .039) after treatment, in alignment with the reduction measured in non–T-cell–derived IL-17 (Figure 1E-F). Importantly, there was negative correlation of collagen I with both macrophages (Spearman correlation coefficient rs = −0.603; P = .015) or activated macrophages (CD68+ vimentin-positive; rs = −0.541; P = .033) after belumosudil treatment (Figure 1G).
ROCK2 inhibition with belumosudil reduces collagen, T cells, and IL-17–producing cells in the oral buccal mucosa. (A) Representative PSR (picrosirius red) staining paired with polarized microscopy of OM sections before and after treatment with belumosudil showing changes in collagen type I (red) and collagen type III (green). White dashed line denotes epithelial-submucosal border. (B) Changes in percentage area of collagen types I and III in the OM (n = 16; paired parametric t test). (C) Representative multiplex immunofluorescence staining for DAPI (blue), CD3 (cyan), CD4 (red), and IL-17 (green) in the OM before and after treatment with belumosudil with close-up view of marked region in yellow. (D) Changes in CD3+ T, CD4+, IL-17+, CD3+CD4+ T, CD3+CD4− (CD8) T, Th17, CD3−CD4+, and CD3− IL-17+ cells after belumosudil treatment (n = 14; Wilcoxon signed-rank test). (E) Representative multiplex immunofluorescence staining for DAPI (blue), Vim (green), CD68 (red), α-SMA (magenta) in the OM before and after treatment with belumosudil with close-up view of marked region in yellow. (F) Changes in macrophages (CD68+) and activated macrophages (CD68+Vim+ cells) after belumosudil treatment (n = 8; Wilcoxon signed-rank test). (G) Scatterplots showing negative correlation between macrophages and activated macrophages with collagen I. P value <.05 is considered significant. Scale bar of 100 μm was used. α-SMA, α-smooth muscle actin; DAPI, 4′,6-diamidino-2-phenylindole; Vim, vimentin.
ROCK2 inhibition with belumosudil reduces collagen, T cells, and IL-17–producing cells in the oral buccal mucosa. (A) Representative PSR (picrosirius red) staining paired with polarized microscopy of OM sections before and after treatment with belumosudil showing changes in collagen type I (red) and collagen type III (green). White dashed line denotes epithelial-submucosal border. (B) Changes in percentage area of collagen types I and III in the OM (n = 16; paired parametric t test). (C) Representative multiplex immunofluorescence staining for DAPI (blue), CD3 (cyan), CD4 (red), and IL-17 (green) in the OM before and after treatment with belumosudil with close-up view of marked region in yellow. (D) Changes in CD3+ T, CD4+, IL-17+, CD3+CD4+ T, CD3+CD4− (CD8) T, Th17, CD3−CD4+, and CD3− IL-17+ cells after belumosudil treatment (n = 14; Wilcoxon signed-rank test). (E) Representative multiplex immunofluorescence staining for DAPI (blue), Vim (green), CD68 (red), α-SMA (magenta) in the OM before and after treatment with belumosudil with close-up view of marked region in yellow. (F) Changes in macrophages (CD68+) and activated macrophages (CD68+Vim+ cells) after belumosudil treatment (n = 8; Wilcoxon signed-rank test). (G) Scatterplots showing negative correlation between macrophages and activated macrophages with collagen I. P value <.05 is considered significant. Scale bar of 100 μm was used. α-SMA, α-smooth muscle actin; DAPI, 4′,6-diamidino-2-phenylindole; Vim, vimentin.
In labial MSG biopsies (11 evaluable pairs), no significant change in collagen (type I or type III) was seen after treatment with belumosudil (Figure 2A-B). Changes in immune cell frequency, including CD3+ T cells, CD3+CD4+ T cells, and CD3+CD4− (CD8) T cells (supplemental Table 2), were not significant. Although a marginal decrease (P = .053) in CD4+ cells was noted, these were mainly found as periductal infiltrates in MSGs. There was a significant decrease in IL-17 expression (P = .03) after treatment. Although Th17 cells are rare in MSGs, there was an upward trend (P = .062; Figure 2C-D). A significant reduction in the frequency of IL-17 production by non–T cells (CD3−IL-17+; P = .042) was identified in MSGs. In addition, myeloid cell (CD3−CD11b+) frequency was significantly reduced (P = .009; Figure 2E-F), whereas the frequencies of NK and NKT cells and IFN-γ protein expression were UN in MSG sections (supplemental Figure 2; supplemental Table 2).
In MSGs, ROCK2 inhibition decreased IL-17 production by non–T cells. (A) Representative PSR staining paired with polarized microscopy of labial MSGs showing collagen type I (red) and collagen type III (green) in biopsies before and after treatment with belumosudil. (B) Percentage area of collagen types I and III in labial MSGs (n = 11; Wilcoxon signed-rank test). (C) Representative multiplex immunofluorescence staining for DAPI (blue), CD3 (cyan), CD4 (red), and IL-17 (green) in labial MSGs before and after treatment with belumosudil with close-up view of marked region in yellow. (D) Changes in CD3+ T, CD4+, IL-17+, and Th17 cells after belumosudil treatment (n = 11; Wilcoxon signed-rank test). (E) Representative multiplex immunofluorescence staining of DAPI (blue), CD3 (cyan), and CD11b (red) in MSGs before and after treatment with belumosudil. (F) Changes in myeloid cells and CD3− IL-17+ non–T cells (n = 11; Wilcoxon signed-rank test). P value < .05 is considered significant. Scale bar of 100 μm was used.
In MSGs, ROCK2 inhibition decreased IL-17 production by non–T cells. (A) Representative PSR staining paired with polarized microscopy of labial MSGs showing collagen type I (red) and collagen type III (green) in biopsies before and after treatment with belumosudil. (B) Percentage area of collagen types I and III in labial MSGs (n = 11; Wilcoxon signed-rank test). (C) Representative multiplex immunofluorescence staining for DAPI (blue), CD3 (cyan), CD4 (red), and IL-17 (green) in labial MSGs before and after treatment with belumosudil with close-up view of marked region in yellow. (D) Changes in CD3+ T, CD4+, IL-17+, and Th17 cells after belumosudil treatment (n = 11; Wilcoxon signed-rank test). (E) Representative multiplex immunofluorescence staining of DAPI (blue), CD3 (cyan), and CD11b (red) in MSGs before and after treatment with belumosudil. (F) Changes in myeloid cells and CD3− IL-17+ non–T cells (n = 11; Wilcoxon signed-rank test). P value < .05 is considered significant. Scale bar of 100 μm was used.
In skin biopsies (14 evaluable pairs), collagen type I (P = .011) was increased after treatment (supplemental Figure 3A-B). Unlike oral biopsies, no measurable changes in the studied immune profile (T cells or non–T cells) or IL-17 production were observed in skin samples after belumosudil treatment (supplemental Figures 2 and 3C-D; supplemental Table 2).
Clinical response to belumosudil was associated with tissue-level differences in responders vs nonresponders
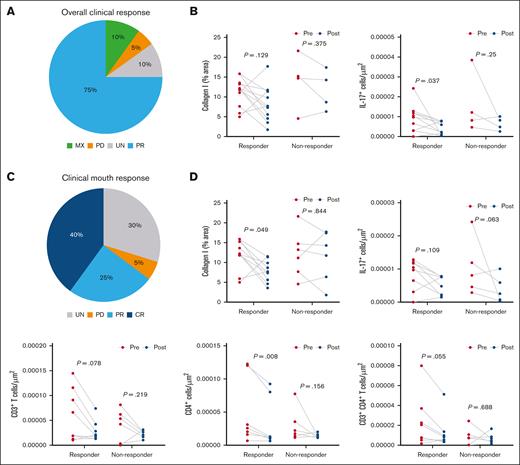
Based on NIH GVHD Consensus Criteria,21 the overall clinical response rate in the OM (n = 20) was mixed response in 10%, PD in 5%, UN in 10%, and PR in 75% of patients (Figure 3A). To understand the immune composition and functionality related to the overall clinical response to belumosudil, we evaluated collagen, immune milieu, systemic immune cells, and salivary cytokines in responders (PR) and nonresponders (mixed response, PD, and UN). A significant decrease in overall OM IL-17 (P = .037) was identified in responders, but no other differences were identified between responders and nonresponders in any tissue (OM, MSG, and skin), in PBMCs, or in the saliva (Figure 3B).
Clinical responders to belumosudil had reductions in OM IL-17 cells, immune cells, and local type I collagen that were not observed in nonresponders. (A) Pie chart showing the percentage of total patients (n = 20) with overall clinical response of MX, PD, UN, or PR. (B) Overall clinical response classification of changes in OM collagen type I and IL-17+ cells between responders and nonresponders with belumosudil (n = 16). Responders included PR, and nonresponders included MX, PD, and UN. (C) Pie chart showing percentage of total patients (n = 20) with clinical mouth response of UN, PD, PR, and CR. (D) Clinical mouth response classification of differences in percentage area of collagen type I, IL-17+, CD3+ T, CD4+, and CD3+CD4+ T cells before and after treatment in the cohort (n = 16) of responders and non-responders in the OM. Responders included PR and CR, and nonresponders included UN and PD. P value <.05 is considered significant. Wilcoxon signed-rank test was performed. MX, mixed response.
Clinical responders to belumosudil had reductions in OM IL-17 cells, immune cells, and local type I collagen that were not observed in nonresponders. (A) Pie chart showing the percentage of total patients (n = 20) with overall clinical response of MX, PD, UN, or PR. (B) Overall clinical response classification of changes in OM collagen type I and IL-17+ cells between responders and nonresponders with belumosudil (n = 16). Responders included PR, and nonresponders included MX, PD, and UN. (C) Pie chart showing percentage of total patients (n = 20) with clinical mouth response of UN, PD, PR, and CR. (D) Clinical mouth response classification of differences in percentage area of collagen type I, IL-17+, CD3+ T, CD4+, and CD3+CD4+ T cells before and after treatment in the cohort (n = 16) of responders and non-responders in the OM. Responders included PR and CR, and nonresponders included UN and PD. P value <.05 is considered significant. Wilcoxon signed-rank test was performed. MX, mixed response.
Next, we explored the clinical mouth response, in which 65% of patients were classified as having a clinical response after belumosudil treatment. In the overall cohort, 40% (n = 20) achieved CR, 25% had PR, 30% were UN, and 5% had PD (Figure 3C). Analysis of the immune and cytokine milieu revealed significant reductions in collagen type I (P = .049) and CD4+ cells (P = .008) in the OM of mouth responders (Figure 3D). However, the frequency of IL-17+ cells in the OM did not differ between mouth responders and nonresponders.
CD4 Treg cells were increased in the peripheral blood and MSG effector tissues
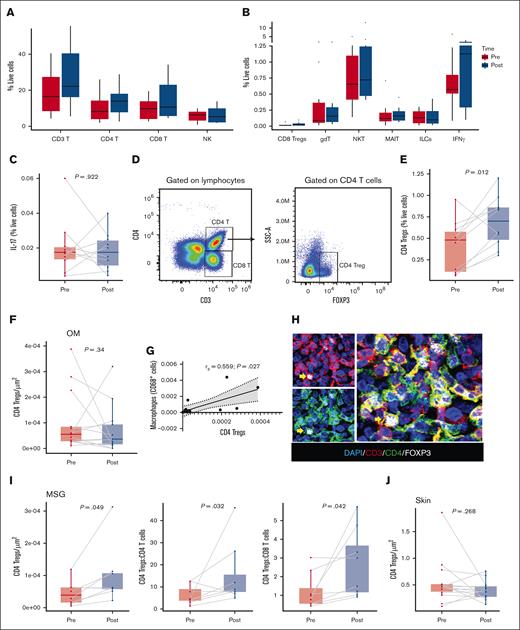
To characterize changes in the peripheral blood immune profile after belumosudil treatment, evaluable PBMCs (n = 10 pairs) were analyzed using spectral flow cytometry. Among circulating cells, no significant changes were observed in the percentages of CD3 T cells, CD4 T cells, CD8 T cells, NK cells, NKT cells, CD8 Treg cells, γδ T cells, innate lymphoid cells, or mucosal-associated invariant T cells after treatment (Figure 4A-B; supplemental Figure 4; supplemental Table 3). T cells were further characterized into the following subsets: naïve (CD27+CD45RA+), central memory (CD27+CD45RA−), effector memory (CD27−CD45RA−), and terminally differentiated effector memory T cells (CD27−CD45RA+). However, no statistically significant differences were identified in T-cell subsets except for an increase in effector memory T cells (P = .027; supplemental Table 3). After treatment with belumosudil, there were no differences in IL-17 (Figure 4C) or IL-17–producing immune cells. In agreement with previous findings,26 an increase in Treg cell (CD3+CD4+FOXP3+) frequency (P = .012) was identified in PBMCs after belumosudil treatment (Figure 4D-E). Tissue analyses revealed no changes in CD4 Treg cells in the OM (Figure 4F); however, there was a significant positive correlation between CD4 Treg cell and macrophage numbers (rs = 0.559; P = .027; Figure 4G). A statistically significant increase in CD4 Treg cells (P = .049) was detected in MSG biopsies (Figure 4H-I). Relationships among CD4 Treg cells and other immune subsets in the MSG showed increased ratios of CD4 Treg cells to CD4 T cells (P = .032), and CD4 Treg cells to CD8 T cells (P = .042; Figure 4I) emphasizing the preponderance of CD4 Treg cells in MSG sections after belumosudil treatment. Conversely, skin biopsies showed no changes in CD4 Treg cells after belumosudil (Figure 4J).
MSG resident and blood CD4 Treg cells increased in frequency after belumosudil therapy. (A-B) Percentages of immune cells (CD3 T, CD4 T, CD8 T, NK, CD8 Treg cells, gdT, NKT, MAIT, ILCs, IFN-γ) in PBMCs before and after treatment with belumosudil measured by spectral flow cytometry. (C) Percentage of IL-17 before and after treatment with belumosudil from PBMCs (n = 10). (D) Gating strategy used for CD4 Treg cells (CD3+CD4+FOXP3+). (E) Percentage of CD4 Treg cells before and after treatment with belumosudil from PBMCs (n = 10). (F) Quantification of CD4 Treg cells in the OM. (G) Scatterplot showing significant positive correlation between CD4 Treg cells and macrophages in the OM. (H) Representative multiplex immunofluorescence staining of DAPI (blue), CD3 (red), CD4 (green), and FOXP3 (white) in labial MSGs. (I) Quantification of CD4 Treg cells and ratios to other T-cell populations in the MSGs. (J) Quantification of CD4 Treg cells in the skin. P value <.05 is considered significant. Wilcoxon signed-rank test was performed. Scale bar of 50 μm was used. FOXP3, forkhead box P3; gdT, γδ T cells; ILC, innate lymphoid cell; MAIT, mucosal-associated invariant T cell.
MSG resident and blood CD4 Treg cells increased in frequency after belumosudil therapy. (A-B) Percentages of immune cells (CD3 T, CD4 T, CD8 T, NK, CD8 Treg cells, gdT, NKT, MAIT, ILCs, IFN-γ) in PBMCs before and after treatment with belumosudil measured by spectral flow cytometry. (C) Percentage of IL-17 before and after treatment with belumosudil from PBMCs (n = 10). (D) Gating strategy used for CD4 Treg cells (CD3+CD4+FOXP3+). (E) Percentage of CD4 Treg cells before and after treatment with belumosudil from PBMCs (n = 10). (F) Quantification of CD4 Treg cells in the OM. (G) Scatterplot showing significant positive correlation between CD4 Treg cells and macrophages in the OM. (H) Representative multiplex immunofluorescence staining of DAPI (blue), CD3 (red), CD4 (green), and FOXP3 (white) in labial MSGs. (I) Quantification of CD4 Treg cells and ratios to other T-cell populations in the MSGs. (J) Quantification of CD4 Treg cells in the skin. P value <.05 is considered significant. Wilcoxon signed-rank test was performed. Scale bar of 50 μm was used. FOXP3, forkhead box P3; gdT, γδ T cells; ILC, innate lymphoid cell; MAIT, mucosal-associated invariant T cell.
Saliva reflected attenuated inflammation in the local oral environment and reduced TGF-β1
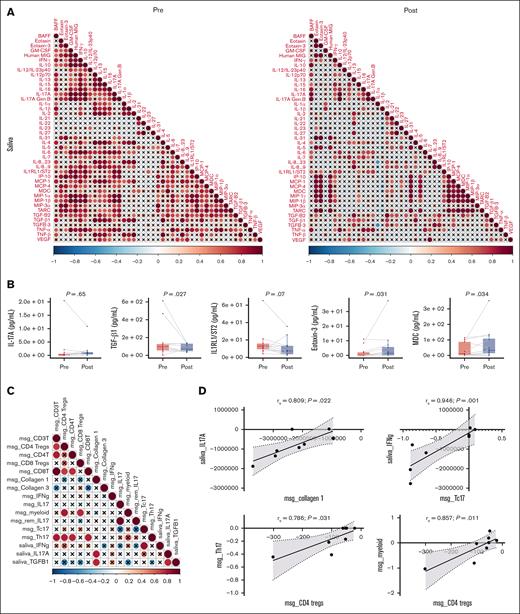
To measure the effect of belumosudil on proinflammatory and inflammatory cytokines in the saliva, we generated a correlation matrix of all salivary cytokines measured in evaluable samples (n = 12) before and after treatment with belumosudil (Figure 5A). This analysis revealed few significant correlations among cytokines after belumosudil, including a decrease in positive correlation between proinflammatory cytokines IL-17 and IFN-γ. A further loss of correlation among IL-17 and eotaxin, granulocyte-macrophage colony-stimulating factor, and monokine induced by IFN-γ was noted after belumosudil (Figure 5A). No changes were observed in salivary IL-17A levels; however, a statistically significant reduction in transforming growth factor β1 (TGF-β1; P = .027) and elevations in eotaxin-3 (P = .031) and macrophage-derived chemokine (P = .034) were identified. Notably, a nonsignificant trend toward reduced salivary IL-1 receptor-like 1/suppression of tumorigenicity 2 levels (P = .07), a predictive biomarker for steroid-refractory GVHD,27 was also observed (Figure 5B).
Saliva reflected attenuated inflammation in the local oral environment and had reduced TGF-β1. (A) Correlation matrix of salivary cytokines in pretreatment and posttreatment cohorts. See correlation matrix key below “∗.” (B) Changes in selected salivary cytokines (IL-17A, TGF-β1, IL-1RL1/ST2, eotaxin-3, and MDC) with belumosudil treatment (n = 12; Wilcoxon signed-rank test was performed). (C) Correlation matrix among the changes in selected salivary cytokines, immune cells, and collagen in labial salivary glands between pretreatment and posttreatment cohorts. See correlation matrix key “∗.” (D) Scatterplots exploring relationships from panel C specific analytes in saliva and labial salivary glands with a significant positive correlation. ∗Correlation matrix key: the intensity of the colors and the diameter of the circles give an indication of the degree of correlation between 2 cytokines and reflect the strength of Spearman ρ correlation coefficient. The crosses represent correlation coefficients that were not statistically significant. Significance was tested using a Spearman rank test and level of significance was set at P value <.05. Paired nonparametric t test was performed. BAFF, B-cell activating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-1RL1/ST2, interleukin 1 receptor-like 1/suppression of tumorigenicity 2; IP, IFN-γ–induced protein; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIG, monokine induced by IFN-γ; MIP, macrophage inflammatory protein; TARC, thymus and activation-regulated chemokine; Tc17, type 17 cytotoxic effector cell; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Saliva reflected attenuated inflammation in the local oral environment and had reduced TGF-β1. (A) Correlation matrix of salivary cytokines in pretreatment and posttreatment cohorts. See correlation matrix key below “∗.” (B) Changes in selected salivary cytokines (IL-17A, TGF-β1, IL-1RL1/ST2, eotaxin-3, and MDC) with belumosudil treatment (n = 12; Wilcoxon signed-rank test was performed). (C) Correlation matrix among the changes in selected salivary cytokines, immune cells, and collagen in labial salivary glands between pretreatment and posttreatment cohorts. See correlation matrix key “∗.” (D) Scatterplots exploring relationships from panel C specific analytes in saliva and labial salivary glands with a significant positive correlation. ∗Correlation matrix key: the intensity of the colors and the diameter of the circles give an indication of the degree of correlation between 2 cytokines and reflect the strength of Spearman ρ correlation coefficient. The crosses represent correlation coefficients that were not statistically significant. Significance was tested using a Spearman rank test and level of significance was set at P value <.05. Paired nonparametric t test was performed. BAFF, B-cell activating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-1RL1/ST2, interleukin 1 receptor-like 1/suppression of tumorigenicity 2; IP, IFN-γ–induced protein; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIG, monokine induced by IFN-γ; MIP, macrophage inflammatory protein; TARC, thymus and activation-regulated chemokine; Tc17, type 17 cytotoxic effector cell; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
In MSGs, to better understand relationships, targeted correlations among cytokines (IFN-γ, IL-17A, and TGF-β1) and immune cell frequency were assessed before and after treatment with belumosudil (Figure 5C). The results revealed the following positive correlations of interest: TGF-β1 with IL-17A (rs = 0.738; P = .046), IL-17A with collagen I (rs = 0.81; P = .022), and TGF-β1 with collagen I (rs = 0.619; P = .022). Strong correlations were observed between salivary IL-17A and collagen type I in MSG tissue (rs = 0.809; P = .022), and between TGF-β1 and salivary IFN-γ in type 17 cytotoxic effector cells (CD3+CD4−IL-17+; rs = 0.946; P = .001; Figure 5D). In addition, a positive correlation was observed between CD4 Treg cells and Th17 cells (rs = 0.786; P = .031) and myeloid cells (rs = 0.857; P = .011; Figure 5D). All findings after belumosudil treatment are summarized in Table 2.
Clinical dose regimen (200 mg once daily vs 200 mg twice daily): related changes in immune milieu
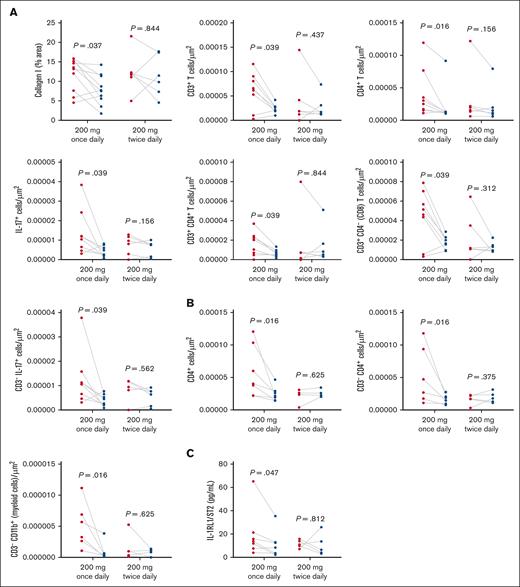
Additional analyses were undertaken to compare outcomes by belumosudil dose regimen (200 mg once daily [n = 11] vs 200 mg twice daily [n = 9]). In the once-daily arm, in OM samples, there were significant posttreatment reductions in collagen I (P = .037), CD3 T cells (P = .039), CD4+ cells (P = .016), CD3+CD4 T cells (P = .039), CD3+CD4− (CD8) T cells (P = .039), IL-17+ cells (P = .039), and non–T cells producing IL-17 (CD3−IL-17+; P = .039; Figure 6A), which were not observed in the twice-daily arm. Similarly, significant reductions in labial MSG CD4+ cells (P = .016), CD3−CD4+ cells (P = .016), and myeloid cells (CD3−CD11b+; P = .016) were measured in the once-daily but not the twice-daily arm (Figure 6B). Finally, when restricted to patients receiving once-daily belumosudil, a reduction in salivary IL-1 receptor-like 1/suppression of tumorigenicity 2 (P = .047) was detected (Figure 6C).
Dose-specific analysis highlighted specific reductions in IL-17, immune cells, and fibrosis only in the 200 mg once-daily belumosudil group. (A) Dose-group levels of collagen type I, CD3+ T, CD4+, IL-17+, CD3+CD4+ T, CD3+CD4− (CD8) T, and CD3− IL-17+ cells in the OM (n = 8) quantified by picrosirius red. (B) Dose-group levels of CD4+, CD3−CD4+, and myeloid cells (CD3−CD11b+) in labial MSGs (n = 6) via immunofluorescence staining. (C) Dose-group levels of IL-1RL1/ST2 in the saliva (n = 7) measured by enzyme-linked immunosorbent assay. P value <.05 is considered significant. Wilcoxon signed-rank test was performed. IL-1RL1/ST2, interleukin 1 receptor-like 1/suppression of tumorigenicity 2.
Dose-specific analysis highlighted specific reductions in IL-17, immune cells, and fibrosis only in the 200 mg once-daily belumosudil group. (A) Dose-group levels of collagen type I, CD3+ T, CD4+, IL-17+, CD3+CD4+ T, CD3+CD4− (CD8) T, and CD3− IL-17+ cells in the OM (n = 8) quantified by picrosirius red. (B) Dose-group levels of CD4+, CD3−CD4+, and myeloid cells (CD3−CD11b+) in labial MSGs (n = 6) via immunofluorescence staining. (C) Dose-group levels of IL-1RL1/ST2 in the saliva (n = 7) measured by enzyme-linked immunosorbent assay. P value <.05 is considered significant. Wilcoxon signed-rank test was performed. IL-1RL1/ST2, interleukin 1 receptor-like 1/suppression of tumorigenicity 2.
Discussion
Tissue-level evidence from effector sites of cGVHD reported here show that belumosudil often targets critical drivers of cGVHD, including IL-17 production and tissue fibrosis. Tissue inflammation and fibrosis are hallmarks of cGVHD and contribute to its significant symptom burden and disability.28 Because organ-level biologic responses are rarely evaluated in clinical trials of cGVHD therapies, this study was designed to investigate the biologic impact of a therapeutic agent in target organs. The OM and MSGs were selected as the key organs for investigation based on preliminary data indicating frequent IL-17+ cells in these cGVHD tissues, in addition to their accessibility for biopsy. The clinical efficacy and safety findings for belumosudil reported in this study are aligned with the results from the main ROCKstar study.19
Fibrosis in cGVHD is related to excess collagen deposition and disorganization, which is driven by several factors including inflammation and TGF-β–dependent and –independent macrophages, and is particularly destructive in tissues (such as the lung and salivary gland) that are reliant on intact structure for their function.29-31 Fibroblasts cultured from cGVHD skin had increased collagen type I gene expression and aberrant TGF-β signaling,32 and cGVHD lacrimal glands contained increased periductal collagen types I and III adjacent to activated fibroblasts.33 Although the role of type I vs type III collagen is not clear in cGVHD, fibrosis is considered to be the end stage of an unchecked inflammatory alloreactivity cascade.29 Sclerotic cGVHD affects the oral cavity, resulting in limited mouth opening and reduced quality of life, with frequent changes in the buccal tissues and fibrosis of the salivary ducts.34-36 In this study, belumosudil treatment reduced TGF-β1 levels in the saliva and significantly decreased types I and III collagen levels in the oral buccal mucosa, with type I collagen reduction being further indicative of clinical response in the mouth.
The rapid turnover of OM epithelial cells (∼14 days) may account for the earlier detection of antifibrotic effects in the OM throughout the study period vs in other tissues, such as the MSGs and skin, the cells of which have a slower turnover rate.37 Notably, patients in this analysis were not required to have skin involvement, although most did, and the skin biopsies were not required to be from active cGVHD sites; 23% of skin biopsies had histopathologic evidence of cGVHD at baseline. A more directed study design is needed to examine belumosudil mechanisms in cGVHD-affected skin.
In a murine model of induced liver fibrosis, belumosudil was effective in reducing liver fibrosis through direct and indirect effects on macrophage function with concomitant reduction of liver IL-17.12 Similarly, in a diabetes model, ROCK2 inhibition suppressed TGF-β expression and downstream profibrotic mediators.38 Additional studies are needed to examine the antifibrotic mechanism of belumosudil in cGVHD tissue.
ROCK2 regulates the proinflammatory cytokine IL-17, which was a major focus of this investigation. The oral cavity is a complex environment, and oral dysbiosis may also contribute to an increased risk of cGVHD concomitantly with IL-17 elevation.39 In this study, belumosudil treatment resulted in a reduction in the total number of IL-17–producing cells accompanied by a significant reduction in local CD8 T cells in oral tissues. Th17 cells were rarely identified; rather, most IL-17 was determined to be produced by non–T cells. Although current panels ruled out NK cells or myeloid cells as the source of IL-17, further investigation is required profile these CD3−IL-17−–producing cells, which may represent γδ T cells, mucosal-associated invariant T cells, or innate lymphoid cells. Finally, responder-level analysis showed that overall clinical responders to belumosudil had significant reductions in total IL-17+ cells in the OM and MSG, which was not observed in non-responders, and suggests that the effect of belumosudil on IL-17–producing cells may be important for its therapeutic impact, warranting further research to clarify the involved cells and mechanisms. Macrophages (CD68+) and activated macrophages (CD68+ vimentin-positive) were reduced after belumosudil treatment, which negatively correlated with type I collagen, suggesting macrophages may promote remodeling in cGVHD OM.40 TGF-β1, reduced in the saliva, is a key driver of fibrosis, whereas eotaxin-3 and macrophage-derived chemokine were elevated in the posttreatment saliva and drive immune cell recruitment and inflammation. Furthermore, the loss of correlation among IL-1, IFN-γ, eotaxin, granulocyte-macrophage colony-stimulating factor, and monokine induced by IFN-γ after belumosudil may indicate a shift toward immune homeostasis, with potential reduction in Th17 cell–driven inflammation, macrophage activation, eosinophilic infiltration, and T-cell recruitment.
Treg cells maintain the balance between autoimmunity and tolerance by suppressing immune cell proliferation and effector function, and their impairment is implicated in the development of cGVHD.41-44 The elevation in CD4 Treg cells observed in the peripheral blood and MSGs this study aligns with previous belumosudil studies showing expansion of Treg cells via upregulation of STAT5 phosphorylation.18 This suggests that Treg cells help balance T-cell subsets in cGVHD.42 The observed shifts in the ratios of CD4 Treg cells to CD4 T cells and CD4 Treg cells to CD8 T cells indicate that changes in the Treg cell compartment were not uniform across all T-cell subsets. Although belumosudil promotes systemic Treg cells expansion in PBMCs and MSGs, its limited effect in the OM may be because of impaired Treg cell recruitment or increased Treg cell exhaustion, highlighting the need for tissue level assessments.17 This cellular interplay may be important in mitigating the pathogenesis of cGVHD and suggests a prominent role of CD4 Treg cells. Additionally, this targeted effect highlights a pivotal role of ROCK2 inhibition in suppressing effector T cells to restore immune homeostasis within affected tissues.
Analysis by clinical dose regimen revealed distinct biological changes in the 200 mg once-daily group that were not observed in the 200 mg twice-daily group. Specifically, the once-daily dose was associated with a reduction in OM collagen I levels, along with total IL-17+ cells, various T-cell types, CD3−IL-17+ cells, MSG myeloid cells, and saliva IL-1 receptor-like 1. The group sizes in these subset analyses were small and were not adjusted for responder frequency or concomitant medications; however, the results suggest that additional investigations are needed to explore a potential regimen-related effect. There is precedent for bell-shaped response curves to immunomodulatory agents, when balance is needed in receptor engagement vs receptor-complex turnover,45 which supports the possibility of a balanced immunomodulation inducing these observations.
Experimental models have markedly improved our understanding of the complex immunobiology of cGVHD46,47 and have had a direct impact in the advancement of clinical agents for people with cGVHD. Although our clinical study is limited by a small sample size, it nonetheless highlights the feasibility and challenges of conducting insightful correlative studies within the context of multicenter clinical trials.
In summary, in this study, belumosudil treatment restored immune homeostasis within cGVHD-affected oral tissues. Reduction in mucosal IL-17+ and other immune inflammatory and effector cells was paired with restoration of peripheral- and tissue-level CD4 Treg cells. These shifts were paralleled with a reduction in local TGF-β and macrophages, key regulators linked to fibrosis, and OM tissue remodeling, which support the proposed mechanisms of action underlying the efficacy of belumosudil for cGVHD. These results highlight the potential of belumosudil to address key pathological processes in oral cGVHD and improve patient outcomes. Finally, this study highlights the feasibility and importance of tissue-level analysis in assessing the effects of cGVHD therapy. Incorporation of effector-site analysis in future cGVHD therapeutic trials could generate evidence to guide patient-specific treatment decisions.48
Acknowledgments
The authors thank all the patients, caregivers, health care professionals, ROCKstar investigators, members of the National Institute of Dental and Craniofacial Research (NIDCR) Imaging Core and NIDCR Dental Clinic teams, and the investigators and team members at the Dana-Farber Cancer Institute, MD Anderson Cancer Center, and Washington University in St. Louis study sites.
The Mays Laboratory is funded by the Intramural Research Program of the NIDCR, National Institutes of Health (NIH).
This study was funded by Kadmon Corporation, LLC, which is now a subsidiary of Sanofi. Medical writing support was provided by Bridget Colvin, Helen Jones, and Andy Brown of Envision Pharma Group, and was funded by Sanofi. Publication tracking by Envision Pharma Group was funded by Sanofi. Additional support was provided by the Intramural Research Program of the NIDCR, the NIH (ZIA DE000747 [J.W.M.]), and the Intramural Program of the National Cancer Institute, NIH (1ZIABC011743 [S.Z.P.]). Imaging support came from the NIDCR Imaging Core (ZIC DE00075).
Authorship
Contribution: S.Z.P. and J.W.M. designed the study; R.S. and J.W.M. designed laboratory analyses; R.S., A.C.C.-d.-S., D.P.M., F.B., and A.J. performed the experiments; R.S., N.G.H., and J.W.M. analyzed and interpreted the data, prepared figures and tables, and wrote the manuscript; N.G.H., I.P., C.C., N.T., R.S.M., A.S.A., N.V., O.S., S.M., Y.Y., R.J., B.H., K.M., B.R.B., S.J.L., S.Z.P., and J.W.M. collected and analyzed the clinical data; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: C.C. reports consulting fees/honoraria from CareDx, CSL Behring, Incyte Corporation, Sanofi, and Syndax; and reports consulting fees and/or equity from Cimeio Therapeutics, Orca Bio, and Oxford Immune Algorithmics. N.T. reports consultancy roles for Alira Health LLC, AstraZeneca, MuReva Phototherapy Inc, and Teladoc Health Inc. R.S.M. reports being an Endpoint Adjudication Committee reviewer for Orca Bio. A.S.A. reports participation in an advisory board for Sanofi. O.S., S.M., Y.Y., R.J., B.H., and K.M. are employees of Sanofi and may hold stock/stock options in Sanofi. B.R.B. reports research funding from BlueRock Therapeutics and Carisma Therapeutics; and reports consulting fees from BlueRock Therapeutics, GentiBio Inc, Janssen Oncology, Legend Biotech, Magenta Therapeutics, and Sandoz. S.J.L. reports consultancy roles for Incyte Corporation, Novartis, and Sanofi; reports research funding from AstraZeneca, Pfizer, Sanofi, and Syndax; reports drug supply from Janssen; reports steering committee memberships for Incyte Corporation and Sanofi; and reports membership of the board of directors of the National Marrow Donor Program (uncompensated). S.Z.P. reports research support from the Center for Cancer Research at the National Cancer Institute through the National Institutes of Health Intramural Research Program (including Clinical Research Development Agreements with Actelion, Celgene, Eli Lilly, Kadmon [now Sanofi], and Pharmacyclics). J.W.M. reports an uncompensated consultancy role for Incyte Corporation. The remaining authors declare no competing financial interests.
The current affiliation for R.S.M. is Division of Cancer Medicine, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, Houston, TX.
Correspondence: Jacqueline W. Mays, National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Dr, Bethesda, MD 20892-1203; email: jacqueline.mays@nih.gov.
References
Author notes
R.S. and N.G.H. are joint first authors
S.Z.P. and J.W.M. are joint senior authors.
Qualified researchers may request access to patient-level data and related documents (including, eg, the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications). Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org.
The full-text version of this article contains a data supplement.